Abstract
The antioxidant capacity of gingerbread plum kernel protein fraction (albumin, globulin and glutelin) hydrolysates (GPKH) was studied. Gingerbread plum kernel protein fractions were hydrolyzed through a combined action of two digestive enzymes (pespsin and trypsin). The hydrolyzed fractions were subjected to antioxidant test via several chemical assays such as: DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity, hydroxyl radical-scavenging activity, reducing power and metal chelating activity. Total phenolic contents, amino acid composition and molecular weight distribution were also evaluated. The glutelin fraction hydrolysate showed the strongest antioxidative activity throughout the entire investigation: 79.09, 58.81, 52.08 % and 40.7 μg/mL GAE for DPPH, hydroxyl radical, chelating activity and total phenolics respectively. GPKH possess a molecular weight ranging from 300 to 4000 Da and also showed much more high reducing power than some common standards such as BHA and α-tocopherol indicating that, hydrolysates derived from gingerbread plum kernel protein could be a new antioxidants source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gingerbread plum is an exceptionally under-explored source of oilseed proteins that thrive in the arid and semiarid regions mainly in the Western part of Africa and Central America particularly Panama. A tree of family Chrysobalanaceae, it is also known by two other names, Neocarya macrophylla (Sabine) Prance and Parinari macrophylla Sabine (Frederick 1961). The tree produces fruits in form of an ellipsoid drupe, glabrous, yellowish-brown with grey warts on the surface, 4–5 cm long and 2.3–3.5 cm across, with a hard stone embedded in a thick pulp (Fig. 1a). The flesh is soft and yellowish when fresh, with a peculiar flavor sometimes likened to avocado. The endocarp contains one or two kernels (embryos) (Arbonnier 2004).
Gingerbread plum kernels are of high nutritional value (about 47 % oil and 20 % protein contents) (Amza et al. 2010) and comparable in proximate chemical composition to those of almond (Prunus dulcis L.) (Ahrens et al. 2005), cashew (Anacardium occidentale, L.) (Fetuga et al. 1974) and peanut (Arachis hypogaea L.) (Khalil and Chughtai 1983) seeds (Table 1). Additionally, they are a good source of certain amino acids, such as lysine, valine and phenylalanine (Amza et al. 2010), which is important for balancing the deficiency of these essential amino acids in cereal-based diets. The kernel is covered by a very thin brownish skin (Fig. 1b) and much of its weight is made up of the cotyledons. It has an average of 21.3 and 6.6 mm of length and width respectively with 115 kernels per 100 g (Amza et al. 2011). The kernels are mostly consumed roasted and enjoyed like cashews or almonds. Some are consumed as snacks, others mixed into cooked dishes, etc. Therefore, to add value to the use of these lesser-known kernels, new opportunities for their application need to be explored.
In recent years, many studies have shown that plant derived protein hydrolysates possess significant antioxidant ability. Reports on the antioxidant properties of the proteins of various oilseeds such as peanut (Hwang et al. 2010), almond (Wijeratne et al. 2006), cashew nut (Andrade et al. 2011) etc. have been published. So far, although gingerbread plum kernels are similar in terms of physicochemical aspects (Table 1) to the above well-known and more investigated oilseeds; information on its antioxidant properties is non-existent.
Nutritional and chemical composition of gingerbread plum fruits and kernels (Cook et al. 2000; Amza et al. 2010), biological activities of the fruits (Audu et al. 2005), functional properties of the kernel proteins (Amza et al. 2011) and antioxidant activities of the fruits and shells (Cook et al. 1998) have been reported. However in-depth, antioxidant characterization in gingerbread plum kernel proteins remains unexplored and was therefore the focus of the current study. In the present work, the antioxidant properties of gingerbread plum kernel various protein fractions were evaluated through several chemical assays: DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity, reducing power, hydroxyl radical-scavenging activity, metal chelating activity and total phenolic contents (TPC). Additionally, amino acid composition and molecular weight distribution were also evaluated to determine their relationship with the antioxidant activity.
Materials and methods
Materials
Gingerbread plum kernels were obtained from Birni N’Gaouré, southern region of Republic of Niger. The kernels were kept dried in a desiccator at room temperature until use. DPPH (2,2-diphenyl-1-picrylhydrazyl), Pyrocatechol Violet, Pepsin (E.C. 3.4.23.1, 800–2500 units/mg) and Trypsin (E.C. 3.4.21.4, trypsin >250 N.F. units/mg) were products of Sigma Chemical Co. (St. Louis, MO, USA). Gallic acid, 1,10—Phenanthroline were produced by Shanghai Chemical Reagents Company of China (Sinopharm chemical reagent Co., Ltd., Shanghai). All other chemicals and reagents were of the highest grade commercially available.
Preparation of defatted gingerbread plum kernels flour
The kernels were milled using a laboratory scale hammer miller. Firstly, the resulting paste was dispersed in n-hexane at paste to n-hexane ratio of 1:5 (w/v) and stirred for 2 h at room temperature. Secondly, the semi defatted flour was fully defatted in a Soxhlet extraction apparatus using n-hexane. After completion of extraction, the flour was spread on a plate for 2 to 4 h at room temperature under a laboratory fume hood to remove the traces of solvent. The defatted flour was triturated, screened through 60 mesh sizes, packed in polyethylene bags and stored in a refrigerator at 5 °C until use (Amza et al. 2011).
Fractionation of gingerbread plum kernel proteins according to the Osborne method
The defatted gingerbread plum kernels flour (50 g) was first fractionated by extracting with 250 mL of distilled water for 60 min and centrifuging at 10,000 rpm for 30 min (High-speed refrigerated centrifuge CR21 III, Hitachi Koki Co., Ltd. Japan) to obtain the albumin fraction (supernatant). The residue obtained after the previous step was extracted with 250 mL of 5 % NaCl solution for 60 min followed by centrifugation at 10,000 rpm for 30 min to obtain the globulin fraction (supernatant). 250 mL of 0.1 M NaOH and 250 mL of 70 % ethanol were used to extract the glutelin (from the residue obtained after globulin extraction) and prolamin (from the residue obtained after glutelin extraction) fractions respectively under the same conditions as described previously. To further improve the yield of the protein fractions, each extraction step was repeated twice.
Salt in the globulin extract was removed by dialysis at 4 °C using cellulose membrane against 20 volumes of de-ionised water, for 72 h with water changes every 24 h. For glutelin fraction, pH of the extract was adjusted to 4.0 with 1 M HCl and centrifuged for 10 min to obtain the precipitate which was then resuspended in five volumes of de-ionised water and adjusted to pH 7.0 with 1 M NaOH. Ethanol in the prolamin fraction was evaporated from the extract using Rotary Evaporator (RV 10 Basic IKA® Rotary Evaporator) at 40 °C. All extracted protein fractions were lyophilized using a lab-scale freeze-dryer (Floor model Freeze Dryer, serial No. 050639219 A, Labconco Co., Kansas, USA) with vacuum collector at −52 °C and absolute pressure at 0.035 mbar and kept at 5 °C until use.
Determination of protein content
Total protein content of gingerbread plum kernel protein fractions (albumin, globulin, glutelin and prolamin) was evaluated using a K06C-type, FOSS nitrogen analyzer and a conversion factor of 6.25.
Hydrolysis of gingerbread plum kernel protein fractions with digestive enzymes
The lyophilized gingerbread plum kernel protein fractions were dissolved at 1 % (w/v) protein in distilled water of pH 2. The pepsin solution was prepared at 0.1 % (w/v) in distilled water of pH 2. The protein solutions were mixed with pepsin solution at the enzyme/substrate ratio of 1/100 (w/w). The mixtures were incubated at 37 °C for 3 h. The pepsin was first inactivated by adjusting the pH to 7 and then the 0.1 % (w/v) trypsin solution was added at the same enzyme/substrate ratio and further incubated at 37 °C for 3 h. Subsequently, the protein solutions were boiled in a water bath at 95 °C for 15 min to inactivate the enzymes and then centrifuged at 10,000 × g for 30 min. The supernatants were lyophilized to obtain protein hydrolysate powders and kept at −20 °C until use (Chanput et al. 2009).
Antioxidant properties of gingerbread plum kernel protein fractions
Determination of total phenolic content (TPC)
Standard solution was prepared using gallic acid solution at a concentration of 0.1–1.0 mg/mL. The reaction mixture is composed of 50 μl of standard or sample solution, 200 μl of freshly prepared Folin–Ciocalteau reagent, and 3 mL of distilled water. The mixture is left at room temperature for 10 min and 500 μl of 20 % sodium carbonate was added. The solution was mixed and incubated in water bath at 40 °C for 20 min and the reaction was stopped in an ice bath. The absorbance was measured at 765 nm and distilled water was used as a blank (Zhou and Yu 2004). TPC contents were quantified and expressed as Gallic Acid Equivalent (GAE) from a calibration curve; y = 0.9362x + 0.0793 (R2 = 0.9892).
Reducing power
The reducing power of gingerbread plum kernel protein fractions hydrolysates (GPKH) was determined according to Ebrahimzadeh et al. (2010). Two milliliters of sample (0, 1, 2, 3, 4 and 5 mg of protein hydrolysate powder/mL of protein solution) was mixed with a phosphate buffer (2 mL, 0.2 M, pH 7.0) and potassium ferricyanide [K3Fe (CN) 6] (2 mL, 1 %). The mixture was incubated at 50 °C for 20 min. A portion (2 mL) of trichloroacetic acid (10 %) was added to the mixture to stop the reaction, which was then centrifuged at 3000 rpm for 10 min. The upper layer of solution (2 mL) was mixed with distilled water (2 mL) and FeCl3 (400 μl, 0.1 %) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power.
DPPH radical-scavenging activity
The scavenging activity of GPKH on DPPH was determined using the method described by Parthasarathy et al. (2009) with slight modifications. This method depends on the reduction of purple DPPH to a pale yellow colored diphenyl picrylhydrazine. The determination of the disappearance of free radicals was done using spectrophotometer. The remaining DPPH which showed maximum absorption at 517 nm was measured. Each protein fraction hydrolysate was prepared at a concentration of 1 mg of protein hydrolysate powder/mL of protein solution using distilled water. Two milliliters of a 0.1 mM DPPH ethanol solution were added to 1 mL of sample solutions. These are test solutions (A1). Two milliliters of ethanol (95 %) were added to 1 mL of sample solutions. These are blank solutions (A0). Two milliliters of DPPH solution plus 1 mL distilled water were used as a negative control (A2). As DPPH is sensitive to light, it is exposed to the minimum possible light. These solutions were allowed to react at room temperature for 30 min. The absorbance values were measured at 517 nm and results were determined using the following equation:
Hydroxyl radical-scavenging activity
The hydroxyl radical-scavenging assay was carried out using the method described by Li et al. (2008) with some modifications. Both 1,10-phenanthroline (1 mL, 0.75 mM) and FeSO4 (1 mL, 0.75 mM) were added to phosphate buffer (2 mL, pH 7.4) and mixed thoroughly. H2O2 (1 mL, 0.01 %) and gingerbread plum kernel protein fractions hydrolysates (1 mL of 2 mg of protein hydrolysate powder/mL of protein solution) were added. The mixture was incubated at 37 °C for 60 min, and the absorbance was measured at 536 nm. Results were determined using the following equation:
Where: A s : absorbance of sample; A 1 : absorbance of control solution containing 1,10-phenanthroline, FeSO4 and H2O2; A 0 : absorbance of blank solution containing 1,10-phenanthroline and FeSO4.
Metal chelating activity
The ability of gingerbread plum kernel protein fractions hydrolysates to chelate prooxidative Cu2+ was investigated according to the procedure described by Amadou et al. (2011). In the chelation test, 1 mL of 2 mM CuSO4 was mixed with 1 mL of pyridine (pH 7.0) and 20 μL of 0.1 % pyrocatechol violet. After the addition of 1 mL of samples (2 mg of protein hydrolysate powder/mL of protein solution), the disappearance of the blue color, due to dissociation of Cu2+ was recorded by measuring the absorbance at 632 nm after 5 min of reaction. The Cu2+ chelating activity of the samples was calculated as:
Determination of molecular weight distribution
Gingerbread plum kernel protein fractions hydrolysates were analyzed for molecular weight distribution according to the procedure described by Li et al. (2008). A Waters TM 600E Advanced Protein Purification System (Waters Corporation, Milford, MA, USA) was used. The hydrolysates were loaded onto TSK gel G2000 SWXL column (7.8 i.d. × 300 mm, Tosoh, Tokyo, Japan), eluted with 45 % (v/v) acetonitrile containing 0.1 % (v/v) trifluoroacetic acid at a flow rate of 0.5 mL/min and monitored at 220 nm. A molecular weight calibration curve was obtained from the following standards from Sigma: cytochrome C (12,500 Da), aprotinin (6500 Da), bacitracin (1450 Da), tetrapeptide GGYR (451 Da), and tripeptide GGG (189 Da). Results were processed using Millennium 32 Version 3.05 software (Waters Corporation, Milford, MA 01757, USA).
Amino acid composition
The lyophilized hydrolysate fractions were digested with HCl (6 M) at 110 °C for 24 h under nitrogen atmosphere. Reversed phase high performance liquid chromatography (RP-HPLC) analysis was carried out using an Agilent 1100 (Agilent Technologies, Palo Alto, CA, USA) assembly system after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μl) was injected on a Zorbax 80 A C18 column (4.6 i.d. ×180 mm, Agilent Technologies, Palo Alto, CA, USA) at 40 °C with detection at 338 and 262 nm. Mobile phase A was 7.35 mmol/l sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mmol/l sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as g of amino acid per 100 g of protein.
Statistical analysis
All experiments were conducted in triplicate with SPSS Inc. software (version 13.0). One-way analysis of variance (ANOVA) was used to determine significant differences between means, with the significance level taken at a = 0.05. Tukey’s HSD test was used to perform multiple comparisons between means.
Results and discussion
Protein contents of gingerbread plum kernel protein fractions
The glutelin (80.2 %) and albumin fractions (75.1 %) of gingerbread plum kernel protein yielded the highest protein content followed by globulin (53.7 %) and prolamin (2.0 %) (Table 2). The relative amounts of the fractionated proteins were calculated as 40.6 %, 27.6 %, 25.8 % and 6.48 % for glutelin, albumin, globulin, and prolamin fractions, respectively. Chanput et al. (2009) found the percent extraction from rice bran protein fractioned by the Osborne method was computable as 32.6 %, 30.9 %, 24.9 % and 11.6 % for glutelin, albumin, globulin, and prolamin fraction, respectively.
Phenolic contents of gingerbread plum kernel protein fractions hydrolysate (GPKH)
The total phenolics contents (TPC) of gingerbread plum kernel protein fraction hydrolysates (albumin, globulin and glutelin) are shown in Table 2. Values for total phenolics content were affected by protein type (14.6 μg/mL–40.7 μg/mL GAE). Chanput et al. (2009) reported a level of 0.46, 0.22, 0.14 and 0.26 mg/mL total phenolic contents present in albumin, globulin, prolamin and glutelin hydrolyzed fractions of rice bran protein respectively. Wijeratne et al. (2006) reported a total phenolic content of 8 mg (quercetin equiv/g of ethanolic extract) in almond whole seed. The marked differences of the results obtained for GPKH when compared with previous studies (Kornsteiner et al. 2006; Wijeratne et al. 2006) can be explained on the basis of two possible factors. First, gingerbread plum kernel protein fractions were subjected to enzymatic digestion (37 °C for 6 h); second, heating at 95 °C for 15 min was applied to inactivate the enzymes (pepsin and trypsin). The digestion and heating processes might cause the degradation of phenolics. Indeed, as reported by Chanput et al. (2009), phenolic contents in protein hydrolysate after enzymatic digestion decreased to almost half compared with those prior to hydrolysis.
Reducing power
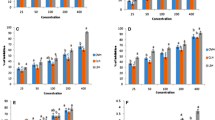
Figure 2 shows the reducing power (measuring the conversion of a Fe3+/ferricyanide complex to the ferrous form) of GPKH examined as a function of their concentration. The reducing power increased with concentration, and the values obtained for all the fractions were higher when compared with the trends observed by Li et al. (2008) and Amadou et al. (2011) for chickpea protein hydrolysate and fermented soy protein meal hydrolysate fractions respectively. At 1.0 mg of protein hydrolysate powder/mL of protein solution, the absorbance values were above 0.10 for all fractions, proving once more to have much more high reducing power than some common standards such as BHA and α-tocopherol. Reducing powers of BHA and α-tocopherol at 20 mM (3.6 and 8.6 mg/mL) were only 0.12 and 0.13, respectively (Mau et al. 2002). On the other hand, reducing properties for albumin and globulin were significantly lower (p < 0.05) than the values obtained for glutelin fraction (Fig. 2). This difference could be explained by the presence of high amounts of reductones (strong reducing agents, thus efficacious antioxidants), which could react with free radicals to stabilize and terminate radical chain reactions.
DPPH radical scavenging activity
The DPPH method is often used to evaluate the ability of antioxidants to scavenge free radicals which are known to be a major factor in biological damages caused by oxidative stress. This assay is known to give reliable information concerning the antioxidant ability of the tested compounds (Parthasarathy et al. 2009). The scavenging percentages (Table 2) on the DPPH radical at a dose level of 1.0 mg of protein hydrolysate powder/mL of protein solution for glutelin, albumin and globulin fractions were found to be 79.1, 49.6 and 47.8 % respectively. Lai et al. (2001) reported a radical scavenging activity (RSA) value of 71.7 % for α-Toc and BHT at a dose level of 0.31 mg/mL. The DPPH assay involves a reduction mechanism; therefore it is possible that albumin and globulin fractions probably had lesser DPPH reductones compared to that of glutelin. This might explain the significant differences (p < 0.05) in RSA values among the samples. Apart from this, variation in the antioxidant mechanism of active compounds in the different samples could also lead to these differences.
Hydroxyl radical-scavenging activity
1,10-Phenathroline (phen) is one of the most commonly used iron chelator for the prevention of iron-mediated ·OH formation in vitro, and also as tool to indicate the participation of iron in oxidative stress processes. Indeed, in the 1980s phen was a key tool to show that iron is involved in the H2O2-induced DNA degradation in cultured mammalian cells (de Avellar et al. 2004).
The ability of GPKH samples to prevent the formation of hydroxyl free radicals is shown in Table 2. At 2 mg of protein hydrolysate powder/mL of protein solution, scavenging effects were 47.3, 46.5 and 58.8 % for albumin, globulin and glutelin respectively. These results indicated that GPKH are good scavengers for hydroxyl free radicals when compared to the values reported by Mau et al. (2002) for BHA (23 %) and α-tocopherol (34 %) at concentration of 3.6 and 8.6 mg/mL respectively. Indeed, as reported by Ebrahimzadeh et al. (2010); in the presence of other competitive chelators, the Fe(phen) 2 +3 complex formation is disrupted with the result that the dark orange color of the complex decreases. This is an indication that, GPKH interfered with the complex formation process, confirming that the samples have the ability to scavenge Fe2+ ions thus disrupt the iron-mediated ·OH formation.
Metal chelating activity
The chelating activity of albumin, globulin and glutelin hydrolysates produced by pepsin and trypsin was measured using pyrocatechol violet (PV) and Cu2+. The complex of PV and Cu2+ absorbs blue light at 632 nm; while PV dissociated with a metal ion does not show this absorption (Saiga et al. 2003). Upon the addition of PV and Cu2+ to GPKH samples (1 mL of 2 mg of protein hydrolysate powder/mL of protein solution), the mixture resulted in a decrease of the blue color and a decline in the absorption at 632 nm. Table 2 shows the Cu2+ chelating activity of albumin, globulin and glutelin hydrolysates. The samples had Cu2+ chelating activity, with the activity of albumin and glutelin hydrolysates being higher (p < 0.05) than that of globulin hydrolysate. As reported by Saiga et al. (2003), some proteins have metal ion chelating activities. For example, Fe ion in hemoglobin is coordinated by the nitrogen in the imidazole ring of His (histidine), and some enzymes retain metal ions by metal chelation by their amino acid residues (Sarkar 1987). Peptides as well as proteins have chelating activity. Thus, the acidic and/or basic amino acids of the peptides in GPKH might play an important role in the Cu2+ chelation.
Molecular weight distribution
The chromatographic data (Fig. 3) showed that gingerbread plum kernel protein fractions digested with pepsin and trypsin (6 h) were composed of low molecular weight peptides with major peaks located at 736–1439 Da (39.06 %) and 384–736 Da (56.08 %) for glutelin; 708–1397 Da (49.84 %) and 390–708 Da (54.63 %) for albumin followed by globulin, 712–1397 Da (50.63 %) and 352–712 Da (53.83). A study on Alcalase hydrolyzed whey protein (Doucet et al. 2003) also revealed that more than 80 % was small peptides with of molecular weight <2000 Da after 5 h of hydrolysis. The glutelin fraction hydrolyzate presenting a high level of peptides in the molecular range of 384–736 Da (56.08 %) also showed the highest antioxidant activity (Table 2). Indeed, studies on whey protein hydrolysates (Peña-Ramos and Xiong 2001) and fermented soybean protein meal hydrolysate (Amadou et al. 2011) have shown that short peptides with molecular weight ranging from 370 to 1500 Da were responsible for higher antioxidant activity.
Amino acid composition
GPKH samples were subjected to amino acid composition analysis (Table 3) in order to determine the possible effect of the amino acid profile on the antioxidant activity. Although the major constituent amino acids of all the hydrolysates were Asp, Glu, Arg and Leu, some differences were remarkable. Glu content was noticeably higher in albumin hydrolysate, whereas glutelin hydrolysate showed the highest contents of Asp, Val, Phe, Ile, Leu and Pro. Based only on the content of these amino acids in the three samples, slight differences in the antioxidant activity of these hydrolysates could be expected. According to previous reports (Ren et al. 2010; Amadou et al. 2011), the antioxidant activity of peptides was highly dependent on their sequence and the amino acid composition. Hydrophobic amino acid residues Val or Leu at the N-terminus end and Pro, Asp, His or Tyr in the sequences of antioxidative peptides have been reported to be important in antioxidative activity (Ren et al. 2010). On the other hand, Met, an important methyl donor, is an efficient scavenger of almost all oxidizing molecules under physiological conditions; whereas, Cys is necessary for GSH synthesis, a cysteine-containing tripeptide, that is a source of dietary sulfur (Atmaca 2004). The glutelin hydrolysate having the highest hydrophobic amino acids content; 34 % (Table 3) clearly showed the highest antioxidant activity (Table 2). Therefore, the presence of these hydrophobic amino acid residues and their probable better positioning in the sequence could explain the high antioxidative activity observed in glutelin hydrolysate compared to albumin and globulin fractions.
Conclusions
According to the results, it is possible to observe a significant correlation between the total phenolic content in GPKH and the antioxidant activities. Glutelin hydrolysate, presenting the highest phenolic content also presented significantly (p < 0.05) higher antioxidant activities. So, the present study demonstrates for the first time that gingerbread plum kernel protein hydrolysates obtained from the digestion of albumin, globulin and glutelin fractions contained phenolic contents, and showed antioxidant activities, such as DPPH radical scavenging activity, hydroxyl scavenging activity, metal chelating activity and reducing power. The antioxidant peptides from gingerbread plum kernel proteins fractions possess a molecular weight ranging from 300 to 4000 Da. Therefore, the results of this study may encourage potential utilization of hydrolysates derived from gingerbread plum kernel protein as a new antioxidants source.
References
Ahrens S, Venkatachalam M, Mistry AM, Lapsley K, Sathe SK (2005) Almond (Prunus dulcis L.) protein quality. Plant Foods Hum Nutr 60:123–128
Amadou I, Le GW, Shi YH, Jin S (2011) Reducing, radical scavenging, and chelation properties of fermented soy protein meal hydrolysate by lactobacillus plantarum LP6. Int J Food Prop 14:654–665
Amza T, Amadou I, Kamara MT, Zhu KX, Zhou HM (2010) Chemical and nutrient analysis of gingerbread plum (Neocarya macrophylla) seeds. Adv J Food Sci Technol 2:191–195
Amza T, Amadou I, Zhu KX, Zhou HM (2011) Effect of extraction and isolation on physicochemical and functional properties of an underutilized seed protein: gingerbread plum (Neocarya macrophylla). Food Res Int 44:2843–2850
Andrade TJAS, Araújo BQ, Citó AMGL, Silva J, Saffi J, Richter MF, Ferraz ABF (2011) Antioxidant properties and chemical composition of technical cashew nut shell liquid (tCNSL). Food Chem 126:1044–1048
Arbonnier M (2004) Trees, shrubs and lianas of West African dry zones. CIRAD, Margraf Publishers GMBH MNHN, Cote d’Ivorie, p 194
Atmaca G (2004) Antioxidant effects of sulfur-containing amino acids. Yonsei Med J 45:776–788
Audu OT, Oyewale AO, Amupitan JO (2005) The biological activities of secondary metabolites of Parinari macrophylla Sabine. Chem Class J 2:19–21
Chanput W, Theerakulkait C, Nakai S (2009) Antioxidative properties of partially purified barley hordein, rice bran protein fractions and their hydrolysates. J Cereal Sci 49:422–428
Cook JA, VanderJagt DJ, Dasgupta A, Mounkaila G, Glew RS, Blackwell W, Glew RH (1998) Use of the trolox assay to estimate the antioxidant content of seventeen edible wild plants of Niger. Life Sci 63:105–110
Cook JA, VanderJagt DJ, Pastuszyn A, Mounkaila G, Glew RS, Millson M, Grew RH (2000) Nutritional and chemical composition of 13 wild plant foods of Niger. J Food Compos Anal 13:83–92
de Avellar IGJ, Magalhães MMM, Silva AB, Souza LL, Leitão AC, Hermes-Lima M (2004) Reevaluating the role of 1, 10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage. Biochim Biophys Acta 1675:46–53
Doucet D, Otter DE, Gauthier SF, Foegeding EA (2003) Enzyme-induced gelation of extensively hydrolyzed whey proteins by Alcalase: peptide identification and determination of enzyme specificity. J Agric Food Chem 51:6300–6308
Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B (2010) Antihypoxic and antioxidant activity of hibiscus esculentus seeds. Grasas Aceites 61:30–36
Fetuga B, Babatunde G, Oyenuga V (1974) Composition and nutritive value of cashew nut to the rat. J Agric Food Chem 22:678–682
Frederick RI (1961) Woody plants of Ghana. Oxford University Press, London, p 868
Hwang JY, Shyu YS, Wang YT, Hsu CK (2010) Antioxidative properties of protein hydrolysate from defatted peanut kernels treated with esperase. LWT Food Sci Technol 43:285–290
Khalil JK, Chughtai MID (1983) Chemical composition and nutritional quality of five peanut cultivars grown in Pakistan. Plant Foods Hum Nutr 33:63–70
Kornsteiner M, Wagner K, Elmadfa I (2006) Tocopherols and total phenolics in 10 different nut types. Food Chem 98:381–387
Lai LS, Chou ST, Chao WW (2001) Studies on the antioxidative activities of hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 49:963–968
Li Y, Jiang B, Zhang T, Mu W, Liu J (2008) Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem 106:444–450
Mau JL, Lin HC, Song SF (2002) Antioxidant properties of several specialty mushrooms. Food Res Int 35:519–526
Parthasarathy S, Azizi JB, Ramanathan S, Ismail S, Sasidharan S, Said MIM, Mansor SM (2009) Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from mitragyna speciosa (Rubiaceae Family) leaves. Molecules 14:3964–3974
Peña-Ramos EA, Xiong YL (2001) Antioxidative activity of whey protein hydrolysates in a liposomal system. J Dairy Sci 84:2577–2583
Ren J, Zheng XQ, Liu XL, Liu H (2010) Purification and characterization of antioxidant peptide from sunflower protein hydrolysate. Food Technol Biotechnol 48:519–523
Saiga A, Tanabe S, Nishimura T (2003) Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem 51:3661–3667
Sarkar B (1987) Metal protein interactions. Prog Food Nutr Sci 11:363–400
Wijeratne SSK, Abou-Zaid MM, Shahidi F (2006) Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem 54:312–318
Zhou K, Yu L (2004) Effect of extraction solvent on wheat bran antioxidant activity estimation. LWT Food Sci Technol 37:717–721
Acknowledgments
This project was funded by the Fundamental Research Funds for the Central Universities (JUSRP10922).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amza, T., Amadou, I., Balla, A. et al. Antioxidant capacity of hydrolyzed protein fractions obtained from an under-explored seed protein: Gingerbread plum (Neocarya macrophylla). J Food Sci Technol 52, 2770–2778 (2015). https://doi.org/10.1007/s13197-014-1297-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1297-7