Abstract
Ozone dose from 0.1 to 0.4 ppm has been proven to be effective in lowering Bacillus cereus count in uncooked and cooked rice. However, it induces physicochemical changes in raw white rice. Physicochemical tests were done to see the effect of ozone treatment towards moisture content, pH, color, hardness of uncooked rice, adhesiveness and hardness of cooked rice, cooking quality and total solids. Results have shown that moisture content, adhesiveness and hardness of cooked rice and uncooked rice have not undergone any significant changes (P > 0.05) in comparison with controlled rice sample. Meanwhile, color (L* and b* value), pH, total solids and cooking quality results have shown significant changes (P < 0.05). These analyses proved that limitations should be applied to ozone concentration and exposure time to minimize any detrimental effects on the physicochemical characteristics of rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice has been the staple food to Malaysians for more than a century. According to statistics given by Padiberas Nasional Berhad (BERNAS), more than 600 million tons of rice are stored every year in BERNAS’ factories all over Malaysia (Anonymous 2009a).
However, insects and fungi create serious quality issues in stored rice and estimated annual storage losses range from 30 to 42 % (Anonymous 2009b). The appropriate method to eradicate pests from food grains is fumigation. Currently, there are only two registered fumigants for stored food grains; methyl bromide and phosphine. However, under Montreal Protocol, developing countries, including Malaysia, have to phase out methyl bromide usage by 2015 (Anonymous 2009b). In the interim, phosphine is currently being reviewed by Crop Protection & Plant Quarantine Division, Ministry of Agriculture, Malaysia. Assuming that phosphine has been legalized, it would be the only licensed fumigant for stored food grains particularly, rice. With only one fumigant remaining, insect and microorganisms resistance becomes a greater risk. It has been shown that some stored product insects are already exhibiting some levels of phosphine resistance and some have shown resistance to methyl bromide (Zettler et al. 1989; Zettler and Cuperus 1990).
Ozone has been reported to have hostile effects towards insects, fungi and its anti-microbial characteristics have been proven to effectively kill 90 % of microbial populations (Ishizaki et al. 1986; Shah et al. 2011). Moreover, ozone has been deemed as a safe fumigation alternative and a good anti-microbial agent and it is preferred in food handling and hygiene industry. The advantage of ozone is the short half life (20 – 50 min in water) compared to other fumigation chemicals and it does not leave a residue. Currently, ozone is being classified as Generally Recognized as Safe (GRAS) (Anonymous 1982).
Loss of resistance to remaining fumigant and a trend by consumers to move away from residual chemicals, necessitates development of additional control strategies, using gaseous ozone. Therefore, the objective of this study is to determine the effects of physicochemical characteristics of ozone-treated rice.
Materials and methods
Production of ozone treated rice
Ozone treatment on rice was performed in a gas tight Lieberg condenser (0.025 m diameter x 0.56 m height) placed in an air-conditioned room of 20 ± 3 °C. Relative humidity measured by a digital temperature/humidity measurement device (Model HT88, CPU, China) with an accuracy of ± 0.1 was recorded at 50 % to ensure uniformity throughout the whole experiment. The glass condenser was connected to an ozone generator (Model OM-1, Top Ozone, Malaysia) through a Serto tube in which ozone was produced from the ambient air. In order to generate the required level of ozone, corona discharge method was used in this type of ozonator. Ozone detector (Aeroqual, New Zealand) and hot wire anemometer (TA5, Airflow, USA) were connected to a Lieberg condenser, where ozone detector was used to determine the levels of ozone exposure on rice and the latter was used to ensure the flow rate of ozone is stable throughout the experiments. Samples were treated, removed and tested for physicochemical properties before being stored in polyethylene bag at room temperature prior to sensory evaluation tests.
Properties of rice
pH measurement
pH of ozone-treated rice was measured according to AACC method 02–52 (AACC 1962). Ground rice samples (1 g) were homogenized in 9 ml distilled water. The rice suspension was placed on a stirrer and was stirred for 60 s. The suspension was filtered with Whatman No. 4 filter paper. pH of the supernatant was measured with pH meter (Model 3305, Jenway Ltd, England) which has been calibrated with buffer pH 4 and 7.
Color measurement
Objective color measurements were done using a spectrophotometer (Spektrophotometer, Ultrascan PRO Hunter Lab USP1092, Virginia, USA) based on three values; L*, a* and b*. The instrument was calibrated against a standard white reference tile (L* = 99.52, a* = 0.01, b* = −0.056). The measurements were taken with a 0.6 cm2 measuring area. The color measurement was first taken for the controlled sample and then again for ozone-treated rice. The color measurements of the controlled sample were used as the standard. L*, a*, and b* values were taken in triplicate.
Moisture content
Moisture content of ozone-treated rice was measured using a digital grain moisture meter (Model SS-6, Satake, Japan) in comparison to the traditional oven method (Pearson 1976). The readings were then compared to the traditional oven method before further moisture content testing was done using this machine. The results were comparable, with the error of ±0.05 for both methods. Thus, this instrument was then used for further testing.
Cooking quality
Water absorption of rice during cooking was determined using Saburlase et al. (1991) method with added modifications. Distilled water (20 mL) and milled rice (2 g) were placed in a test tube which was covered with a cotton plug and heated at 80 °C in a covered thermostatically-controlled water bath (PROTECH, Malaysia). The milled rice samples were allowed to cook for 30 min, cooled, drained and placed upside down for 1 h and carefully weighed. The increase in weight was calculated and the water absorption was reported as g of water per g of milled rice.
Total solids
To measure total solids, cooking water (10 mL), was obtained after rice was cooked for 30 min at 80 °C, and placed into a previously dried and weighed porcelain dish. The sample was then dried in an oven for 8 h at 130 °C. The total solid was calculated using Eq. 1 (Saburlase et al. 1992; Sirisoontaralak and Noomhorn 2006a, b).
Where,
- TS:
-
Total solids
- SR:
-
Solids recovered from the cooking water (g)
- Sample:
-
Rice sample used (100 g)
Hardness of uncooked rice
Texture Analyzer (Model TA.XT2i, Stable Micro Systems, England) was used to measure the hardness of uncooked rice equipped with 5 kg-capacity compression load cell. The crosshead speed was 0.5 mm/s. The plunger test cell consisted of a plastic of 0.5 mm diameter and 3 cm in length. Selected uncooked grains of rice were placed on a cell base consisting of stainless steel cylinder of 8 cm in diameter. The plunger was allowed to move downward until the sample was compressed to 90 % of its size. The hardness of uncooked rice was determined from the maximum extrusion force in terms of kg force (kgf). The breaking strength was measured and read on the force versus deformation curve by the value of the first peak force (kg) of rice penetration.
Hardness and adhesiveness of cooked rice
Twenty cooked rice granules were equilibrated for 1 h in a closed plastic bottle at 25 °C to measure the hardness and adhesiveness of cooked rice granules using a texture analyzer (TA-XT2, Stable Micro Systems, Surrey, England). Analysis of textural characteristics was based on the procedure of Mua and Jackson (1997). Each cooked rice granule was compressed to 90 % deformation of its original height with a 20 mm diameter probe at a constant speed (0.5 mm/s). Each test sample was compressed thrice, each compression being followed by decompression. The time interval between the end of the first compression and the second compression was 3 s. The first peak force (gf) was termed hardness, and the negative force area of the curve during retraction of the probe was termed adhesiveness (gf).
Scanning electron microscopy (SEM)
To verify the grain structure, milled rice were broken by hand and attached to a Scanning Electron Microscopy (SEM) stubs. Specimens were then coated with gold by a Sputter Coater Baltec (SCD 005 SEM Coating Unit, Liechtenstein, Germany). The cross–section was observed by SEM (Philips XL30 ESEM, Eindhoven, Netherlands) at a magnification of 1000 and 2500 times. Images were then recorded and visually analyzed.
General statistical analysis
Full factorial design with two fixed levels and triplicate were done in order to collect data. Normal probability plotting was then performed in order to assess any outliers to the populations. One way analysis of variance (ANOVA) was done using SPSS Release 12.0 software and followed by a post-hoc analysis using Tukey’s HSD test with 95 % confidence interval to see the difference in comparison with untreated samples. Pearson’s correlation coefficients were calculated for all physicochemical properties tests.
Results and discussion
Preliminary studies
This subchapter presents the output obtained from preliminary tests to determine the initial value of physicochemical analysis on controlled samples. Each of these tests was performed independently, i.e. using different sample sets. The results obtained provide a guide to the actual investigation on effects of different ozone treatments on rice grains.
The values presented in Table 1 were considered as a standard in comparing the results of ozone-treated rice samples. Moisture determination in this study is important to maintain the optimum weight and density of rice after it is cooked. Moisture content for the controlled sample measured using dry basis was at 13.43 %. Generally, the milled rice will be kept and maintained at 12 % dry moisture content to avoid pest and micro-infestation. However, it was difficult to conform to the ideal moisture content in relative humidity of Malaysian weather. Thus, throughout this study, the effect of ozone on the moisture content of treated rice will be monitored closely. Compared means of ozone-treated and controlled rice samples will then ascertain the effects of ozone treatments onto physicochemical and grain structure of rice.
Effects of ozone exposure levels on physicochemical characteristics of rice
pH of controlled sample (without ozone treatment) was recorded at 6.15. However, as the ozone concentrations and exposure time increased, pH of ozone-treated rice samples were observed to decrease generally. From Fig. 1a, the range of pH decreased to 5.43, 5.34, 5.30 and 5.23 for 0.1, 0.2, 0.3 and 0.4 ppm ozone concentrations level respectively. The lowest value for pH was recorded at 0.4 ppm and 360 min of exposure time with pH value of 5.23. However, all the results from the recorded data were not significantly different (P < 0.05) when compared to controlled sample.
A decreasing trend of pH of ozone-treated rice was detected and it might occur from the oxidation of rice compound by ozone leading to the formation of acidic products. Langlais et al. (1991) stated that ozone can oxidize carbohydrates, amino acids and unsaturated fatty acid compounds. This phenomenon could be explained by hydroxyl molecules that were produced as ozone by–product. Ozone treatment is highly efficient at low temperature and high humidity as the free radicals (−O) would react with the hydrogen molecule (−H) from the ambient air which in the end will produce hydroxyl radicals (−OH). This would cause the ozone-treated rice samples to have low pH value. This, however, did not have any detrimental effect on the quality of rice. It nevertheless, gave an added advantage by impeding Bacillus cereus count in raw white rice, since Bacillus cereus does not thrive (it dies slowly) in an environment with pH value less than 6 (Brown 2000; Shah et al. 2011). Decomposition of ozone increases at high pH due to the reaction of ozone with hydroxyl ions, resulting in the formation of free radicals (Kim et al. 1999). El-Din et al. (2006) found that the oxidation of linoleic acid and abietic acids in the presence of ozone (7 to 11 mg/L) increased as pH increased from 8 to 11 due to the production of highly reactive intermediates such as hydroxyl radicals.
Figure 1b shows a general trend of decreasing moisture content of ozone-treated rice in a period of 420 min. The moisture content of controlled sample ranges from 13.40 to 13.43 % (db). And the moisture content observed after 420 min of ozonation is at 12.97, 12.86, 12.47 and 12.29 % for 0.1, 0.2, 0.3 and 0.4 ppm of ozone concentration respectively. Rice treated with ozone at high ozone concentration and exposure time retained the least moisture. Results from ANOVA shows that ozone concentration (P < 0.05) has more significant impact on the moisture content after 420 min of ozone treatment when compared to exposure time (P > 0.05).
With stipulations of moisture migration and retention after ozone treatment, it was noted that rice treated with ozone for a short time retained more moisture compared to rice treated for a longer period (P > 0.05). Moisture loss was more severe for rice sample treated for 420 min compared to 360 min of ozone treatment. Sixty minutes of extra ozone exposure caused 5.85 % of moisture difference with similar ozone concentration used. Another observation noted was moisture content of ozone-treated rice tended to decrease after a certain period of storage. This depreciation might be the result of oxidation from leftover ozone exposure that were captured inside the polyethylene bag where the rice were kept immediately after the ozone treatment to avoid cross – contamination. The higher the ozone concentration used for treatment, the lower will be the resulting moisture content after a certain period of storage. From the test, it was observed that the rice treated at the same amount of time had lower moisture content percentage as the ozone concentration increased.
This result was supported by Zheng and Lan (2007), as it was noted that ozone caused oxidation to rice kernel that eventually lowers surface moisture content of rice. This is an added-advantage to pest control management as lower moisture content discouraged insect infestation in the grain. It was also reported by Pan et al. (2008) that rice grain are recommended to be kept in lower moisture content (12 – 13 %) than it is normally stored (at 13.5 – 14 %) to avoid pest infestation in rice especially Sitophilus oryzae (L.) which are prevalent in rice storage. Thus, it is recommended that the moisture content of ozone-treated rice to be monitored closely as not to cause any detrimental effect on the rice quality. In a study done by Zheng and Lan (2007), it was observed that moisture content of rice had a direct relation to taste quality. Fatty acid content of rice increased as moisture content drops which results in a higher degree of rice aging and restrains starch gelatinization during rice cooking. This sequentially, leads to degradation of rice taste.
The whiteness and translucence of rice is important for consumer perception of ‘fresh’ rice. Thus, the yellowness of rice kernels plays an important part to ensure that the rice is highly valuable. Yellowness of rice in this study is being valued in b* value. The range of b* value runs from yellow (positive values) to blue (negative values) using CIE L* a* b* model. Whereby, lightness of rice is being valued from the range of 0 (black) to 100 (superior white). In this study, it was reported that the color of milled rice changed from creamy white to yellow after treatment of ozone and yellowness (b* value) of milled rice increased with ozone dosage (concentration x exposure time) (Fig. 1c). The average b* value of controlled rice was 10.56. After being treated with ozone, even at the lowest dose (0.1 ppm), it increased significantly to 12.59 (P < 0.05). A significant increase was also detected ranging from 12.58 for 0.1 ppm to 13.74 for 0.4 ppm. According to Tukey’s test, these results were significantly different from each level of exposure time and when compared to the controlled specimen’s values.
This is in agreement with a study by Mendez et al. (2003) where it was found that the husk of rice treated with ozone at 50 ppm for 3 days was a darker shade of brown when compared to the controlled treatments. The author made an assumption that the color degradation was caused by the formation of nitric acid formed from the use of ambient air as an oxygen source to generate ozone. Whereby, Rich (1992) reported nitric acid formation from corona discharge generators was caused by humidity in the air. Thus, this observation indicates that the design of a commercial ozone generator should include a method to remove acidic by – products from the ozonated air stream to reduce yellowness of rice. However, yellowness could also occur because of aging or higher protein content. These observations may be explained by the breakdown of glycosidic and peptidic linkages promoted during ozone treatment. Breakdown products such as carbonyl and amino compounds react (Maillard reaction) further forming colored compounds (Sirisoontaralak and Noomhorn 2006b). Another probable cause of yellowing in ozone-treated rice is melanoidins formed due to oxidation of phenols (Roy et al. 1991).
Chalk or lightness is the opaque area in the rice grain and is deemed undesirable to the end user. Chalky areas cook differently from translucent areas, but only a very clever palate could detect the difference. In this study, it was shown in Fig. 1d, the lightness of ozone-treated rice increased as the ozone concentrations increased when compared to that of controlled rice. The average L* value for controlled rice samples were 69.39, based on random readings of rice without any ozone treatment. Meanwhile, the L* value for the treated sample showed an increase from 70.93 to 76.5 for 0.1 ppm ozone concentration. The L* value increased from 66.85 to 77.93 for 0.2 ppm, from 72.38 to 77.67 for 0.3 ppm and the maximum value were detected at 78.02 for 0.4 ppm from the initial readings of 67.41. All the results unless stated proved significant (P < 0.05) using ANOVA analysis after a cross reference testing were made against the controlled readings.
The mean differences detected between different concentrations were the highest at 0.4 ppm and the lowest at 0.1 ppm. A significant increase (P < 0.05) in L* value could have resulted from malformed starch granules with air spaces between them. These results were consistent with SEM images of broken rice kernel (Fig. 2a-e), where, it can be seen that ozone treatment caused a less-defined polygonal boundaries and smaller granule size to the overall rice structure. This will instigate changes in water binding capacity, starch solubility, total solids leached to cooking water, pasting viscosity and starch damage values.
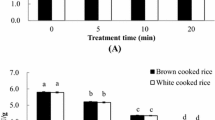
Figure 1e shows the cooking quality of treated rice affected by ozone treatment. The general trend showed a slight linear relationship between ozone concentration and exposure time. It was clearly shown that cooking quality increased as ozone dosages were increased intermittently. The average initial cooking quality of controlled rice samples were between 1.42 and 1.79 g water per g of rice. After 420 min of ozone treatment (0.1 – 0.4 ppm), the water absorbed ranged from 1.76 to 2.17 g/g. The average increment of cooking quality were detected between 17 and 24 %. A significant increase (P < 0.05) was seen at doses above 0.3 ppm. The change of cooking quality implied that the ozone-treated rice had the same characteristics of oxidized rice. Water uptake of rice treated with ozone as compared to those of controlled rice samples, significantly increased after ozone treatment at an interval of 60 min per treatment.
The increasing trend is in agreement with studies done by Saburlase et al. (1991) with gamma irradiation of raw rice. Protein and starch fragmentation from ozone caused an increase in the number of water binding sites. Thus, ozone treatment may disrupt the protein matrix around the starch granule which serves as a physical barrier to water absorption (Juliano et al. 1965). Chaudry and Glew (1973) claimed that oxidation may modify granules and enable water to penetrate with relative ease and after the physical impairment, smaller particle size of the starch increases the available surface area for hydration. This is also in conformity with a study done on aged rice (Gujral and Kumar 2003), where it was reported that aged rice had the characteristics of increased water uptake, elongation and width expansion.
Significant differences (P < 0.05) between total solids in cooking water from controlled and ozone-treated rice samples were also observed at doses above 0.3 ppm. A clear linear relationship can be discerned between ozone concentrations and total solids where high coefficients of correlations for all ozone concentrations were observed. R 2 ranged from 0.9661 to 0.9975 (Fig. 1f). It can be seen that the amount of solids loss of rice after processing increased, with the increase of ozone concentration and exposure time. Total solids calculated as gram of solids per 100 g of rice, were observed to have an end result of 2.89, 2.51, 2.03 and 1.81 (× 10−3) g solids/100 g of rice for 0.1, 0.2, 0.3 and 0.4 ppm from the reference average of 9.68 × 10−3 g solids/100 g rice. The increase of total solids in residual cooking water after ozone treatment concurred with Saburlase et al. (1991). Sirisoontaralak and Noomhorn (2006b) explanation of the increase in total solids in cooking water after ozone treatment was due to aging. Aging is a sol–gel transformation of colloidal starch and protein into a more stable, water–insoluble physical form during storage. Hogan (1963) reported increases in water–insolubility of rice starch during aging. Decrease in solids in cooking water of aged rice was confirmed by Gujral and Kumar (2003). Amylose and amylopectin structures disrupted to smaller linear fragments by ozone treatment which could result in difficulty of colloidal starch changing to a water insoluble form (Sirisoontaralak and Noomhorn 2006b). As a consequence there is greater vulnerability of ozone-treated rice to disintegrate during cooking. This explains why the cooked ozone-treated rice had more length ratio and distension compared to controlled rice. This phenomenon was also elucidated using Scanning Electron Microscopy (SEM) of uncooked rice kernels (Fig. 2a-e). It can be seen that irregular starch granules and bigger water binding sites of ozone-treated rice samples allowed release of starch and protein components.
Texture of raw, ozone-treated rice was observed as the gram force needed to compress 90 % of one rice kernel. The peaks of texture profile from Texture Analyzer were monitored in triplicate in order to see the normality profile of fit. It was shown that the coefficient of determination (R2) for each concentration was low and ranged between 0.0172 and 0.5384. This result is elucidated in the ANOVA analysis where it can be spotted that the ozone concentration and exposure times had a significant impact (P > 0.05) on the hardness of uncooked rice. Based on a study done by Singh et al. (1997), it was reported that chalky and aged rice kernel had lower adhesiveness, cohesiveness and maximum force values than the translucent kinds. The difference in textural properties may be attributed to differences in the amylase content, ratio of long/short chain of amylopectin chains and granular structures. Hardness of uncooked rice was observed to be related to the moisture content of the rice kernel itself. It was noted that as the moisture content of ozone-treated rice kernels decreased, so did the hardness of uncooked rice. This could be due to the brittleness that was caused by low moisture content of the rice kernel.
Texture profiling was done using Texture Analyzer with a compression of 90 % strain to emulate complete mastication during eating. It is described as the force needed to chew down cooked rice. Data analyzed using ANOVA showed that the impact of ozone concentrations (P < 0.05) were significant compared to exposure time (P > 0.05) that was subjected to the rice samples. The hardness of cooked, ozone-treated rice was less than that of cooked, controlled rice. Changes in the hardness of cooked, ozone-treated rice were more noticeable at 0.1 and 0.2 ppm, where less significant results were found when rice was subjected to 0.3 or 0.4 ppm of ozone concentration (Fig. 1h). The softer texture of cooked, ozone-treated rice was due to greater water absorption during cooking. Texture was relatively more slippery contributing to less extrusion peak force. During storage, the texture of cooked, ozone-treated rice became less sticky, less glossy, fluffier and harder. As described earlier, decreased moisture levels of ozone-treated rice samples were the cause of decreased raw rice hardness, although at an insignificant level, which ultimately led to decreased cooked rice hardness. The hardness decreased by 64, 63, 33, 48 % in 0.1, 0.2, 0.3 and 0.4 ppm at 12.65 % moisture content. This was in contrast to a study by Perez and Juliano (1979), where it was reported that hardness of cooked rice increased as moisture content decreased. However, the result of this study is in parallel with the results produced by irradiated raw rice (Sirisoontaralak and Noomhorn 2006b). It was reported that cooked rice hardness decreased as much as 37 % after 2.0 kGy of irradiation dosage were subjected to raw, low–amylase rice.
The main attributes of cooked rice are hardness and adhesiveness, with the adhesiveness parameter often more difficult to measure precisely than hardness. Adhesiveness was measured on ozone treated milled rice compressed by 90 % strain. The negative peaks from the second compression profile were taken as adhesiveness value (Fig. 1i). No statistical differences (P > 0.05) were observed in peak forces of adhesiveness between the ozone-treated rice compared to the control treatment. The data suggested that ozone treatment of rice does not affect its quality as measured by adhesiveness. Among the various treatment subjected to rice samples, it was noted that the highest ozone concentration (0.4 ppm) had the lowest adhesiveness of cooked rice. And 0.2 ppm had the lowest changes (P = 0.638) compared to adhesiveness of cooked, ozone-treated rice. This correlated with the moisture content and hardness of cooked, ozone-treated rice. As moisture content of ozone-treated rice decreased, it too, decreased the adhesiveness of cooked rice. This was supported by Mendez et al. (2003), where it was reported that there was no significant difference (P > 0.05) observed in peak force of adhesiveness between the ozone – treated rice compared to the controlled treatment.
Correlation between physicochemical properties of ozone-treated rice
The correlation coefficients between ozone dosages and rice properties are summarized in Table 2. Ozone concentration was positively correlated (P < 0.01) with b*, L* value, cooking quality and total solids. It was negatively correlated with hardness of cooked and uncooked rice and moisture content. It did not correlate significantly (P > 0.05) with pH and cooked rice adhesiveness. There was negative correlation with pH of ozone-treated rice. However, there were different findings about the correlation of these properties from different studies. For ozone-treated rice, it was reported that there was positive correlation between ozone concentration and adhesiveness of cooked rice (Mendez et al. 2003). However, it was reported in the same study that there was positive correlation (P < 0.05) with color of the rice grain treated with 50 ppm of ozone for 30 days. This is in agreement with the findings in this study.
Grain structure
Rice grain contains 90 % (dry basis) starch. For controlled rice sample, the polygonal–shaped starch granules of rice were clearly seen (Fig. 2e). Ozone induces less well–defined polygonal boundaries (Fig. 2a-d). Fusion of adjacent starch granules, composite clusters with a homogeneous interior, less well–defined polygonal boundaries and voids among the starch granules were observed after ozone treatment. The SEM images also showed reduction of the granule size. Many researchers reported these observations, similar the grain structure observed in irradiation treatment. It was also reported that irradiation may cause damage to starch granules and molecular structure as indicated by changes in water binding capacity, starch solubility, total solids leached to cooking water, pasting viscosity, absolute density and starch damage values. These properties were also demonstrated by ozone-treated rice samples, regardless of ozone dosage. In this study, changes of starch granule enable water to penetrate readily and allow release of starch or protein components. Reduction of granular size increases the surface area for hydration. These observations are consistent with the results in increased water absorption and total solids in residual cooking water after ozone-treatment. The released starch components could also be recognized by the slimy texture of cooked rice. This remark is in conformity with studies done on chalkiness, irradiation and aged rice. Chalky grain showed the presence of a single rather than a compound amyloplasts, air spaces and disordered granular structure (Perez and Juliano 1979).
Conclusion
As a conclusion, ozone has effectively shown its anti-microbial and fumigation characteristics that are invaluable in food industry, where effective applications to ensure safer food products are highly prioritized. Based on this study, sound advantages of ozone applications can be seen on rice. Even though, it does not leave any residue due to quick decomposition of its structure, certain after-effects on the rice, has been reported and restrictions should be applied through limitation of ozone concentrations or exposure time.
References
AACC (1962) Approved methods of AACC. Method 02–52. American Association of Cereal Chemists, St-Paul
Anonymous (1982) United States food and drug administration (1982) GRAS status of ozone. Fed Regist 47:50209–50210
Anonymous (2009a) Padiberas Nasional Berhad, Kuala Lumpur, Malaysia. Accessed: 25th July 2009
Anonymous (2009b) Ministry of Agriculture and Agro-based Industry, Putrajaya, Malaysia. Accessed: 25th July 2009
Brown KL (2000) Control of bacterial spores. Br Med Bull 56(1):158–171
Chaudry MA, Glew G (1973) The effect of ionizing radiation on some physical and chemical properties of Pakistani rice. J Food Technol 8:295–303
El-Din MG, Smith DW, Momani FA, Wang W (2006) Oxidation of resin and fatty acids by ozone: kinetics and toxicity study. Water Res 40:392–400
Gujral HS, Kumar V (2003) Effect of accelerated aging on the physicochemical and textural properties of brown and milled rice. J Food Eng 59:117–121
Hogan JT (1963) Rice research at Southern laboratory. Rice J 66:38–41
Ishizaki K, Shinriki N, Matsuyama H (1986) Inactivation of Bacillus spores by gaseous ozone. J Appl Bacteriol 60:67–72
Juliano BO, Onate LU, Del Mondo AM (1965) Relation of starch composition, protein content and gelatinization temperature to cooking and eating qualities of milled rice. J Food Technol 19:1006–1011
Kim JB, Yousef AE, Chism QW (1999) Use of ozone to inactivate microorganisms in lettuce. J Food Saf 19:17–34
Langlais B, Reckhow DA, Brink DR (1991) Ozone in water treatment application and engineering. Lewis Publisher Inc., Michigan
Mendez F, Maier DE, Mason LJ, Woloshuk CP (2003) Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. J Stored Products Res 39:33–44
Mua JP, Jackson DS (1997) Relationship between functional attributes and molecular structures of amylase and amylopectin fractions from corn starch. J Agri Food Chem 45:3848–3854
Pan Z, Khir R, Godfrey LD, Lewis R, Thompson JF, Salim A (2008) Feasibility of simultaneous rough rice drying and disinfestations by infrared radiation heating and rice milling quality. J Food Eng 84:469–479
Pearson D (1976) The chemical analysis of foods, 642–643. Chemical Publishing Company Inc, New York
Perez CM, Juliano BO (1979) Indication of eating quality for nonwaxy rice. J Food Chem 4:185–189
Rich T (1992) Basic comparison of ozone technology. Water Technol Magazine 17:51–52
Roy MK, Ghosh SK, Chatterjee SR (1991) Gamma irradiation of rice grains. J Food Sci Technol Mysore 28:337–340
Saburlase VC, Liuzzo JA, Rao RM, Grodner RM (1991) Cooking quality of brown rice as influenced by gamma irradiation, variety and storage. J Food Sci 56:96–98
Saburlase VC, Liuzzo JA, Rao RM, Grodner RM (1992) Physicochemical characteristics of brown rice as influenced by gamma irradiation. J Food Sci 57:143–145
Shah NNAK, Rahman RA, Mat Hashim D, Ling TC (2011) Application of gaseous ozone to inactivate Bacillus cereus in processed rice. J Food Proc Eng 34:2220–2232
Singh, N., Pisarczyk, K.S. and Sigmund, J.J. (1997) Catalytic destruction of ozone at room temperature. Paper presented at 90th Annual Meeting & Exhibition, Air & Wave Management Association, Toronto, Ontario, Canada, June 8–13
Sirisoontaralak P, Noomhorn A (2006a) Changes in physicochemical and sensory-properties of irradiated rice during storage. J Stored Prod Res 43(3):282–289
Sirisoontaralak P, Noomhorn A (2006b) Changes to physicochemical properties and aroma of irradiated rice. J Stored Prod Res 42:264–276
Zettler JL, Cuperus GW (1990) Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J Econ Entomol 83:1677–1681
Zettler JL, Halliday WR, Arthur FH (1989) Phosphine resistance in insects infesting stored peanuts in the southeastern United States. J Econ Entomol 82:1508–1511
Zheng X, Lan Y (2007) Effect of drying temperature and moisture content on rice taste quality. CIGR J 4:1–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, N.N.A.K., Rahman, R.A. & Hashim, D.M. Changes in physicochemical characteristics of ozone-treated raw white rice. J Food Sci Technol 52, 1525–1533 (2015). https://doi.org/10.1007/s13197-013-1111-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1111-y