Abstract
Physical properties of gelatin extracted from Unicorn leatherjacket (Aluterus monoceros) skin, which is generated as a waste from fish processing industries, were optimised using Response Surface Methodology (RSM). A Box-Behnken design was used to study the combined effects of three independent variables, namely phosphoric acid (H3PO4) concentration (0.15–0.25 M), extraction temperature (40–50 °C) and extraction time (4–12 h) on different responses like yield, gel strength and melting point of gelatin. The optimum conditions derived by RSM for the yield (10.58%) were 0.2 M H3PO4 for 9.01 h of extraction time and hot water extraction of 45.83 °C. The maximum achieved gel strength and melting point was 138.54 g and 22.61 °C respectively. Extraction time was found to be most influencing variable and had a positive coefficient on yield and negative coefficient on gel strength and melting point. The results indicated that Unicorn leatherjacket skins can be a source of gelatin having mild gel strength and melting point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gelatin is a high molecular weight biopolymer obtained by partial hydrolysis of collagen which is found in the bones, scale, skin, connective tissue and intestines of animal (Mohtar et al. 2010). Therefore, gelatin is normally manufactured from the waste generated during the animal slaughtering and processing such as skin and bone. The wide applications of gelatin in food, pharmaceutical, and photographic industry is because of its unique functional and technological properties (Karim and Bhat 2009) and compatibility with wide variety of ingredients. So far the main source of gelatin is limited to those of land origin such as bovine or porcine skin and bone. However, gelatin obtained from porcine skins or bones cannot be applied for some foods due to esthetic and religious objections (Sadowska et al. 2003). Therefore, alternative sources, especially fish processing wastes including skin, bones or scale have been paid increasing attention for its extraction (Pranoto et al. 2007). Skins, scales and bones contribute about 30% of waste generated in fish processing industries (Kittiphattanabawon et al. 2005). Several authors have reported extraction of gelatin from waste of several cold water fishes such as cod (Gudmundsson and Hafsteinsson 1997), hake (Gomez-Guillen et al. 2002), pollock (Zhou and Regenstein 2004), Atlantic salmon (Arnesen and Gildberg 2007), rainbow trout (Tabarestani et al. 2010) and warm water fishes like megrim (Montero and Gomez-Guillen 2000), tilapia (Jamilah and Harvinder 2002), shark (Cho et al. 2004), nile perch (Muyonga et al. 2004), yellow fin tuna (Cho et al. 2005), big eye snapper and brown stripe red snapper (Jongjareonrak et al. 2006), channel catfish (Yang et al. 2007) grass carp (Kasankala et al. 2007), skipjack tuna (Aewsiri et al. 2008) and authors also reported and compared the properties of obtained gelatin with gelatin from various marine sources as well as mammalians. It has been suggested that gelatin from warm water species has an advantage over those extracted from cold water species, providing nearly similar rheological properties to mammalian gelatin (Cho et al. 2005; Gilsenan and Ross-Murphy 2000). Scientific literature about different alternative sources and new functionalities of collagen and gelatin has experienced a boom in the environmental friendly management of industrial wastes (Gómez-Guillén et al. 2011).
In present study, gelatin was extracted from the skin of Unicorn leatherjacket (Aluterus monoceros), which is a reef associated subtropical fish occurring in the continental shelf down to 50 m depth and belongs to the family Monacanthidae and the order Tetraodontiformes. It is widely distributed in Atlantic, Indian and Pacific oceans. This species has been used for fillet production and for the export in frozen condition in India. As a consequence, a large amount of skin is produced as fish processing waste. The skin of Unicorn leatherjacket is relatively thick and can form an ideal raw material for gelatin production. Recently, Ahmad and Benjakul (2011) studied the characteristics of gelatin extracted from the skin of Unicorn leatherjacket (Aluterus monoceros), as influenced by phosphoric and acetic acid pretreatment and extraction time. However, optimization of the extraction conditions for achieving better yield and functional properties of gelatin were not attempted. The main objective of the present study was to optimize the extraction of gelatin conditions by Response Surface Methodology (RSM) for achieving enhanced response variables.
Materials & methods
Materials
Unicorn leatherjacket (Aluterus monoceros) skin waste was collected from Sassoon Dock Landing Centre, Mumbai, India and transported in ice to the laboratory. The skin was washed thoroughly using 10 ppm chlorinated water and cut into pieces of 5–6 cm size, placed in polyethylene bags and stored at −20 °C until used. The storage time was less than 4 months. All chemicals and reagents used in this study were of analytical grade. Phosphoric acid, GR (85% Cat. No. 1.00573) and sodium hydroxide, GR (Cat. No. 1.06498) were procured from MERCK (Germany).
Experimental design
To optimize gelatin extraction from Unicorn leatherjacket skin, the Response Surface Methodology (RSM) was adopted and for determining the effects of the independent variables on gelatin extraction, the Unscrambler Software (CAMO, Norway) was employed for Box-Behnken Design (BBD) of RSM (Box and Behnken 1960). Based on preliminary experiment three independent variables, molar concentration of H3PO4 (A) pre-treatment, hot water extraction temperature in °C (B) and extraction time in h (C), were used to determined the response pattern against the dependent variables i.e. yield, gel strength and melting point. Each independent variable had three levels −1, 0, +1 and the experiment as shown in Table 1 was conducted as 15 experimental points. A second-order polynomial equation, such as:
was obtained using response surface regression:
where Yi is the predicted response, xi and xj are the independent variables, βi is the ith linear variable coefficient, βij is the ijth interaction coefficient. In this experiment, the independent variables are termed A (phosphoric acid, H3PO4, molar concentration), B (extraction temperature, °C), and C (extraction time, h), and the predicted response are Y1 (yield, g), Y2 (gel strength, g) and Y3 (melting point, °C).
Extraction of gelatin
Gelatin was extracted from the skin of Unicorn leatherjacket (Aluterus monoceros) following the procedure described by Jongjareonrak et al. (2006) with slight modification. Thawed skin was soaked in 0.1 M NaOH with a skin / solution ratio of 1:7 (w/v). The mixture was kept at 6 ± 1 °C for 2 h with occasional stirring and the alkaline solution was changed every 1 h interval in order to remove non collagenous proteins and pigments. Alkaline treated skin was then washed with potable water until neutral or faintly basic pH of wash water was obtained. The skin was then soaked in 0.15–0.25 M H3PO4 with a skin/solution ratio of 1:7 (w/v) for 24 h with occasional stirring at 6–7 °C. The acidic solution was changed every 12 h to swell the collagenous material in the fish skin matrix. Acid pre-treated skin was washed thoroughly with potable water until wash water became neutral or faintly basic. To extract gelatin, the swollen skin was soaked in distilled water with a skin/water ratio of 1:3 (w/v) in a temperature-controlled water bath at 40–50 °C for 4–12 h with an occasional stirring. The mixture was then filtered using two layers of cheese cloth. The filtrate was further filtered using a Whatman No. 4 filter paper (Whatman International Ltd., England; Cat.No.1003 150), followed by drying the solution in thermostatically controlled hot air oven at temperature 80 ± 2 °C for 18 h. The powder obtained, referred to as Unicorn leatherjacket skin gelatin, was calculated based on the weight of dry gelatin in comparison with that of fresh skin and was stored at room temperature (25 ± 5 °C) for further analysis.
Determination of gel strength
Gel strength was measured according to the method described by Duan et al. (2011) with slight modification. Samples of gelatin gels were prepared by dissolving 6.67% (w/v) of dry gelatin in distilled water and then heated at 60 °C in a water bath for 30 min until gelatin was completely dissolved. The gelatin solutions were cooled at room temperature (25 ± 5 °C) for 30 min and the gelatin solution (60 ml) was filled in 100 ml capacity corning beakers before being chilled in a refrigerator at maturation temperature 6–7 °C for 18 h. The samples were assumed to be at a temperature of 7 °C since the gel strength was measured immediately after being removed from refrigeration using a Model TA.XT2 Texture Analyser (Stable Micro Systems, Surrey, UK) fitted with a 12.7 mm diameter cylindrical delrin probe at a speed of 1 mm/s with 5 Kg load cell. The gel strength was expressed as maximum force (in g), required for the plunger to penetrate by 4 mm into the centre of gelatin gels. The measurement was performed in triplicate.
Melting point
Determination of melting point was based on the JIS K6503 (JSA 1996) method with slight modification. Solutions containing 6.67% (w/v) gelatin were prepared in thin walled (12 mm × 75 mm) screw cap test tubes. The test tubes were filled to leave some headspace and closed. The dissolved samples were held in a refrigerator (7 °C) for 16–18 h, after which they were transferred into a water bath (10 °C). One or two drops of mixture of 75% chloroform and 25% reddish brown dye (food colour) were added in all test tubes to observe the melting point. The temperature of water bath was increased gradually at the rate of 1 °C per min from its initial temperature 10 °C. The temperature was read using an electronic digital thermometer (Fischer Scientific, Germany). The water of bath was constantly stirred manually using a plastic spatula in order to maintain uniform temperature of bath. The temperature at which the drops began to move freely down the gel was taken as the melting point. The measurements were done in triplicate.
Results and discussion
Optimization of gelatin
The yield, gel strength and melting point of the extracted gelatin were measured at 15 experimental points obtained by Box- Behnken design (Table 2). All the quadratic coefficients were observed highly significant (p < 0.05) in all the models except the term BB (quadratic effect of extraction temperature) of Y2 (gel strength) and Y3 (melting point) (Table 4). On the other hand all the interaction coefficients except the terms AB (interaction effect of molar phosphoric acid concentration and extraction temperature) and AC (interaction effect of molar phosphoric acid concentration and extraction time) of Y1 (yield) were not found significant (p > 0.05) whilst in case of linear coefficient the C (extraction time) term of Y1, Y2 and Y3 was significant (p < 0.05) and the other linear coefficients were not significant. In order to develop the fittest response surface model equations, all insignificant terms (p > 0.05) were ignored and the fitted models are shown in Table 3. All the independent variables A, B and C have negative linear and quadratic effects in all surface models except B and C of yield (Table 4). The coefficients of determination (R2) for Y1, Y2 and Y3 were 0.971, 0.977 and 0.942 respectively, which indicates that the model is suitable to explain true relationships among the selected reaction parameters (Table 3). The R2 values for all the models were extremely high.
Analysis of variance (ANOVA)
The ANOVA for the models that explain the response of three dependent variables Y1, Y2 and Y3 are shown in Table 4. The total regression models of yield, gel strength and melting point were highly significant (p < 0.01, p < 0.001, p < 0.01) respectively at 99% probability level. The lack of fit values of Y2 and Y3 did not show a significant p value (p = 0.2407, p = 0.1276 respectively) at 95% probability level (Table 4). The lack of fit value indicates the fitness of the model. Therefore, response surface model represented as quadratic polynomial equation was statistically significant.
Response surface plots and the effect of factors
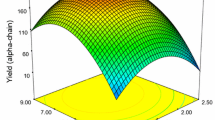
Figure 1 shows the estimated response function and the effect of the independent variables (A, B, C) on the dependent variables (Y1, Y2, Y3). The plots are represented as a function of two factors at a time, holding other factor at a fixed level (middle level). Figures 1a, b, 2a, b, 3a and b depict the effect of independent variables on Y1 (yield), Y2 (gel strength) and Y3 (melting point). As the level values of three independent variables were closed to zero, yield increased. The effect of factor C (extraction time) was statistically significant. The three dimensional plot shows that yield and gel strength increased with an increase in extraction temperature and acid concentration to optimum point whereby further increase leads to a decrease. This decrease could be due to the production of short protein fragments and lowering gelling ability (Ledward 1986; Normand et al. 2000). Moreover, high temperature yields low molecular weight protein fragments (Cho et al. 2004). The effects of acid concentration and extraction time on the melting point of gelatin are shown in Fig. 3a, b. The three dimensional plots show that there has been indirect relationship between extraction time and melting point.
Gelatin yield
Gelatin yield is considered to be one of the most important parameters for the gelatin industry because of its potential economic importance. Gelatin yield is influenced by all the independent variables like H3PO4 concentration, extracting temperature, and extraction time. The model obtained by RSM produced a satisfactory fit to the data with regard to the gelatin extraction R 2 = 0.971, p = 0.0025 (Table 3). Extraction time is the most significant factor on yield. Based on response surface analysis, the predicted maximum yield was 10.58% at extraction condition of H3PO4 concentration 0.2 M, extraction time 9.0 h and extraction temperature 45.83 °C (Table 5). Ahmad and Benjakul (2011) reported a yield of 6.12 and 11.54% (wet weight basis) for the Unicorn leatherjacket (Aluterus monoceros) by using 0.2 M acetic acid and 0.2 M phosphoric acid respectively which was 1% higher than yield (10.5%) found in the present study (Table 1). This may be due to differences in proximate components in the skin as this property seems to vary within the species and age of the fish (Muyonga et al. 2004). Gomez-Guillen et al. (2002) reported the yield of 8.3, 7.4, 7.2 and 6.5% gelatin extracted from different fish species sole, megrim, cod and hake respectively. In the case of other species, Grossman and Bergman (1992) reported a yield of 15% for tilapia skin. Similarly, Gudmundsson and Hafsteinsson (1997) also reported a gelatin yield of about 14% for cod skins. However, Jamilah and Harvinder (2002) reported that the yields of gelatin for red and black tilapia were 7.81% and 5.39%, respectively. From the present study it was revealed using RSM that the yield was increased as the extraction times and temperature increased. The results were in accordance with Arnesen and Gildberg (2007) who reported that increasing extraction times were associated with the increase in yield of gelatin from Atlantic salmon skin. During heating hydrogen bonds stabilizing triple helix of mother collagen were destroyed, leading to helix-to coil transition. This resulted in the conversion of collagen to soluble gelatin (Benjakul et al. 2009).
Gel strength
Gel strength is the most important attribute of gelatin and determines the quality of produced gelatin. The application of gelatin was determined by the range of gel strength values. Usually, the gel strength of commercial gelatins ranges from 100 to 300 g, but gelatins with gel strength of 250–260 g are the most desirable and suitable for a wide range of applications in the food industry especially in the processing of jellies, canned meat, marshmallows and yoghurts (Holzer 1996). Fish gelatin typically has a gel strength ranging from as low as zero to 426 g, compared to 200–300 g for bovine or porcine gelatin (Karim and Bhat 2009). Some species of warm-water fish gelatins have been reported to exhibit high gel strengths, close to that of porcine gelatin while cold water fish has poorer physical properties (Gudmundsson and Hafsteinsson 1997). According to the results of gel strength the extraction temperature (B) and extraction time (C) are the most influencing factors. However, the effect is negative. Higher extraction temperature and longer extraction time give low gel strength. This may be due to shorter peptides fragment (Gomez-Guillen et al. 2002). The gel strength of gelatin from the skin of Unicorn leatherjacket, which is a sub tropical fish, was 138.54 g. This value is low compared to that of gelatin from the skins of warm water fish such as grass carp (267 g) (Kasankala et al. 2007), tilapia (328 g) (Songchotikunpan et al. 2008), giant catfish (153 g) (Jongjareonrak et al. 2010). It may be due to limited imino acids content which might have resulted in less triple helical structures resulting in lower bloom strength. Bloom strength not only depends on average molecular weight of gelatin but also is dependent on other factors such as the chemical treatment of raw collagen material, type and concentration of the gelatin and the time/temperature history of the sample (Babin and Dickinson 2001; Kolodziejska et al. 2004). But the result obtained is higher than the values obtained from cold-water fish species such as salmon (108 g) (Arnesen and Gildberg 2007), cod 70–90 g (Gomez-Guillen et al. 2002), while that for Alaskan pollock it was 98 g (Zhou et al. 2006). The extracted gelatin with low gel strength may find application in microencapsulation, light sensitive coatings, low-set time glues and as shampoo ingredient (Rustard 2003). Also fish gelatin with low bloom value and low melting point can accelerate the flavour release than high bloom value gelatin (Zhou and Regenstein 2007).
Melting point
The melting point of gelatin is the point at which the gelatin gel starts melting when the temperature increases above a certain point. The model developed for predicting melting point could explain 94.2% of the variation observed in this functional property (Table 3). The melting point of gelatin extracted from Unicorn leatherjacket skin was 22.61 °C in optimum condition which was higher than the results of cod skin (8–10 °C) (Gudmundsson and Hafsteinsson 1997), hake (14 °C), sole (19.4 °C), megrim (18.8 °C) (Gomez-Guillen et al. 2002) and grass carp (19.5 °C) (Kasankala et al. 2007). Typical melting points for fish gelatins range from 11 to 28 °C (Karim and Bhat 2009). It was well recognized that the melting temperature of gelatin prepared from the skins of warm blooded animals and warm water fish is generally higher than that of gelatin from the skin of fish living in cold-water (Gilsenan and Ross-Murphy 2000). In this study the obtained melting point was lower than some gelatin such as black tilapia skin gelatin 28.9 °C (Jamilah and Harvinder 2002). For porcine and bovine gelatins, the melting points range from 20 to 25 and 28 to 31°C, respectively (Karim and Bhat 2009). The results obtained were consistent with the finding of Choi and Regenstein (2000) that the increase in gel strength of a gelatin gel is accompanied by an increase in melting point.
Conclusions
Response Surface Methodology allowed the development of polynomial equation for optimization of gelatin extraction from skin waste of Unicorn leatherjacket. The generated model was able to explain adequately the activity of dependent variables by changing independent variables. The skin of Unicorn leatherjacket can be a possible material for gelatin production, which can be used in products requiring low gel strength like soft gelatin capsules, mousse, pies, table creams, ice cream etc. However, further study can be performed for improving the gel strength by using crosslinkers such as transglutaminase to have wider applications, with added advantages of avoiding risk of BSE and winning confidence from Koshers and Halal. It is also concluded that the application of RSM may be followed for optimizing the extraction parameters and adapting the results for commercial extraction of gelatin from Unicorn leatherjacket fish skins.
References
Aewsiri T, Benjakul S, Visessanguan W, Tanaka M (2008) Chemical compositions and functional properties of gelatin from pre-cooked tuna fin. Int J Food Sci Technol 43(4):685–693
Ahmad M, Benjakul S (2011) Characteristics of gelatin from the skin of Unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocolloids 25(3):381–388
Arnesen JA, Gildberg A (2007) Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour Technol 98:53–57
Babin H, Dickinson E (2001) Influence of transglutaminase treatment on the thermoreversible gelation of gelatin. Food Hydrocolloids 15(3):271–276
Benjakul S, Oungbho K, Visessanguan W, Thiansilakul Y, Roytrakul S (2009) Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem 116:445–451
Box G, Behnken D (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Cho SM, Kwak KS, Park DC, Gu YS, Ji CI, Jang DH (2004) Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocolloids 18:573–579
Cho SM, Gu YS, Kim SB (2005) Extracting optimization and physical properties of yellowfin tuna (Thunnus albacares) skin gelatin compared to mammalian gelatins. Food Hydrocolloids 19:221–229
Choi SS, Regenstein JM (2000) Physicochemical and sensory characteristics of fish gelatine. J Food Sci 65:194–199
Duan R, Zhang JJ, Xing FF, Konno K, Xu XJ (2011) Study on the properties of gelatins from skin of carp (Cyprinus carpio) caught in winter and summer season. Food Hydrocolloids 25:368–373
Gilsenan PM, Ross-Murphy SB (2000) Viscoelasticity of thermoreversible gelatin gels from mammalian and piscine collagen. J Rheology 44:871–882
Gomez-Guillen MC, Turnayb J, Ferandez-Diaz MD, Ulmo N, Lizarbe MA, Monteroa P (2002) Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocolloids 16:25–34
Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP (2011) Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocolloids 25:1813–1827
Grossman S, Bergman, M (1992) Process for the production of gelatin from fish skins. US patent 5,093,474
Gudmundsson M, Hafsteinsson H (1997) Gelatin from cod skins as affected by chemical treatments. J Food Sci 62(1):37–39
Holzer D (1996) Gelatin production. US patent 5,484,888
Jamilah B, Harvinder KG (2002) Properties of gelatins from skins of fish—black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem 77(1):81–84
Japanese Standard Association (J.S.A.) (1996) Japanese industrial standard animal glues and gelatins. JIS K 6503, Japan
Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M (2006) Skin gelatin from bigeye snapper and brownstripe red snapper: chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocolloids 20(4):1216–1222
Jongjareonrak A, Rawdkuen S, Chaijan M, Benjakul S, Osako K, Tanaka M (2010) Chemical compositions and characterisation of skin gelatin from farmed giant catfish (Pangasianodon gigas). LWT - Food Sci Technol 43:161–165
Karim AA, Bhat R (2009) Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 23:563–576
Kasankala LM, Xue Y, Yao W, Hong SD, He Q (2007) Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour Technol 98:3338–3343
Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005) Characterization of acid soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem 89:363–372
Kolodziejska I, Kaczorowski K, Piotrowska B, Sadowska M (2004) Modification of the properties of gelatin from skins of Baltic cod (Gadus morhua) with transglutaminase. Food Chem 86(2):203–209
Ledward DA (1986) Gelation of gelatin. In: Mitchell JR, Ledward DA (eds) Functional properties of food macromolecules. Elsevier Applied Science Publishers, London, pp 171–201
Mohtar NF, Perera C, Quek SY (2010) Optimisation of gelatine extraction from hoki (Macruronus novaezelandiae) skins and measurement of gel strength and SDS-PAGE. Food Chem 122:307–313
Montero P, Gomez-Guillen MC (2000) Extracting conditions for megrim (Lepidorhombus boscii) skin collagen affect functional properties of the resulting gelatin. J Food Sci 65(3):434–438
Muyonga JH, Cole CGB, Duodub KG (2004) Extraction and physicochemical characterisation of Nile perch (Lates niloticus) skin and bone gelatine. Food Hydrocolloids 8:581–592
Normand V, Muller S, Ravey JC, Parker A (2000) Gelation kinetics of gelatin: a master curve and network modelling. Macromolecules 33:1063–1071
Pranoto Y, Lee CM, Park HJ (2007) Characterizations of fish gelatin films added with gellan and k-carrageenan. LWT J Food Sci Technol 40(5):766–774
Rustard T (2003) Utilization of marine by-products. Electronic J Envt Agric Food Chem 2:458–463
Sadowska M, Kolodziejska I, Niecikowska C (2003) Isolation of collagen from the skins of Baltic cod (Gadus morhua). Food Chem 81:257–262
Songchotikunpan P, Tattiyakul J, Supaphol P (2008) Extraction and electrospinning of gelatin from fish skin. Int J Biol Macromol 42:247–255
Tabarestani SH, Maghsoudlou Y, Motamedzadegan A, Sadeghi Mahoonak AR (2010) Optimization of physico-chemical properties of gelatin extracted from fish skin of rainbow trout (Onchorhynchus mykiss). Bioresour Technol 101(15):6207–6214
Yang H, Wang Y, Jiang M, Oh J, Herring J, Zhou P (2007) 2-Step optimization of the extraction and subsequent physical properties of channel catfish (Ictalurus punctatus) skin gelatin. J Food Sci 72(4):c188–c195
Zhou P, Regenstein JM (2004) Optimization of extraction conditions for pollock skin gelatin. J Food Sci 69(5):393–398
Zhou P, Regenstein JM (2007) Comparison of water gel desserts from fish skin and pork gelatins using instrumental measurements. J Food Sci 72:C196–C201
Zhou P, Mulvaney SJ, Regenstein JM (2006) Properties of Alaska pollock skin gelatin: a comparison with Tilapia and pork skin gelatins. J Food Sci 71:313–321
Acknowledgments
The authors thank Dr. W.S. Lakra, Director of Central Institute of Fisheries Education for providing all the facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanjabam, M.D., Kannaiyan, S.K., Kamei, G. et al. Optimisation of gelatin extraction from Unicorn leatherjacket (Aluterus monoceros) skin waste: response surface approach. J Food Sci Technol 52, 976–983 (2015). https://doi.org/10.1007/s13197-013-1075-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1075-y