Abstract
Pseudomonas fluorescens BJ-10, a kind of psychrotrophic bacteria, was isolated from raw milk. It produced an extracellular protease of 47 kDa by SDS-PAGE. The crude proteases were purified by ammonium sulfate fractionation, ion-exchange and gel filtration chromatography. The specific activity of purified protease increased 61.38-fold. The optimum pH and temperature were pH 7.0 and 30 °C, respectively. The purified protease was partially inhibited by DL-dithiothreitol, and the activity increased a little upon Fe2+ addition. The protease showed typical heat-stable behavior. After treatment at 100 °C for 3 min, more than 94% activity remained. This work might lay the foundation for possible relationship between the heat stable protease and gelation of UHT milk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Storage of raw milk in refrigerated tanks has largely contributed to the amelioration of microorganisms in raw milk. However, some psychrotrophic bacteria have ability to grow at low temperature (3–7 °C) and to hydrolyze and use large molecules of proteins and lipids for growth (Kumerasan et al. 2007; Sperber and Doyle 2010). Numerous genera of psychrotrophic bacteria have been isolated from milk, both Gram negative: Pseudomonas, Aeromonas, Serratia, Acinetobacter, Alcaligenes, Achromobacter, Enterobacter and Flavobacterium, and Gram positive: Bacillus, Clostridium, Corynebacterium, Microbacterium, Micrococcus, Streptococcus, Staphylococcus and Lactobacillus (Sorhaug and Stepaniak 1997; Alatossava and Alatossava 2006; Zacharov and Halpern 2007). Pseudomonas species are the most common organisms in raw milk at the time of the spoilage. The Pseudomonas species like P.fluorescens, P. putida, P. fragi, P. putrefaciens, and less frequently P. aeruginosa constitute the predominant microorganisms limiting the shelf life of processed fluid milk (Liu et al. 2009). A common and well studied strain of Pseudomonas species that grow in raw milk is Pseudomonas fluorescens (Cousin 1982). They often become the predominant microbial flora during cold storage of raw milk.
Psychrophils cannot survive under pasteurization, but many species produce heat-resistant extracellular proteases which cause defects in dairy products. It is especially associated with proteolysis and lipolysis in milk and in dairy products . The extracellular enzymes can resist pasteurization (72 °C for 15 s) and even ultra high temperature processing (UHT: 138 °C for 4 s) (Sorhaug and Stepaniak 1997). Proteases are associated with bitterness and gelation of UHT sterilized milk, and reduced yields of soft cheese.
In the previous reports, researchers described different proteases from different strains of P. fluorescens. These proteases differ in biochemical and physicochemical properties. The purified enzyme from P. fluorescens T16 is a monomer with a molecular weight of 38,000 ± 2,000 Da. Alpha-casein is a preferred substrate, with a K m of 0.05 mmol/L (Petal et al. 1983). P. fluorescens RM12 produce an extracellular heat resistant protease with an estimated molecular weight of 45 kDa. Mn2+ has positive effect on activity and can increase the heat resistance capability, while Ca2+ has a negligible effect (Mu et al. 2009). P. fluorescens CY091 cultures produce an extracellular protease with an estimated molecular mass of 50 kDa. Production of this enzyme (designated AprX) was observed in media containing CaCl2 or SrCl2. Over 20% of the enzyme activity was retained in the AprX sample which had been heated in boiling water for 10 min, indicating that the enzyme is highly resistant to heat inactivation (Liao and Mccallus 1998).
Gelation of UHT milk is thought to be related to proteolysis. The amount of protease required and the degree of proteolysis necessary for gelation to occur, have not been determined. In the present study, the characteristics of a protease produced by P. fluorescens BJ-10 were investigated. Characterization of a protease produced by a psychrophil grown in milk should permit future work on the possible relationship between this type of protease and gelation of UHT milk.
Materials and methods
Bacterial culture
Five psychrotrophic strains (BJ-2, BJ-5, BJ-8, BJ-10, M) were isolated from 60 raw milk samples in the region of Beijing, China. The isolates were tested for the production of proteolytic activity by agar diffusion assays at 28 °C for 72 h, nutrient broth as control. Proteolytic enzyme production was tested by using skim milk agar (1% skim milk powder, 0.5% yeast extract, 1.5% agar). The presence of clear zone around the wells was indicative of proteolysis. The strians were identified by the methods API 20NE and 16 s rDNA sequence analysis. The strains were cultured in nutrient broth (Luqiao, China) at 28 °C for 48 h.
Protease and protein assay
The protease activity was measured by azocasein assay with some modifications (Ewings et al. 1984). One millilitre of suitable dilution of an enzyme solution was added to 1 mL of phosphate buffer saline (PBS) of pH 7.4 and 2 mL of 0.5% azocasein (Sigma, USA). After 2 h incubation at 30 °C, the reaction mixture was terminated by the addition of 4 mL of 12% (w/v) trichloroacetic acid and keeping for 30 min at room temperature. The mixture was then centrifuged at 4500 × g for 15 min. Finally, the absorbance of supernatant was measured at 345 nm in a spectrophotometer (U-3010, Hitachi, Japan). One unit of enzyme activity was defined as the amount of protein that resulted in an increase in absorbance at 345 nm of 0.01 in 1 h under the assay conditions used.
Protein assay was performed in microtitre wells according to the Bradford method with bovine serum albumin (Guangda, China) as the standard (Bradford 1976).
Purification
Ammonium sulfate precipitation
Cells were removed from the culture medium by centrifugation (10000 × g for 10 min at 4 °C) and the supernatant (1 L) was collected. The protein was precipitated using solid ammonium sulfate (80%). The precipitated protein was dissolved in 10 mmol/L PBS (pH 7.4) and dialyzed over night with three changes using the same buffer.
Ion-exchange chromatography
For ion-exchange chromatography, the dialyzed material was loaded onto an ion-exchange column (DEAE-Sepharose Fast Flow, Pharmacia, Uppsala, Sweden) (1 cm × 20 cm) previously equilibrated with 10 mmol/L PBS (pH 7.4). The column was then washed with 300 mL of equilibration buffer, and bound proteins were eluted with a discontinuous gradient of increasing ionic strength (0–1 mol/L NaCl) at a flow rate of 1.8 mL/min. Active fractions were pooled, dialyzed overnight against the same buffer, lyophilized, and resuspended in 10 mmol/L PBS (pH 7.4).
Gel filtration chromatography
For gel filtration chromatography, the dialyzed material was loaded onto a gel column (Sephacryl S-100 HR, Pharmacia, Uppsala, Sweden) (1 cm × 75 cm) previously equilibrated with 10 mmol/L PBS (pH 7.4). The column was then washed with 180 mL of the same buffer, and bound proteins were eluted with the same buffer at a flow rate of 15 mL/h. Fractions (3 mL) were collected, and positive fractions were pooled, lyophilized and resuspended against the same buffer.
Molecular weight determination
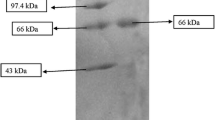
SDS-PAGE was performed according to the method of Laemmli (1970). Separating gel (12%, pH 8.8) and stacking gel (4.0%, pH 6.8) were used and 20 μg enzyme protein was loaded. Electrophoresis was run for 2 h with a current of 15 mA. For the molecular weight determination by SDS-PAGE, the standards used were phosphorylase B (97 kDa; Sigma), bovine serum albumin (66 kDa; Sigma), ovalbumin (45 kDa; Sigma), carbonic anhydrase (30 kDa; Sigma), trypsin inhibitor (20.1 kDa; BioRad) and α-lactalbumin(14.4 kDa; Sigma). The gel was stained with Coomassie Brilliant BlueR 250 (CBB) and destained by conventional procedures.
Characterization of the protease
Effect of temperature and pH on protease activity
The optimal temperature on protease activity was determined by performing the standard assay procedure at 20, 30, 40, 50 and 60 °C. The optimal pH for the proteolytic activity using azocasein as the substrate was determined by changing the pH of the reaction mixture in the range of pH 5.0 to pH 10.0. Protease activity was measured at 30 °C using the following buffer: 0.2 mol/L citrate buffer (pH 5.0), 0.2 mol/L phosphate buffer (pH 6.0 to pH 8.0), and 0.2 mol/L glycine–NaOH buffer (pH 9.0 to pH 10.0). Results are means of three replications. Error bars indicate the standard deviation of the means.
Effect of inhibitors and metal ions on the protease activity
The effect of metal ions and known protease inhibitors was investigated (Yao et al. 2011). Metal ions and the concentrations tested were as follows: Ca2+, Co2+, Cu2+, Mg2+, Mn2+, Zn2+, Pb+, Hg2+ and Ni2+ at 1 and 5 mmol/L. Inhibitors and the concentrations tested were as follows: 1 and 5 mmol/L Phenyl Methane Sulphonyl Fluoride (PMSF), Ethylene Diamine Tetra Acetic acid (EDTA), DL-Dithiothreitol (DTT). The residual enzyme activity was determined at 30 °C for 2 h by using azocasein substrates.
Heat-stable analysis
Protease in plastic tubes was treated in a water bath at 80 °C for 10, 20, 30 min and at 90 °C and 100 °C for 1, 2 and 3 min. Heated tubes were cooled quickly in ice-water mixture, and the residual protease activity was determined by standard assay procedures. All values were expressed as percentage of protease activity of unheated control. The residual activity represents the average of three experiments (Matta and Punj 1998).
Statistical analysis
All experiments were conducted in triplicate and an analysis of variance (ANOVA) was performed. The least significant difference at p < 0.05 was calculated using the Duncan Multiple Range Test on Statistical Analysis System (SAS Institute Inc., Cary, NC, USA). The data were expressed as mean ± SD.
Results and discussion
Determination of proteolytic activity
Protease production is reported in Fig. 1. Five milk derived psychrotrophic microbial cultures were screened for the ability to produce proteases in skim milk agar. Of all of them, only one strain, P. fluorescens BJ-10 produced extracellular protease at 28 °C for 72 h inculation. It performed a clear proteolysis zone around the wells. Therefore, the protease from BJ-10 was used for purification and characterization.
Protease purification
The process of purifying a major protease was developed (Table 1). The ammonium sulfate precipitated enzyme (80% saturated) on dialysis yielded 83% recovery and 2.5 fold increase in the specific activity. The obtained protein solution was loaded on a DEAE-Sepharose FF column and eluted, which resulted in three separate peaks. Proteins in peak 3 (Fig. 2a) had proteolytic activity, which was obtained by gradient eluting at 0–0.5 mol/L sodium chloride (NaCl). Proteins in peak 3 were pooled and concentrated. The enzyme was further purified using Sephacryl S-100 HR column, which resulted in two separate peaks, peak I and peak II. Peak II between 30th and 55th fractions was the major protein peak (Fig. 2b). Results from the purification process (Table 1) showed that the enzyme was purified 61.38 fold with a total yield of 23.63% of original activity. A single protein (Fig. 3) with approximate molecular mass of 47 kDa was obtained by SDS-PAGE.
Characterization of purified protease
The protease was active over a wide range of temperature from 20 to 60 °C, with an optimum at 30 °C (Fig. 4a).The purified enzyme although active over a wide pH range (pH 5.0 to pH 10.0), had an optimum at pH 7.0 (Fig. 4b). In this respect, BJ-10 protease was similar to the protease of P. fluorescens Rm12 (Mu et al. 2009) and P. fluorescens M3/6 (Kohlmann et al. 1991). The optimum temperature for the protease of Pseudoalteromonas sp. NJ276 (Wang et al. 2008) was 30 °C, which is consistent with the present study.
As it can be seen from Table 2, Mg2+, Ca2+, Fe2+, Mn2+, Zn2+, Pb+ had no inhibitory effect on the enzyme activity. Only Cu2+ exhibited an inhibition as compared to original activity. However, Fe2+ had a slight stimulatory effect, since protease activity increased by 12.59% when Fe2+ concentration was 5 mmol/L. Petal et al. (1983) reported that the majority of proteases from psychrophils were metalloproteases requiring either Ca2+ or Zn2+ for optimum activity. The protease Ht13 from P. fluorescens Rm12 showed optimum activity in the presence of Mn2+ (Mu et al. 2009). The protease from P. fluorescens T16 showed optimum activity in the presence of either Ca2+or Mn2+. However, Fe2+ had an effect of stimulus on the protease of P. fluorescens BJ-10. Thus the protease from P. fluorescens BJ-10 differs in this respect from other P. fluorescens proteases. The purified protease was not inhibited by PMSE or EDTA, which indicated that the enzyme was not a metalloprotease. Purified protease was slightly inhibited by DTT. DTT can reduce disulfide bonds, which resulted in protease inactivation. This phenomenon gave an indication that BJ-10 protease contained disulfide bonds. Similar conclusions were drawn by Mu et al. (2009).
Heat stability data are presented in Table 3. After treatment at 80 °C for 10, 20, 30 min and 90 °Cand 100 °C for 1, 2 and 3 min, more than 93% of protease activity remained. So, the protease showed typical heat-stable behavior. The purified enzyme retained more than 94% activity even after heat treatment at 100 °C for 3 min. Mayerhofer et al. (1973) reported that the extracellular protease of P. fluorescens P26 was found to be more stable at pasteurization temperatures, and more stable in milk than in water. The enzyme’s heat stability can be attributed to both the whey and casein components of milk. Heat stability of proteas of psychrotrophic bacteria at higher temperature is well documented (Matta et al. 1994; Muller and Fehlhaber 1995). So, residual heat stable proteases may play an important role in determining the shelf-life of milk and dairy products.
Conclusions
Extracellular proteases from psychrotrophic strains of P. fluorescens BJ-10 were purified and characterized. Characteristics of the purified protease were found to be similar with those previously described for proteases isolated from various P. fluorescens species. It hydrolysed k-casein preferentially micelles. This property, combined with the high thermal stability, makes the enzyme an interesting subject for research in milk spoilage. Detection and control of protease activity prior to processing are critical because the enzyme is able to resist pasteurization and to remain active at the pH and temperature of fluid and fermented dairy products.
Extracellular protease of psychrotrophic microorganisms have been studied because of their relationship to milk quality. More information is needed concerning proteases produced by milk psychrophils and their role in milk. Amounts of these proteases present in milk and their heat stabilities, are some of the areas for which information is needed.
References
Alatossava PM, Alatossava T (2006) Phenotypic characterization of raw milk Associated psychrotrophic bacteria. Res Microbiol 161:334–346
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Cousin MA (1982) Presence and activity of psychrotrophic microorganisms in milk and dairy products. J Food Prot 45:172–207
Ewings NK, O’Conner RE, Mitchell GE (1984) Proteolytic microflora of refrigerated raw milk in south east Quecnsland. Aust J Dairy Technol 39:3275–3283
Kohlmann KL, Nielsen SS, Ladisch MR (1991) Purification and characterization of an extracellular protease produced by Pseudomonas fluorescens M3/6. J Dairy Sci 74:4125–4136
Kumerasan G, Annalvilli R, Sivakumar K (2007) Psychrotrophic spoilage of raw milk at different temperatures of storage. J Appl Sci Res 3:1383–1387
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(259):680–685
Liao CH, McCallus DE (1998) Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol 64(3):914–921
Liu M, Cao ZJ, Mu ZS, Li YM (2009) Study on isolation and identification of thermotolerant protease-producing psychrophiles in milk. Food Sci 05:184–186
Matta H, Punj V (1998) Isolation and partial characterization of a thermostable extracellular protease of Bacillus polymyxa B-17. Int J Food Microbiol 42:139–145
Matta H, Punj V, Kalra MS (1994) Isolation and partial characterization of a heat stable extracellular protease from Pseudomonas sp. AFT-36. Milchwissenschaft 49:186–189
Mayerhofer HJ, Marshall RT, White CH, Lu M (1973) Characterization of a heat-stable protease of Pseudomonas fluorescens P26. Appl Microbiol 25:44–48
Muller U, Fehlhaber K (1995) Studies into thermoresistance of Bacillus protease. Fleischwirtaschaft 75:1427–1429
Mu ZS, Du M, Bai Y (2009) Purification and properties of a heat-stable enzyme of Pseudomonas fluorescens Rm12 from raw milk. Eur Food Res Technol 228:725–734
Petal TR, Jackman DM, Bartlett FM (1983) Heat-stable protease from Pseudomonas fluorescens T16: purification by affinity column chromatography and characterization. Appl Environ Microbiol 46:333–337
Sorhaug T, Stepaniak L (1997) Psychrotrophs and their enzymes in milk and dairy products:quality aspects. Trends Food Sci Technol 8:35–41
Sperber WH, Doyle MP (2010) Microbiological spoilage of dairy products. In: Compendium of the microbiological spoilage of foods and beverages. Springer, New York, pp. 41–68
Wang QF, Hou YH, Xu Z, Miao JL, Li GY (2008) Purification and properties of an extracellular cold-active protease from the psychrophilic bacterium Pseudoalteromonas sp. NJ276. Biochem Eng J 38:362–368
Yao DW, Qu J, Chang PW, Tao YH, Yang DJ (2011) Production and characterization of alkaline proteasefrom hemoglobin-degrading Bacillus pumilus NJM4 to produce fermented blood meal. J Food Sci Technol. doi:10.1007/s13197-010-0205-z
Zacharov EH, Halpern M (2007) Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lypolytic traits. Appl Environ Microbiol 73(22):7162–7168
Acknowledgements
This work was supported by Special Fund for Agro-scientific Research in the Public Interest (200903043), National Natural Science Foundation of China (31071575).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Lv, J. Purification and properties of heat-stable extracellular protease from Pseudomonads fluorescens BJ-10. J Food Sci Technol 51, 1185–1190 (2014). https://doi.org/10.1007/s13197-012-0620-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0620-4