Abstract
Aloe vera gel powder was produced through dehumidified air drying of Aloe vere gel at optimized conditions of temperature, relative humidity and air velocity of 64 °C, 18% and 0.8 m.s−1, respectively. The powder was packed in three different packaging materials viz., laminated aluminum foil (AF), biaxially oriented polypropylene (BOPP) and polypropylene (PP). The shelf-life of the powder was predicted on the basis of free flowness of product under accelerated storage condition (38 ± 1 °C, 90 ± 1% relative humidity) and was calculated to be 33.87, 42.58 and 51.05 days in BOPP, PP and AF, respectively. The storage stability of powder in terms of colour change was studied. The magnitude of colour change of Aloe vera gel powder during storage suggests that AF was better than BOPP and PP. The colour change of powder during storage followed first order reaction kinetics with a rate constant of 0.0444 per day for AF, 0.075 per day for BOPP and 0.0498 per day for PP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aloe vera (Aloe barbadensis Miller), an industrial crop belongs to the Aloe genus of herbaceous, shrubby, perennial xerophyte succulents belonging to Liliaceae family. It has long been used in health foods and for medical and cosmetic purposes (Grindlay and Reynolds 1986; Koga 1998). A newly developed aloe product as natural health food supplement attracts many health-conscious consumers. Aloe vera is widely used for manufacturing food products, beverages, pharmaceuticals and cosmetics because of its aromatic properties, bitter taste, the cathartic activity of anthraquinones and other pharmacological activities such as emolliency, reduction of inflammation and acceleration of wound healing (Pisalkar et al. 2010). Because of its medicinal and therapeutic values it is well known as miracle plant, first aid plant, burn plant and stick of heaven. It helps to cure diabetes, ulcer, and heart disease. Aloe vera gel powder is being used by food processing industries in preparation of yogurt and other food products (Smitha and Pratima 2007).
Besides medicinal values this plant contains a number of nutrients such as vitamins, minerals, amino acids, sugars, enzymes, fatty acids and saponins, which have positive effects on human body (Choi and Chung 2003) which could be used in food formulations as functional ingredient for health benefits.
In food industry, Aloe vera is used as a resource of functional food. A few examples of product applications for food and beverage products are aloe soft drink (with electrolytes), diet drink with soluble fiber, hangover drink, healthy vegetable juice, tropical fruit juice with Aloe vera, yogurt and yogurt drinks, Aloe vera jelly desserts with chunks of aloe, instant Aloe vera granules, Aloe vera gums for sore or bleeding gums, Aloe vera candy, Aloe vera sorbet with citrus juice and Aloe vera smoothies (Ramachandra and Rao 2008).
Now, the processing of Aloe vera gel, derived from the leaf pulp of the plant, has become a big industry worldwide due to application in food industry. Aloe vera gel processed either by traditional hand filleting and/or whole leaf process can be reduced to powder form which improves shelf-life compared to liquid products and eliminate the cost of transport and storage (Ahlawat and Khatkar 2011).
Presently, spray-drying and freeze-drying technologies are commonly used in the aloe industry to produce aloe powder. However, despite the good-quality powder produced, these methods are limited in use because they are time consuming and expensive (He et al. 2002). To find an alternative production technology, dehumidified air drying is employed in the present investigation to retain the essential food and pharmacological properties of Aloe vera gel to a greater extent. The finished dried material resembles spongy loaf, which has to be scooped out as flakes. Dehumidified air drying of Aloe vera gel does not require pre-concentration of the gel, thereby eliminating one step of the process. In addition, when aloe is gently dried in a natural fillet form, the macromolecules do not break down, as they occur in mechanical processing. As a result the powder, when rehydrated, returns to the natural slippery form which it had inside the leaf. In the present paper, the shelf-life and colour change kinetics of Aloe vera gel powder produced through dehumidified air drying process packed in three different packaging material viz. biaxially oriented polypropylene (BOPP), polypropylene (PP) and laminated aluminum foil (AF) pouches and stored under accelerated storage conditions (38 ± 1 °C, 90 ± 1% relative humidity) is discussed.
Materials and methods
Preparation of dehumidified air dried Aloe vera gel powder
The leaves of Aloe vera grown at research farm of Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur, India, were selected according to uniformity in colour, size and freshness. Hand filleting was done to separate fillet from the rind, and the fillet was cut into uniform flat slabs of 10 ± 1 mm in thickness. The flat slabs of Aloe vera were dried and Aloe vera gel powder was produced in a dehumidified air dryer developed at the Agricultural and Food Engineering Department, Indian Institute of Technology, Kharagpur, India. The schematic diagram of the experimental set-up used in drying is shown in Fig. 1. It consisted of two units, viz., a dryer (1) and a dehumidifier (2) (manufacturer: Bry Air (Asia), Gurgaon, India; model: BA-1.5A) connected together through a duct (3) having facilities to regulate the ratio of ambient and dehumidified air sucked by the dryer. The dryer consisted of an air blower (4), air heating chamber (5), a plenum chamber (7), a drying chamber (9) and power supply and control panel. The dehumidifier was used to reduce the relative humidity of air. Two mercury-in-glass thermometers were inserted at the inlet and exhaust end of the dryer for measuring the dry bulb and wet bulb temperatures of incoming and outgoing air to the dryer. These two temperatures were used to determine the relative humidity of inlet and exhaust air. The temperature of the drying air was controlled by programmable temperature controller (make: Dolphin Automation; model: DTC 113/113P). A thermocouple for measuring the air temperature was placed in the drying chamber and connected to a digital temperature indicator. Relative humidity of the drying air, calculated by using psychrometric chart was also verified with the help of humidity/temperature meter (make: Lutron; model: HT 3006A).
Schematic diagram of dehumidified air drying set-up. Legend 1) Dryer 2) Dehumidifier 3) Duct 4) Air blower 5) Air heating chamber 6) Heaters 7) Plenum chamber 8) Baffles 9) Drying chamber 10) Perforated wall (SS) 11) Drying air exhaust 12) Insulation (Asbestos rope) 13) Rotating desiccant bed 14) Rotating shaft 15) Motor 16) Driving chain and pulley 17) Dehumidification compartment 18) Reactivation compartment 19) Processed air inlet 20) Processed air outlet 21) Reactivation air inlet 22) Reactivation air outlet 23) Divider 24) Heater
The air velocity at the product surface in the drying chamber was measured by using a pre-calibrated anemometer (make: Kanomax; model: 24–6111). Three circular holes of 20 mm diameter each provided on the front door panel of the dryer were used for inserting the sensing probe of anemometer and humidity/temperature meter. During the drying operation these holes were kept closed by using rubber corks. The drying temperature, relative humidity and air velocity range of 40 to 70 °C, 15 to 30% and 0.5 to 1.5 m.s−1, respectively, were adopted during the present investigation. A central composite rotatable design (CCRD) was employed for conducting drying experiments (Erbay and Icier 2009).
The process parameters were optimized using response surface methodology (RSM) (Myers and Montgomery 1995) using a commercial statistical package ‘Design Expert’ version 7.0.0 (Stat-Ease 2000) and the Aloe vera gel powder was produced at an optimum temperature, relative humidity, and air velocity of 64 °C, 18%, and 0.8 m.s−1, respectively. Margarita et al. (2009) investigated the influence of temperature on the drying kinetics, physicochemical properties and antioxidant capacity of Aloe vera and concluded that a drying temperature of 60–70 °C will resulted in the production of high-quality gel. The optimization was mainly based on minimum aloin content (8.74 mg.kg−1), drying time (478.14 min), specific energy consumption (227.28 MJ.kg−1) and colour values; maximum L * (60.31) and minimum a * and b * (2.20 and 19.57).
Determination of sorption characteristics of dried Aloe vera gel powder
The procedure suggested by Iglesias and Chirife (1982) was followed for deriving adsorption isotherms for dehumidified air dried Aloe vera gel powder at 39 ± 1 °C. Dehumidified air dried Aloe vera gel powder sample (2 g) placed in sterilized glass weighing dishes were kept in eight separate desiccators containing saturated solutions of different salts for maintaining the relative humidity (RH) levels from 10 to 90 (Greenspan 1977). The air inside the desiccators was removed with the help of a vacuum pump (model DC 52, Torrlits Engineering Co., Kolkata, India). The level of the vacuum used was 0.05 mm of Hg. The desiccators containing the samples were placed in an incubator maintained at 39 ± 1 °C. A dish containing 5 ml of toluene was placed in the desiccator with relative humidity higher than 75% to prevent the molds growth (Labuza 1984). Samples were weighed periodically till they attained equilibrium after which they were analyzed for moisture content. To establish moisture sorption isotherms, the equilibrium moisture content (EMC) values were plotted against water activity (aw). The GAB model (Eq. 1) was used for fitting the experimental aw and EMC data.
Where M o is the monolayer moisture content (d.b.), X is the equilibrium moisture content (d.b.) and C g , K g are constants. The values of the constants (M o , C g , K g ) were calculated following the non-linear fitting of the data using Origin 7.5 software (Origin Lab Corp., Northampton, Mass).
Assessment of shelf-life of dried Aloe vera gel powder
Samples of dried Aloe vera gel powder weighing 20 g was placed in three packaging materials, viz., AF (area:13.25 × 10−3 m2), BOPP (area:12.75 × 10−3 m2), and PP (area:14.38 × 10−3 m2) and were closed by heat sealing taking care that the minimum possible air space remained in the packets and that the packets were leak proof. Seven sealed pouches were kept in a desiccator maintained at 90 ± 1% relative humidity using a saturated solution of potassium nitrate and placed in an incubator maintained at 38 ± 1 °C under dark. Pouches were placed in vertical pouch holder to ensure that they should not contact each other and all are exposed to the same environment.
One packet was taken out from the desiccator at 7 days interval up to 49 days and analyzed for moisture content. The shelf-life was calculated using the following equation reported by Crank (1999).
Where θ = shelf-life (days), W s = weight of the dry solids (g), P* = saturated vapour pressure of water at ambient temperature (Pa), k = permeability of packaging material (kg.m−2 day−1 Pa−1), A p = surface area of the packaging material (m2), RH = relative humidity of the environment in which the package is placed (%), a w = water activity of the product, X i = initial moisture content (%, d.b.) and X c = critical moisture content (% d.b.). The initial value of the moisture content, X i , should normally be lower than the monolayer value, M o (kg water per kg dry solid) of the powder and it can be obtained from the GAB equation (Eq. 1). As the moisture migrates into the packaging material, the value of a w will rise and that of (RH-a w ) will reduce in Eq. 2.
The initial value of the integral of Eq. 2 was estimated numerically by using the GAB equation (Eq. 1). Graphical relationship between the moisture content X s (kg water per kg dry solid) and the corresponding storage time θ (day), obtained from Eq. 2 was compared with the experimental values of X s , and θ. To check the validity of this model, the mean relative percent deviation modulus, E (%) was calculated by the relationship reported by Lemon et al. (1985).
Where E is the mean relative percentage of deviation modulus, N is the total number of observations, e i is the experimental value, p i is the predicted value using the developed model. A value of E less than 10% is considered as reasonably good fit for the model and real values (Lemon et al. 1985).
Measurement of permeability of packaging material
The water vapour permeability, k (kg.m−2 day−1 Pa−1) of packaging material was computed by Eq. 4 (Labuza 1984).
Where d w /dθ p = slope of the straight-line plot between the time θ p (day) and weight (kg) of the silica gel kept within the packaging material, A p = surface area of the packaging material (m2), and P* = saturation vapour pressure of water at 38 ± 1 °C (Pa) which is the temperature of the environment in which the packaging material is kept.
Assessment of colour change in dried Aloe vera gel powder during storage
One packet was taken out from desiccator at regular intervals and analyzed for moisture content, water activity and colour. Moisture content was determined following AOAC Method 934.06 (AOAC 1990) by using a vacuum oven (temperature: 70 °C and pressure: 37.3 kPa) and analytical balance. The colour of the dehumidified air dried samples was measured with colourimeter (model CR-400/410 chromameter, Konica Minolta Holdings Inc., Tokyo, Japan) and results were expressed in accordance with the CIE Lab system (Hutchings 1994) with reference to illuminant D65 and a viewing angle of 10° (Calvo et al. 2001). The L *, a *, and b * values of the fresh as well as stored powder were measured. The total colour difference (∆E s ) between dehumidified air dried Aloe vera gel powder initially and the stored powder was calculated using Eq. 5.
Where, \( L_i^{*} \), \( a_i^{*} \) and \( b_i^{*} \) are the initial and \( L_r^{*} \), \( a_r^{*} \) and \( b_r^{*} \) are the corresponding values for stored powder.
Kinetics of colour change during storage
The Aloe vera powder produced at optimized conditions of drying temperature, relative humidity and air velocity of 64 °C, 18%, and 0.8 m.s−1, respectively, was packed in three different packaging materials, viz., AF, BOPP and PP. The pouches were kept in environment maintained at 90 ± 1% relative humidity and 38 ± 1 °C using saturated potassium nitrate solution in an incubator. After every 7 days one of the pouches was taken out of the control environment and the L * , a * , and b * values of the stored powder was measured. The values of the overall colour difference, ∆E s between the stored powder obtained from 0th and the θ days of storage were computed using Eq. 5. It is expected that the value of ∆E s will gradually increase with time of storage and will reach the maximum value of \( \Delta E_s^{*} \).
Considering that the rate of change of (\( \Delta E_s^{*} - \Delta {E_s} \)) with storage period θ s will follow a first order kinetics, we can write (Chen and Ramaswamy 2002);
Where k (day−1) is the reaction rate constant for the colour change of Aloe vera gel powder. Solving Eq. 6 with the limits of ∆E s varying from ∆E s to \( \Delta E_s^{*} \) and the corresponding storage time varying from 0 to θ s we get,
From the estimated values of ∆E s , the value of k (day−1) was estimated by finding the slope of the best fit line of the plot between θ and \( \ln \left( {1 - \frac{{\Delta {E_s}}}{{\Delta {E^{*}}}}} \right) \), using this value of k.
Results and discussion
Permeability of packaging materials
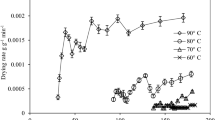
The water vapour permeability of packaging material was determined by using Eq. 4 for AF, BOPP and PP. Figure 2 depicts the gain of weight by silica gel packed inside the three packaging materials stored in an environment maintained at 90 ± 1% relative humidity and 38 ± 1 °C temperature. The slopes of the best-fit linear plot of the Fig. 2 was 2 × 10−4 for all three packaging materials. The permeability of packaging material was found as 2.67 × 10−6, 2.37 × 10−6, and 2.57 × 10−6 kg.m−2 day−1 Pa−1, respectively for BOPP, PP and AF. The value of water vapor permeability for AF was higher than the reported value of 5.4 × 10−8 kg.m−2 day−1 Pa−1 by Jaya and Das (2005). The increased value may be attributed due to low thickness (40 μm) of the packaging material used in the present investigation in comparison to (90 μm) thickness of packaging material used by Jaya and Das (2005).
Variation of moisture content and shelf-life of Aloe vera gel powder
The variation in the moisture content and changes in quality characteristics of dehumidified air dried Aloe vera gel powder during accelerated storage is shown in Fig. 3. There was a gradual increase in moisture content of the samples in three different packaging materials. ANOVA for moisture (Table 1) indicated that the types of packaging material and storage time significantly (P < 0.01) affected the moisture gain by Aloe vera gel powder. The moisture uptake depends on water vapour permeability of the packaging film. The moisture gain by powder packed in BOPP and PP were more than that in the AF pouches and the moisture content values after 35 days of storage were 9.17, 8.74 and 7.78% (d.b.), respectively. The water activity of the powder was determined using a water activity meter (AquaLab, make), it was increased from 0.232 to 0.639, 0.232 to 0.640 and 0.232 to 0.641 during 49, 42 and 35 days of storage for AF, PP and BOPP, respectively (Table 2).
The dehumidified air dried powder samples exhibited a caking tendency at moisture contents of 9.13–9.17% (d.b.). The sticky point temperature was also measured at this moisture content and it was recorded as 38 °C whereas at 5% moisture content (d.b.) it was 53 °C. The samples therefore lost their free flowing characteristics and formed lumps at moisture contents of 9.13–9.17% (d.b.). This moisture content was considered as critical moisture content and the corresponding water activity was calculated using the GAB parameters. Four sorption models namely Smith, Oswin, Peleg and GAB were fitted to the experimental data and found that GAB model is fitting well. The models were selected on the basis of their effectiveness in describing the sorption isotherms of several foods (Basu et al. 2006). The attainment of moisture content of 9.13% (d.b.) by powder samples during the storage was therefore used as an index for deciding the shelf-life. Similar results were obtained by Kumar and Mishra (2004) for mango fortified yogurt powder.
The values of GAB parameters M o , C g and K g for AF were 0.0862, 0.226 and 21.848, respectively, for PP the parameters were 0.0865, 0.216 and 21.742, respectively, and for BOPP the parameters were 0.089, 0.222 and 20.528, respectively. The area A p of the three pouches BOPP, PP and AF were 12.75 × 10−3 m2, 14.38 × 10−3 m2 and 13.25 × 10−3 m2, respectively. The amount of the dry solids, W s in the 0.02 kg powder was 0.0169 kg. The integral part of Eq. 2 was estimated between the limits of initial moisture content of 0.05 kg per kg dry solids and critical moisture content 0.0913 kg per kg dry solids. By putting the values of W s , P * , k, A p in Eq. 2, the shelf-life θ s of the dehumidified air dried Aloe vera gel powder was calculated as 51.05, 42.58 and 33.87 days in AF, PP and BOPP pouches respectively, under accelerated storage conditions.
The actual shelf-life obtained was 49, 42 and 35 days in AF, PP and BOPP pouches, respectively. The mean relative percentage deviation modulus, E% (Eq. 3) between the calculated values of shelf-life by using GAB model and the experimental value of shelf-life was calculated as 4.17, 1.37 and 3.41%, respectively for AF, PP and BOPP pouches. Since the value of E is below 10%, it may be conclude that the Eq. 2 can be used for finding out the shelf-life of the dehumidified Aloe vera gel powder. Similar results were obtained by Jaya and Das (2005) for mango powder.
Kinetics of colour change in dried Aloe vera gel powder during storage
Kinetic modelling is necessary to derive basic information for a system in order to describe the reaction rate as a function of storage time and hence, to predict colour changes in a particular food during storage. Hunter L *and b * values of dried Aloe vera gel powder decreased whereas the a * values increased during accelerated storage condition and were significantly (P < 0.01) affected by both the packaging material and storage time (Tables 1 and 3). After storage for 49 days at 38 ± 1 °C and 90 ± 1% RH, there was a greater colour change of 22.73, 25.37 and 19.62 for PP, BOPP and AF, respectively. Similar trend of results were observed by Anupama et al. (2007) for sweet whey powder.
Figure 3 shows the changes in quality characteristics of Aloe vera gel powder packed in AF, BOPP and PP packaging material during storage at 38 ± 1 °C. It shows increase in overall colour difference (∆E s ) with increase in storage time θ s (day). Based on the experimental data, a straight line plots (Fig. 4) between storage time θ s (day) and \( \ln \left( {1 - \frac{{\Delta {E_s}}}{{\Delta {E^{*}}}}} \right) \)was made for AF, BOPP and PP packaging materials.
Table 4 shows the kinetic parameter of colour change obtained from first order kinetics. The negative sign of k indicates that the value reduces with increase in storage time θ s . The high value of rate constant for BOPP (k 1 = −0.075) and PP (k 1 = −0.0498) pouches in comparison to AF pouches (k 1 = −0.0444) means that more changes take place in powder packed in BOPP and PP than AF (Table 4).
Conclusions
This study demonstrated that during storage under accelerated conditions (38 ± 1 °C, 90% RH), the predicted shelf-life of dehumidified air dried Aloe vera gel powder was 33.87, 42.58 and 51.05 days in BOPP, PP and AF pouches, respectively. Change in colour was affected by both storage time and packaging material. The total colour difference was increased from 4.64 to 17.17 in PP, 2.45 to 11.15 in AF and 7.95 to 22.74 in BOPP. Colour change of powder during storage followed first order reaction kinetics with a rate constant of −0.0444 per day for AF, −0.075 per day for BOPP and −0.0498 per day for PP. The negative sign of k indicates that the value reduces with increase in storage time. Kinetic constants for colour change were more for powder packed in BOPP and PP pouches than in AF pouches suggesting that AF is better suited for packaging of dried Aloe vera gel powder.
References
Ahlawat KS, Khatkar BS (2011) Processing, food applications and safety of Aloe vera products: a review. J Food Sci Technol doi:10.1007/s13197-011-0229-z
AOAC (1990) Method 934.06: moisture in dried fruits. In official methods of analysis. Gaithersburg, Md.: Association of Official Analytical Chemists
Anupama D, Mark RE, Scott AR (2007) Kinetics of browning during accelerated storage of sweet whey powder and prediction of its shelf-life. Internl Dairy J 17(2):177–182
Basu S, Shivhare US, Mujumdar AS (2006) Model for sorption isotherm forfood: a review. Drying Technol 24(8):917–930
Calvo C, Salvador A, Fiszman SM (2001) Influence of colour intensity on the perception of colour and sweetness in various fruit flavoured yogurts. European Food Res Technol 213(2):99–103
Chen CR, Ramaswamy HS (2002) Color and texture change kinetics in ripening of bananas. Lebensm Wiss Technol 35(5):415–419
Choi S, Chung M (2003) A review on the relationship between Aloe vera components and their biologic effects. Semin Integr Med 1(1):53–62
Crank J (1999) The mathematics of diffusion, 2nd edn. Oxford University Press, Oxford, pp 89–103
Erbay Z, Icier F (2009) Optimization of hot air drying of olive leaves using response surface methodology. J Food Eng 91(4):533–541
Greenspan L (1977) Humidity fixed point of binary saturated aqueous solutions. J Res Natl Bureau of Standards 81A(1):89–96
Grindlay D, Reynolds T (1986) The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol 16(2–3):117–151
He Q, Zhang T, Lieu (2002) Study on technology of aloe gel freeze-dried powder. Food and Fermentation Industries (Chenses) 28(6):49–52
Hutchings JB (1994) Food colour and appearance. Blackie Publication, Glasgow
Iglesias HA, Chirife J (1982) Handbook of food isotherms: water sorption parameters for food and food components. Academic, New York
Jaya S, Das H (2005) Accelerated storage, shelf-life and colour of mango powder. J of Food Proc Preserv 29(1):45–62
Koga T (1998) Cosmetic compositions containing aloe polysaccharides for inhibition of spots, darkening and wrinkles. Japanese Patent 10101541
Kumar P, Mishra HN (2004) Storage stability of mango soy fortified yoghurt powder in two different packaging materials: HDPP and ALP. J of Food Eng 65(4):569–476
Labuza TP (1984) Typical sorption isotherms. In moisture sorption: practical aspects of isotherm measurement and use, American Association of Cereal Chemists. St. Paul, Minn, pp: 8–21
Lemon CJ, Bakshi AS, Labuza TP (1985) Evaluation of food moisture sorption isotherm equations, Part I. Fruit, vegetable and meat products. Lebensm Wiss Technol 18:111–117
Margarita M, Maureira H, Katia R, Antonio VG (2009) Influence of temperature on the drying kinetics, physicochemical properties and antioxidant capacity of Aloe vera (Aloe Barbadensis Miller) gel. J of Food Eng 91(2):297–304
Myers R, Montgomery DC (1995) Response surface methodology. John Wiley and Sons, New York
Pisalkar PS, Jain NK, Jain SK (2010) Osmo-air drying of Aloe vera gel cubes. J Food Sci Technol 48(2):183–189
Ramachandra CT, Rao PS (2008) Processing of Aloe vera leaf gel: a review. American J Agril and Biol Sci 3(2):502–510
Smitha G, Pratima A (2007) Nutrient composition and physico-chemical characteristics of Aloe vera (Aloe barbadensis Miller) powder. J of Food Sci Technol 44(2):224–225
Stat-Ease Inc (2000) Design expert user guide. The Stat-Ease Inc, USA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramachandra, C.T., Rao, P.S. Shelf-life and colour change kinetics of Aloe vera gel powder under accelerated storage in three different packaging materials. J Food Sci Technol 50, 747–754 (2013). https://doi.org/10.1007/s13197-011-0398-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0398-9