Abstract
Neuroendocrine carcinoma rarely occurs in the duodenum, and most cases of neuroendocrine carcinoma in the duodenum show rapid progression of the disease. Such cases have poor prognosis even with radical surgery with or without chemotherapy with low 5-year survival rate. We present a case of a 52-year-old man who presented with abdominal pain of 1-month duration and one episode of vomiting. Upper gastrointestinal endoscopy revealed polypoidal lesions in the first and second part of the duodenum. Whipple’s procedure was performed. Diagnosis of poorly differentiated neuroendocrine carcinoma was made with extension to pancreas with peripancreatic lymph node metastases. The patient expired on post operative day 17 following cardiac arrest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NETs) of the gastroenteropancreatic (GEP) system are epithelial neoplasm with predominantly neuroendocrine differentiation and originate from diffuse endocrine system located in the gastrointestinal (GI) tract and in the pancreas [1, 2]. NETs occur in the small and large intestine, pancreas, appendix and lung [1]. NETs are graded as well differentiated, moderately differentiated NET and poorly differentiated neuroendocrine carcinoma (NEC) [3]. There is a great difference in pathological grading at different sites. Most NETs from oesophagus, colon and gall bladder are poorly differentiated NEC [4]. Duodenal neuroendocrine tumours (d-NET) are rare neoplasms that originate from the enterochromaffin cells of the gastroenteropancreatic (GEP) neuroendocrine system [3]. Duodenal NETs account for only 2.6% of all the neuroendocrine neoplasms [3, 5]. They most commonly occur in the 6th decade of life with male preponderance [6]. Most of the GEP-NET are non-functional and are discovered incidentally on endoscopy, done for symptoms like abdominal pain, upper gastrointestinal bleeding, anaemia or jaundice [5, 7]. Various investigations that can be performed for the diagnosis of NET are upper gastrointestinal endoscopy which is the gold standard [6]. Ultrasound abdomen, endoscopic ultrasonography (EUS) and EUS-fine-needle aspiration and biopsy (EUS-FNAB) can also be done along with other radiological modalities such as computerised tomography (CT) and magnetic resonance imaging (MRI) [8, 9]. Surgical intervention includes Whipple’s procedure or pancreaticoduodenectomy with Roux-enY anastomosis. A 5-year survival rate for patients with well differentiated NET is 85%, but patients with poorly differentiated NEC have unfavourable prognosis [6]. Histologically, NETs are small blue round cell tumours showing uniform cells having round to oval nucleus with stippled chromatin and eosinophilic granular cytoplasm. Immunohistochemical markers include chromogranin A, synaptophysin and neuron-specific enolase [10].
Here, we present a case of 52-year-old male patient with duodenal poorly differentiated neuroendocrine carcinoma.
Case History
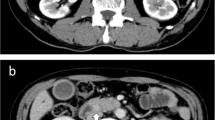
A 52-year -old man presented with pain abdomen of 1-month duration and one episode of vomiting. Clinical examination did not reveal mass per abdomen or abdominal tenderness. There were no other significant systemic findings. Ultrasound abdomen and CT scan showed thickening of the stomach wall in the distal portion and a mass measuring 8 cm × 3.6 cm in the duodenum. Liver showed normal echotexture. Multiple lymph nodes in peri pancreatic region were noted. PET scan was not done due to monetary constraints. Laboratory investigations showed mild anaemia (11.7 g/dl haemoglobin) and liver function tests showed mild decrease in albumin/globulin ratio (1.16) with other parameters being normal.
Upper gastrointestinal endoscopy showed multiple polypoidal lesions in the first and second part of the duodenum (Fig. 1). Biopsy was taken from polypoidal lesions, which on histopathology showed features of well differentiated NET (Fig. 2).
Whipple’s surgery was performed. On gross examination, multiple polypoidal umbilicated grey white to yellow tumour with mucosal bulgings were seen involving the entire length of duodenum with extension to muscularis propria. Tumour extension to head of pancreas was also noted (Fig. 3). Histologically, tumour cells were arranged predominantly in, ribons (Fig. 4), insular pattern (Fig. 5) and sheets (Fig. 6) and rosettes, having round to oval nucleus with stippled chromatin and scant to moderate amount of cytoplasm. Tumour was infiltrating head of pancreas with involvement of 18 out of 30 peripancreatic lymph nodes. Resected margins were free of tumour. The tumour was graded as poorly differentiated NEC (grade 3) with pathological stage III.
The patient had massive intraoperative bleeding, multiple episodes of desaturation in the post operative period and succumbed to cardiac arrest on post operative day 17
Discussion
Duodenal NET is extremely rare and accounts for only 2.6% of all NETs [3, 5]. Duodenal NETs (d-NETs) can be classified into five different tumour types: non-functional d-NETs, duodenal gangliocytic paraganglioma, high-grade poorly differentiated duodenal NEC (d-NEC), duodenal gastrinomas and somatostatinomas [5]. d-NEC represents 6–8% of all d-NETs and is primarily located in periampullary region [6]. Broadly, it is important to note that 90% of d-NETs are not associated with a functional clinical syndrome and more than 90% occur in the first or second part of the duodenum [5]. These tumours are mostly non-functional and may not cause any signs or symptoms in early stage of the disease. Symptoms appear once the tumour grows into surrounding tissues. Early symptoms may be non-specific, which makes NEC difficult to diagnose.
They most commonly occur in the 6th decade of life with male preponderance. Most of the GEP-NETs are non-functional and present clinically with symptoms like abdominal pain, upper gastrointestinal bleeding, anaemia or jaundice [6]. Our patient was a 52-year-old male, presenting with abdominal pain.
CT scans are often the initial imaging study for a patient presenting with signs or symptoms suggestive of a NET. There are no studies comparing the effectiveness of CT and MRI in detecting primary d-NETs and liver metastases [6]. However, according to European Neuroendocrine Tumour Society (ENETS) guidelines, MRI is considered superior for the detection and follow-up of both primary tumours and liver metastases when compared to CT. In the present case, CT scan revealed a circumferential thickening of the pylorus and 8 × 4 cm mass arising from the second part of duodenum along with enlarged peripancreatic lymph nodes, without any evidence of liver metastaes.
Other imaging modalities which are necessary for staging include 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) [5]. In our case, PET scan was not done due to monetary constraints. Endoscopic ultrasonography (EUS) has an important role in assessing the depth of tumour invasion and lymph node assessment. EUS can detect tumours located in the submucosa that cannot be seen during upper gastrointestinal endoscopy. The ability to perform ultrasound guided fine-needle aspiration (FNA) for d-NETs in deeper layers is another advantage of EUS. Upper gastrointestinal endoscopy with biopsies represents the gold standard in the diagnosis of d-NETs [6]. Upper gastrointestinal endoscopy in our patient showed multiple polypoidal lesions in the first and second part of the duodenum.
All sporadic d-NETs should be considered for resection, unless medical co-morbidities preclude any improvement in overall patient survival or in the presence of distant metastases. Endoscopic resection can be performed on small non-gastrinoma d-NETs, after excluding lymph node metastases by EUS or other tumour localisation studies. Surgery in the form of local resection with lymphadenectomy or pancreaticoduodenectomy is usually employed for patients with duodenal gastrinomas or large d-NETs (> 2 cm), d-NETs invading beyond the submucosa, lymph node metastases or d-NET in the periampullary region [3]. Our patient underwent pancreaticoduodenectomy with Roux-en Y anastomosis with resection of pyloric end of stomach, duodenum, head, neck and uncinate process of pancreas, proximal portion of jejunum and gall bladder.
Gross examination showed multiple polypoidal umbilicated grey white to yellow tumour with mucosal bulgings involving the entire length of duodenum with extension to muscularis propria. Tumour extension to head of pancreas was also noted.
Histologically, NETs are small blue round cell tumours showing uniform cells having round to oval nucleus with stippled chromatin and eosinophilic granular cytoplasm. Histopathology in the present case showed tumour cells arranged predominantly in sheets, insular pattern, ribbons and rosettes, having round to oval nucleus with stippled chromatin and scant to moderate amount of cytoplasm, with tumour in filtration into head of pancreas and involvement of peripancreatic lymph nodes.
Immunohistochemical markers for confirmation of diagnosis include chromogranin A, synaptophysin and neuron-specific enolase [10].
The diagnosis of poorly differentiated neuroendocrine carcinomas of the second part of the duodenum (d-NEC) with extension to the head of pancreas was made.
d-NECs are very aggressive tumours, diagnosed in advanced stage of the disease with lymph node or liver metastases [7].
In 2010, the World Health Organisation (WHO) updated its classification of NETs based on the histopathology of the tumour and the assessment of proliferation fraction and/or mitotic count [7, 8]. The proliferative rate of the neoplasm is the most important feature used for grading. It is assessed as the percentage of neoplastic cells showing positive immunostaining for the proliferation marker Ki-67 and by counting mitotic figures as shown in Table 1 [6, 11].
Approximately 40 to 60% of patients at the time of diagnosis have metastases to regional lymph nodes, while liver metastases occur in less than 10% of patients [7]. A 5-year survival rate for patients with well differentiated NET is 85%, but patients with poorly differentiated NEC have unfavourable prognosis. Our patient had massive intraoperative bleeding, multiple episodes of desaturation in the post operative period and he succumbed to cardiac arrest on post operative day 17.
Survival rates vary between 65 and 75% in the case of lymph node involvement and 20–40% in patients with liver metastases [6].
Conclusion
Neuroendocrine carcinoma of the duodenum is very rare tumour and quite challenging to suspect clinically. It commonly occurs in the 6th decade of life with male preponderance. Preoperative imaging, endoscopy and the accompanying detailed histological examination are useful to diagnose NEC. Pancreaticoduodenectomy can provide status of lymph node metastases and also infiltration to neighbouring organs which is important for the staging of the neuroendocrine tumour. Poorly differentiated forms have an aggressive behaviour and should be diagnosed at the earliest for immediate intervention.
References
Sata N, Tsukahara M, Koizumi M, Yoshizawa K, Kurihara K, Nagai H, Someya T, Saito K (2004) Primary small-cell neuroendocrine carcinoma of the duodenum – a case report and review of literature. World Journal of Surgical Oncology 2:28. https://doi.org/10.1186/1477-7819-2-28.
Uppin MS, Uppin SG, Sunil CS, Hui M, Paul TR, Bheerappa N (2017) Clinicopathologic study of neuroendocrine tumors of gastroenteropancreatic tract: a single institutional experience. J Gastrointest Oncol 8(1):139–147
Kim SH, Park CH, Ki HS, Jun CH, Park SY, Kim HS, Choi SK, Rew JS (2013) Endoscopic treatment of duodenal neuroendocrine tumors. Clinical Endoscopy 46(6):656–661. https://doi.org/10.5946/ce.2013.46.6.656
Qui X, Liu M, Liu M, Yang Z, Liu J, Meng F et al (2017) Analysis of primary site and pathology on 903 patients with neuroendocrine neoplasms. Chinese journal of gastrointestinal Surgery 20(9):993
Chin JL, O’Toole D (2017) Diagnosis and management of upper gastrointestinal neuroendocrine tumors. Clinical Endoscopy 50(6):520–529. https://doi.org/10.5946/ce.2017.181
Strinovic M, Kruljac I, Dabelic N, Nikolic M, Ljubicic N, Cugura JF (2016) Duodenal neuroendocrine tumors (d-NETs): challenges in diagnosis and treatment. Endocrine Oncology and Metabolism 2(3):174–93
Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G (2010) Neuroendocrine tumors of the small bowels are on the rise: early aspects and management. World J Gastrointest Endosc 2(10):325–334
Yang K, Kim S, Shin N, Park Y (2017) Clinicopathological features and surgical outcomes of neuroendocrine tumors of ampulla of Vater. BMC Gastroenterol 17:70
Imamura N, Nanashima A, Hiyoshi M, Fujii Y (2017) Report of two cases of large cell neuroendocrine carcinoma of duodenal ampulla with contrasting outcomes following pancreaticoduodenectomy according to the use of adjuvant chemotherapy. Int J Surg Case Rep 31:132–138. https://doi.org/10.1016/j.ijscr.2017.01.031
Freis P, Graillot E, Rousset P, Hervieu V, Chardon L, Walter T (2017) Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers ; Vol 7: 40609
Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E (2017) Four neuroendocrine tumor types and neuroendocrine carcinoma of the duodenum: analysis of 203 cases. Neuroendocrinology 104:112–125
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dewan, P., Bhat, S.P., Kishan Prasad, H.L. et al. Neuroendocrine Carcinoma of Duodenum—an Uncommon Tumour at an Unusual Site. Indian J Surg Oncol 10, 199–203 (2019). https://doi.org/10.1007/s13193-018-0834-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-018-0834-7