Abstract
We studied the effect of nutrient additions and hydrology on the above-ground production and shoot nutrient contents of representative species of the conservative (Carex acuta) and competitive (Glyceria maxima) plant functional types in a minerotrophic wet grassland. Above-ground samples were collected in May, late June (time of maximum biomass) and August (re-growth following cutting) from plots subjected to nutrient addition treatments in years with differing hydrology, ranging from drought to prolonged flooding. Net above-ground primary production (NAPP) and shoot nutrient contents (C, N, P) were determined separately for C. acuta and G. maxima. Between-year and species differences were analyzed by repeated measures ANOVA and generalized linear mixed models (GLMM). NAPP decreased with hydrologic stress with the proportion due to C. acuta increasing with flooding, while G. maxima responded positively to nutrient additions. As expected, C% was greater, but N and P% were lower, in C. acuta shoots compared to G. maxima. Hydrology affected above-ground production and shoot nutrient contents more than nutrients, but both interacted with plant functional type, with likely impacts on ecosystem processes. Future studies must consider multiple factors to predict the effect of climate and management changes on wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change is expected to affect hydrologic regimes through changes in precipitation amounts and seasonal patterns. This is especially relevant for Central Europe, which is predicted to have drier conditions with changed precipitation patterns as a result of climate change (IPCC 2014). Such changed hydrologic patterns are expected to affect plant species composition with subsequent repercussions on ecosystem functions (Garssen et al. 2017). Other human actions, including increased nutrient inputs resulting from agricultural intensification (Vitousek et al. 1997), can interact with site hydrology resulting in changed plant species composition and diversity (Wisheu and Keddy 1992; McJannet et al. 1995; Bollens et al. 2001; Brinson and Malvarez 2002) as well as affecting plant biomass and nutrient allocation patterns (Saggar et al. 1997; Detenbeck et al. 1999; Gough et al. 2000; Pezeshki 2001) with such changes ultimately impacting ecosystem processes and functions (Grigulis et al. 2013; Kaštovská et al. 2015).
Changing environmental conditions affect ecosystem processes through impacts on plant species traits, as well as influencing the dominance of various functional types (Diaz et al. 2004; Minden and Kleyer 2015). The conservative and competitive plant functional types represent end points of a growth-survival gradient predicted by life history theory (Grime 1988; Metcalf et al. 2006; White et al. 2016; De Deyn 2017). Compared to conservative species, competitive species tend to grow in nutrient-richer habitats, have greater photosynthesis and nutrient uptake rates, leading to faster growth of tissues with higher N contents, which results in faster decomposition and nutrient turnover rates (Orwin et al. 2010; de Vries et al. 2012; Baxendale et al. 2014).
Although it is well-known that both internal (e.g., primary production, biomass and nutrient allocation patterns) and external conditions and processes affect plant growth and development (Wardlaw 1990; De Deyn 2017), most of the studies conducted on this topic have focused on the impacts of single factors. However, ecological systems, including wetlands, are influenced by multiple, co-occurring factors, which may interact in a non-additive manner (Dieleman et al. 2012; Sierra et al. 2015). Multifactorial studies may therefore provide greater and more realistic insights into how ecosystems respond to changing conditions brought about by various human activities including climate change (Reese et al. 2018).
Manipulative field experiments offer one means for investigating the effect of multiple, interacting factors on plant growth and ecosystem processes. Such an experiment was established in two wet grasslands in the South Bohemia region of the Czech Republic to study the effect of long-term (seven years, from 2006 to 2013) nutrient inputs on plant-soil interactions (Picek et al. 2008; Edwards et al. 2015). Wet grasslands are graminoid-dominated wetlands (Joyce and Wade 1998; Joyce 2014) that, in Europe, are maintained by human activities. Therefore, the composition of these habitats is vulnerable to any change in management practices (Joyce and Wade 1998; Tallowin and Jefferson 1999; Prach 2008), which can then cascade to affect ecosystem processes and functions (Kaštovská et al. 2015, 2017).
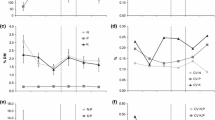
In addition to the nutrient addition treatments, there were uncontrolled changes in the hydrologic conditions over the seven years of the study, with the first three years being drier while the wet grasslands were flooded for long periods in the later years (Edwards et al. 2015). Here, we present results from one of the grasslands in which representative species of the conservative and competitive plant functional types, specifically Carex acuta and Glyceria maxima, respectively, were co-dominants. Carex acuta and G. maxima are tall emergent macrophytes forming stands on flat or gently sloping parts of wetlands on gleyed clays or seasonally shallowly flooded or waterlogged organogenic sediments (Hejný and Husák 1978; Hroudová and Zákravský 2002). Both species have mean Ellenberg moisture indicator values of 8 (Chytrý et al. 2018), but differ in their mean Ellenberg indicator values for nutrients, being 4 and 9 for C. acuta and G. maxima, respectively. Carex acuta replaces short sedges such as C. nigra in formerly oligotrophic wetlands subject to nutrient additions (Prach 1993, 2008), but it tends to be outcompeted by reeds including G. maxima under eutrophic conditions. Data are from three years (2007, 2008 and 2012) differing in their hydrologic conditions. Site hydrologic conditions were quite dry in the 2007 and 2008 growing seasons with minimum water levels being far below the rooting zone (Fig. 1). These two seasons differed in that 2007 followed a wetter year while the year preceding 2008 was also dry. These years differed from the 2012 growing season, in which the water level rarely fell below the rooting zone with periods of prolonged flooding (Fig. 1).
Monthly mean water levels in the study wet grassland from the start of long-term nutrient additions in 2006 to the end of the experiment in 2013. Water levels were measured at 15 s intervals by a water level sensor (Fiedler-Magr, Czech Republic) in a well located in the center of the site. Daily means were calculated from these data and then these were used to calculate the monthly means. Blocks indicate the 2007, 2008 and 2012 growing seasons analyzed in this study. The solid line at zero represents the soil surface while the dashed line at 200 mm below the soil surface represents the lower boundary of the root zone. Gaps in the water level data are when the water level sensor was malfunctioning
The aim of the study was to determine how nutrient additions and site hydrology interact in affecting above-ground plant production and shoot nutrient contents in representative species of the conservative and competitive plant functional types and to provide insight into how these two species seemingly can co-exist (Baxendale et al. 2014; Cortois et al. 2016). We hypothesized that nutrient supply and site hydrology interact to affect above-ground plant production and shoot nutrient contents, but that the effect is specific to the functional type. Therefore, the conservative species C. acuta is expected to be favored under more stressful conditions of low nutrients and less-than optimal water level conditions (including both drought and prolonged flooding), but that increased nutrient supplies coinciding with less stressful hydrologic conditions will favor the more competitive species G. maxima.
Methods
Study Site
Nutrient addition experiments were conducted in a wet grassland with mineral soil in the Třeboň Basin Biosphere Reserve (TBBR: 49o 10’N, 14o 46′E), Czech Republic. A complete description of the study site is given in Picek et al. (2008) and Edwards et al. (2015). The wet grassland is located in the floodplain of a small river (Nežárka) and is frequently flushed. Floodplain wetlands with mineral soil tend to predominate on frequently flushed sites (Hejný and Segal 1998). The site has a silt-sand alluvial substrate and is dominated by G. maxima and C. acuta with few other species present (L. Rektoris, pers. comm.). This particular wet grassland is managed as an active agricultural area used for hay production and contains no rare, threatened nor endangered plant species. It was chosen as our study site because of the apparent co-existence of two dominant species belonging to different plant functional types. As an actively managed agricultural field, the site is mown two times during the growing season, in early-mid June and August, which correspond to the mowing times of the neighboring fields. Altitude of the study site is 410 masl.
Experimental Design
A field experiment was established in Spring 2006 and ran until the end of 2013. See Picek et al. (2008) for a full description of the experimenal design. Briefly, four blocks were established in the site, with three treatment plots (12.25 m2) per block. Fertilization treatments (0, 65 and 300 kg NPK ha−1 yr.−1, corresponding to an unfertilized control, and Low and High fertilization treatments, respectively) were administered to randomly selected plots using a commercial NPK fertilizer (Lovofert 15:15:15 NPK, Lovochemie, a.s.). The fertilizer was added in two half doses during the growing season (mid-May and July) to simulate normal agricultural practices. Adjacent plots were separated by at least 1.5–2 m wide buffer strips. Nutrient addition occurred when there was no standing water in the sites and when weather forecasts predicted that at least the following two days would be without precipitation. Laboratory analyses of the soil from the sites showed that the added nutrients were immobilized into the plants and microbes within 48 h. Thus, the manner of nutrient addition and buffer width were deemed to be sufficient to prevent the spread of the applied fertilizer to neighboring plots.
Continuous water level measurements were taken from a well established in the center of the site. Water levels were taken at 15 s intervals using the STELA system, which consisted of a field datalogger (M4516) with a GSM/GPRS modem (MG40) and water level sensor (Fiedler-Magr, Electronics for Ecology, Czech Republic). For this study, daily water level means were calculated from the continuous water level readings using MOST (Monitoring Station) version 2.3 (Fiedler-Magr, Electronics for Ecology, Czech Republic).
As noted by Edwards et al. (2015), 2007 and 2008 were dry years (Fig. 1) with mean water levels during the respective growing seasons being below the root zone, which was visually estimated to be the top 20 cm of the soil profile. This sharply differed in 2012 when the sites had standing water for most of the growing season (Fig. 1). Therefore, the plants growing in the wet grassland were subjected to drier conditions in the first two years of the study, but then had to contend with periods of prolonged flooding in 2012.
Data Collection
Above-ground plant samples were collected from randomly selected quadrats (two 25 * 25 cm quadrats) in 2007, 2008 and 2012, representing 2, 3 and 7 years of nutrient additions (see Picek et al. 2008; Zemanová et al. 2008 and Edwards et al. 2015 for fuller descriptions). Quadrats were separated by at least 10 cm in a plot and were never re-sampled during the study. Sampling occurred at approximately monthly intervals during the 2007 and 2008 growing seasons (early May to October), but only three times during the 2012 sampling period (beginning of the growing season (late April), time of maximum above-ground biomass (early July) prior to the first cutting, and in August following re-growth but before the second cutting). In all years, the harvested above-ground matter was separated into live, standing dead and litter components, with the live material further divided by species. The number of live shoots were counted per quadrat as a measure of shoot density. These harvested materials were dried at 70o C for 48 h in a forced air oven (Memmert, Germany) and weighed. The dry weights (DW) and shoot density counts were then extrapolated to a meter square basis (e.g., g DW m−2).
Data Analyses
Plant Density, Size and Production
Shoot density (shoot number m−2) and average shoot weight (above-ground biomass divided by shoot number in a particular plot) were calculated for C. acuta and G. maxima for all harvests in the three growing seasons.
Mean NAPP (g DW m−2 yr.−1) for the three growing seasons was calculated from the total dried living, standing dead and litter material for each treatment type in each year (see Picek et al. 2008 and Káplová et al. 2011). Since the site was mown twice during the growing season, live production was calculated as the difference between the mean live stem DW in the sampling period just before the site was mown minus the mean initial DW (LIVEPre-cut – LIVEInitial). To this value was added the difference between the post-mowing sampling period with the maximum mean live DW minus the mean live DW immediately following the mowing, which was assumed to be similar to the initial live DW value. Therefore, total mean live production (LIVEPROD) for each treatment per site was calculated as LIVEPROD = [(LIVEPre-cut – LIVEInitial) + (LIVEPost-cut – LIVEInitial)]. The production of dead material was determined by adding the standing dead and litter DWs collected in each quadrat and calculating the mean dead DW for each nutrient treatment. Differences in mean dead DW per treatment were calculated between subsequent sampling dates with positive differences included in estimating NAPP. NAPP for each treatment type was calculated by adding together the total live production (LIVEPROD) with those positive differences in dead matter (NAPP = LIVEPROD + DEADPositive).
Nutrient contents (percentages of C, N, P) were determined for the harvested above-ground biomass samples, with the exception of the June 2007 sampling which was omitted from the nutrient analyses (see below). C and N were analyzed using a CN analyzer (ThermoQuest, Italy) while P was determined using a flow injection analyzer (FIA, Lachat QC8500, Lachat Instruments, USA). The molar contents of C, N and P were then used to determine the respective stoichiometric ratios (C:N, C:P, N:P; Sterner and Elser 2002). The standing stocks for C, N and P were calculated by multiplying the CNP percentages with the above-ground live biomass at each harvest.
Repeated measures ANOVAs were conducted in SYSTAT v. 11 to determine whether the individual calculated parameters (shoot density and average shoot weight, above-ground biomass, NAPP, nutrient percentages, their stoichiometric ratios and nutrient standing stocks) changed over the years. In addition, repeated measures ANOVAs were run to determine whether the proportion of total above-ground production contributed by C. acuta and G. maxima differed over time. When needed, the ANOVAs were run following natural logarithm or square root data transformations to achieve normality and variance homogeneity. Scheffé’s comparison of means test was used in the case of significant nutrient treatment effects while Bonferroni corrections were used for determining the significance of the temporal effect (Sokal and Rohlf 1995).
Environmental Effects
In addition to the nutrient treatments, several water level parameters were determined from the daily mean water level measurements. From the water level data we were able to determine the mean, minimum and maximum water levels during a particular period of the growing season preceding the harvesting of plant biomass in any particular year, as well as the amplitude of water level fluctuation within any particular period (= maximum – minimum). Similar to Edwards et al. (2015), we also calculated two relative water level indicators, the number and proportion of days in a particular period preceding the selected harvesting of plant biomass in which the water level was below the root zone (the top 20 cm of the soil layer). These relative parameters were included due to their greater ecological relevancy than the basic water level parameters.
The influence of the nutrient treatments and water levels, as well as their possible interaction, on the collected above-ground plant production, shoot density and shoot nutrient content data in each site, were determined using generalized linear mixed models (GLMM) in R v. 3.3.0 (R Core Team 2016) with the nlme package (Pinheiro et al. 2016). Appropriate hydrologic measures to be included in the models for each site were determined by calculating variance inflation factors (vif) in the car package (Fox and Weisberg 2011) to remove highly correlated variables (vif > 3). For NAPP, water level factors were determined for the entire growing season in a particular year, running from April 1 to the date of that year’s last harvest. Shoot biomass and nutrient contents were analyzed for each selected harvest (May, June and August). For the first period, water level data were obtained from the start of the growing season (April 1) to the date of the first harvest. Subsequent periods were from the date of the previous harvest to the day of the selected harvest. Hydrologic measures for the full growing season analyses (NAPP, species proportion of site NAPP) were highly correlated to each other (r > 0.97), therefore the mean water level for the growing season was selected as the hydrologic measure for these analyses. For the shoot biomass and nutrient content analyses, water level amplitude and the number of days in which the water level was below the root zone (a measure of drought) were chosen by the vif analysis for those models.
Mixed models were then run separately on each dependent variable (NAPP, proportional NAPP, above-ground biomass of the dominant species as well as their CNP percentages, the resulting C:N, C:P, and N:P ratios and the CNP standing stocks). Species, the nutrient addition treatments and selected hydrologic measures were used as the explanatory (fixed) variables. Year and block were random variables tested to determine the possible influence of within-site temporal and/or spatial differences on the dependent variables. The block effect was never significant and thus was omitted from the analyses. All of the mixed effect models were run following the procedure outlined in Zuur et al. (2009). The best model for each tested dependent variable was chosen based on comparison of Akaike information criteria (AIC) scores (Akaike 1987).
Results
Above-Ground Production
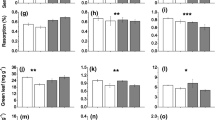
Total site NAPP significantly decreased from 2007 to 2008 and then remained similar in the 2012 growing season (Fig. 2). Based on the mixed model analysis (GLMM), this significant between-year difference in NAPP was due solely to the nutrient treatments (Table 1), likely due to the greater production in the High fertilization treatment plots in 2008 (Fig. 2).
Average (mean ± 1 SD) net above-ground primary production (NAPP; g dry weight * m−2 * yr.−1) as affected by nutrient addition treatments for a wet grassland with mineral soil. NAPP was calculated at the time of maximum biomass in the 2007, 2008 and 2012 growing seasons. Nutrient treatments: Unfert = unfertilized control (0 kg NPK m−2 yr.−1); Low Fert = low fertilization (65 kg NPK m−2 yr.−1); High Fert = high fertilization (300 kg NPK m−2 yr.−1). Significant differences between treatments in a growing season: * - p < 0.05. Different letters represent significant differences (α = 0.05) between years
The proportion of total NAPP attributable to the two co-dominant species in the wet grassland site (C. acuta, G. maxima) differed over time, with that of C. acuta increasing from 2007 to 2012 (from a minimum of 21% to a maximum of 78%) while it decreased for G. maxima (Fig. 3). Both species responded differently to the nutrient treatments and changes in site hydrology (significant species*nutrient and species*hydrology interactions; Table 1). For example, the proportion of total NAPP attributed to C. acuta increased with greater flooding (2012) while that for G. maxima decreased, but the species had opposite reactions to the nutrient treatments, which were more apparent in 2007 and 2012 (Fig. 3). Similarly, between-species differences were noted in the shoot densities of the respective species, with C. acuta density significantly increasing from 2007 to 2012 (p < 0.001) while G. maxima showed the opposite trend (Fig. 4), mostly in their response to site hydrology (significant species*mean water level interaction; GLMM analysis, Table 1). In addition, there was a weak nutrient effect on G. maxima shoot density (t = 2.560; p = 0.093), with the density significantly decreasing in 2012 in all nutrient treatments, but with maximum densities occurring in 2008 in the Low and High fertilization treatments (Fig. 4). The average shoot size did not change for either species over time (repeated measures ANOVA, p > 0.154), with G. maxima shoots always being significantly larger than that of the average C. acuta shoot (Table 1; Fig. 4). However, there was a significant increase in average G. maxima shoot size in the High fertilization treatment over the years, resulting in a weak significant species * nutrient interaction (Table 1).
The proportion of total net above-ground primary production (NAPP) represented by the dominant plant species (C = Carex acuta; G = Glyceria maxima) in a wet grassland with mineral soil subjected to nutrient addition treatments. NAPP was measured as the sum of sequential harvests; see text for details. Different letters represent significant between-year differences determined by repeated measures ANOVAs. Nutrient treatments: Unfert = unfertilized control (0 kg NPK m−2 yr.−1); Low Fert = low fertilization (65 kg NPK m−2 yr.−1); High Fert = high fertilization (300 kg NPK m−2 yr.−1). Between-treatment differences in 2012 are indicated by different letters for each species
The effect of nutrient treatments and time on shoot density (a, c) and average shoot size (b, d) of the two dominant species in a wet grassland at the time of maximum aboveground biomass in the 2007, 2008 and 2012 growing seasons. Species: Carex acuta (a, b); Glyceria maxima (c, d). Nutrient treatments: Unfert = unfertilized control (0 kg NPK m−2 yr.−1); Low Fert = low fertilization (65 kg NPK m−2 yr.−1); High Fert = high fertilization (300 kg NPK m−2 yr.−1). Different letters at top of each graph represent significant differences (α = 0.05) between years. Between-treatment differences between years were calculated using repeated measures ANOVA
Shoot Nutrient Contents
Season and changes in the relevant environmental conditions significantly affected the shoot nutrient contents (CNP percentages, stoichiometric ratios and CNP standing stocks; appendix S1). Shoot C% significantly increased, while N and P% decreased, from the beginning of the growing season to the time of maximum biomass before the first cutting in late June / early July. These then remained stable or slightly increased to the second peak in biomass before the second cut in August (Fig. 5; appendix S1). C% was the lowest in 2008, the driest year, but the greatest in 2012 in young shoots at the beginning of the growing season. Early season N and P% increased over the years, indicating a positive effect of the nutrient treatments. Similar trends were seen at the time of the second biomass peak in August (Fig. 5). However, yearly changes in the CNP contents at the time of the first cutting in June/July differed from those of the other seasonal measures with C% also being greater in 2012 than 2008. However, N and P% showed species-specific changes, decreasing over the years in C. acuta while the opposite trend was observed for G. maxima.
Seasonal shoot CNP percentages (mean ± SE) for the two dominant graminoid species in the wet grassland, the conservative species Carex acuta (filled symbols) and the competitive species Glyceria maxima (open symbols). Seasons: May (early) = circle; June (time of maximum biomass) = square; August (late) = inverted triangle. Means were calculated by combining data from the three nutrient treatments for any sampling period in a particular growing season since most of the nutrient data did not significantly differ. See appendix S1 for all of the data
Nutrient treatments significantly affected only the N% and the C:N ratio. For G. maxima shoots, these factors significantly differed between nutrient treatments early in the 2008 and 2012 growing seasons (May samples) and at the time of maximum biomass (June) but only in 2008. Early season N% was significantly greater in the unfertilized control treatment while the C:N ratio showed the opposite trend (Figs. 5 and 6; appendix S1) implying that plants in the fertilized treatments began to grow earlier than those that did not receive additional nutrients. However, shoot N% and the C:N ratio differed significantly in the Low fertilization treatment in the June 2008 sampling. Significant nutrient effects on the nutrient contents of C. acuta shoots were observed only late in the growing season (August sampling). As with G. maxima, N% and the C:N ratio were significantly different in the Low fertilization treatment in 2008 while all standing stocks were significantly greater in the unfertilized control treatment in 2007 (appendix S1).
Seasonal stoichiometric ratios (mol mol−1; mean ± SE) in shoots of the two dominant graminoid species in the wet grassland, the conservative species Carex acuta (filled symbols) and the competitive species Glyceria maxima (open symbols). Seasons: May (early) = circle; June (time of maximum biomass) = square; August (late) = inverted triangle. Means were calculated by combining data from the three nutrient treatments for any sampling period in a particular growing season since most of the nutrient data did not significantly differ. See appendix S1 for all of the data
All nutrient content parameters (CNP percentages, the stoichiometric ratios, standing stocks) were significantly affected by both the nutrient addition treatments and site hydrology (Table 2), both singly for most of the nutrient content parameters, with the exception of C standing stock, as well as there being significant species-specific responses (appendix S1). Compared to the competitive species G. maxima, C. acuta, the conservative species, had significantly greater shoot C%, but lower N and P%, with corresponding differences in the appropriate stoichiometric ratios (Figs. 5 and 6). The species also differed in the seasonality of maximum N and P stocks, with G. maxima stocks being the highest in June (time of maximum biomass) while C. acuta plants reached their maximum later in August (appendix S1). Only N% and the C:N ratio were significantly influenced by the nutrient addition * hydrology interactions (Table 2).
The species also differed in their responses to changing site hydrologic conditions. The changes in shoot nutrient contents were more strongly connected to the drought measure (number of days in a period the water level was below the root zone) than the amplitude of changing water levels. The stronger connection was especially notable for C and P%, and the C:N and N:P ratios (Table 2). However, changes in the CNP standing stocks were more connected to water level fluctuations (amplitude), due to the stronger effect on shoot biomass.
Discussion
Plant growth, production and allocation patterns are important parameters that influence how plants affect ecosystem functioning (Ehrenfeld et al. 2005; Orwin et al. 2010; Baxendale et al. 2014). These plant parameters are in turn influenced by external environmental factors as well as internal factors such as the functional type of the given species, which is linked to life history strategies (Grime 1988; Metcalf et al. 2006; De Deyn 2017). In agreement with our original hypothesis, we noted significant interactions between these internal and external variables, but not always in the ways that were initially predicted.
Environmental Effects
In our study, both the nutrient addition treatments and changes in site hydrology significantly affected NAPP and shoot nutrient contents. However, our results indicate that changes in site hydrology can outweigh the effects of nutrient additions, as noted in other wetlands (Güsewell et al. 2003; Hefting et al. 2004).
As expected, site NAPP was significantly greater in the High fertilization treatment compared to the unfertilized control. The significant increase in N and P% in young shoots at the beginning of the 2008 and 2012 growing seasons further show the positive effect of the nutrient treatments.
The significant decrease in total site NAPP, with the continued dry conditions in 2008 and the prolonged flooding in the 2012 growing season, shows the importance of site hydrology on plant growth and production. While both the 2007 and 2008 growing seasons were characterized by dry conditions with water levels far below the root zone, 2007 was preceded by a year (2006) with wetter conditions in the growing season (Fig. 1) while 2008 represented a second successive growing season with dry conditions. Thus, the prolonged dry conditions of 2008 may have resulted in decreased photosynthesis and growth, as found for several tree species (Sala et al. 2012; Klein et al. 2014; Wu et al. 2018; Dietrich et al. 2019). Future studies should focus on the influence of past hydrologic conditions on the growth and production of non-woody wetland plant species and how the strength of this effect may change through interactions with other environmental factors.
The decrease in total site NAPP with increased hydrologic stress and the observed changes in shoot nutrient contents during the growing season related to plant development (Dykyjová and Úlehlová 1998) are very common and expected results. What is more interesting was the response of the two plant species, representative of different plant functional types, to changing environmental conditions and how these species seemingly co-exist in the study site.
Role of Plant Functional Type
We predicted that the conservative species C. acuta would be better able to handle adverse, stressful conditions, but this was only partially supported by our results. Contrary to expectations, the significant decrease in total site NAPP in the 2008 growing season was due largely to the negative response of C. acuta to the continued dry conditions, most likely related to reduced plant nutrient uptake leading to decreased photosynthetic rates and growth brought about by the more stressful hydrologic conditions (Dykyjová and Úlehlová 1998; Lambers et al. 1998). This negative response was seen particularly in plants subjected to the Low fertilization treatment, which produced significantly fewer shoots and contributed significantly less to total site NAPP than plants in the other nutrient treatments (Figs. 3 and 4), while also having lower shoot N and P percentages at the time of maximum biomass in 2008. The different response of the C. acuta plants in the Low fertilization treatment plots was a surprise given that the treatment plots were randomly located within blocks spread across the wet grassland site and with no significant block effect for any of the tested parameters.
The situation was reversed under the prolonged flooding conditions in 2012, in which G. maxima growth and production were significantly negatively affected while that of C. acuta rebounded to levels similar to those of the 2007 growing season. This result agrees with the findings of Menges and Waller (1983) who classified Carex species as stress-tolerators especially in terms of their ability to survive prolonged flooding in riparian areas. Several Carex species, including C. acuta, have developed adaptations that allow them to tolerate flooded conditions, including the capacity to switch growth form and produce tussocks (Perelman et al. 2003; Crain and Bertness 2006), as well as being able to release large amounts of oxygen from their roots to the rhizosphere (Mainiero and Kazda 2005). This results in the formation of an aerobic zone in the rhizosphere that leads to increased nutrient availability and uptake rates (Končalová 1990; Visser et al. 2000; Colmer 2003).
The significantly larger average size of G. maxima shoots and the greater contribution of this species to total site NAPP with increased nutrient supply under prolonged flooding conditions implies possible nutrient*hydrology interactions which, however, were not observed in the GLMM analyses. Such a possibility is supported by the opposite response of the C. acuta plants, which likely was due to the increased cost of maintaining a large belowground structure, which should put it at a competitive disadvantage under nutrient-richer conditions (Wedin and Tilman 1990; Kaštovská et al. 2017). Ecological theory predicts that more competitive species should dominate under conditions of greater nutrient supply due to greater allocation to above-ground, photosynthesizing tissues and more efficient nutrient uptake resulting in faster growth compared to conservative species (van der Werf et al. 1993; Aerts and Chapin 2000). The apparent co-existence of these two species in our study site is likely due to interactions between multiple, co-occurring factors (Sierra et al. 2015). Based on the results of our study, the response of C. acuta and G. maxima to changing nutrient and water levels, resulting in either dominance of one of the species or co-existence, is shown in Fig. 7.
Carex acuta would have the competitive advantage under wetter, especially flooded, conditions, because of its extensive root system with well-developed aerenchyma, many fine roots and large starch reserves (Končalová 1990; Vítková et al. 2017). However, the negative effect of flooding on G. maxima can be mitigated to a degree by increased nutrient supplies, which would result in the shoots of this species having significantly larger biomass (Fig. 4). Under drier conditions, increased nutrient levels would favor the competitive species G. maxima, because it can more efficiently use the higher nutrient supplies for growth (Westlake 1966; Hejný and Husák 1978; Hroudová and Zákravský 2002). However, C. acuta shoot density was greater in the High fertilization treatment even with low water levels. Since shoot density can be used as a proxy for fitness (Shipley et al. 2016), our results imply a possible increased competitive ability of C. acuta under conditions of low water, but high nutrient levels, which could result in species co-existence. Intermediate levels of both environmental factors would also favor co-existence.
Concluding Remarks
The effect of site hydrology and nutrient levels, in combination with plant functional type, interact to affect plant growth, production and nutrient content with the response of particular species being context and biologically dependent (Sorrell et al. 2002; Pennings et al. 2005). Species of different functional types can co-exist in wetland habitats such as our wet grassland because fluctuating environmental conditions are an inherent feature of many wetlands (Middleton 1999; Mitsch and Gosselink 2000) with multiple factors interacting to affect plant species composition, community structure and eventually ecosystem functions. Which species is predominant at any point in time will then determine plant inputs to the soil, as seen by the significantly different shoot nutrient contents noted in this study, as well as similar nutrient differences in their belowground structures (Edwards 2015) and rhizodeposits (Kaštovská et al. 2017). Management actions that would increase water supply to the site, with increased flooding depth and duration, would favor the conservative C. acuta at the expense of the competitive G. maxima (Fischer et al. 2016). On the contrary, the expected drier conditions for Central Europe (IPCC 2014) would more likely negatively impact C. acuta than G. maxima, resulting in the predominance of the latter species at least over the short term. Such predominance of the competitive, faster growing species would be enhanced following re-wetting events, which are often followed by a pulse of available nutrients (Bloor and Bardgett 2012).
This illuminates the need for multivariable studies in the future. From such studies, we would be better able to predict the effects of changing climatic conditions and/or management actions on wet grasslands and other wetland systems.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30:1–67
Akaike H (1987) Factor analysis and AIC. Psychometrika 52:317–332
Baxendale C, Orwin KH, Poly F, Pommier T, Bardgett RD (2014) Are plant-soil feedback responses explained by plant traits? New Phytologist 204:408–423
Bloor JMG, Bardgett RD (2012) Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: interactions with plant species diversity and soil nitrogen availability. Perspectives in Plant Ecology, Evolution and Systematics 14:193–204
Bollens U, Güsewell S, Klötzli F (2001) Vegetation changes in two Swiss fens affected by eutrophication and desiccation. Botanica Helvetica 111:121–137
Brinson MM, Malvarez AI (2002) Temperate freshwater wetlands: types, status, and threats. Environmental Conservation 29:115–133
Chytrý M, Tichý L, Dřevojan P, Sádlo J, Zelený D (2018) Ellenberg-type indicator values for the Czech flora. Preslia 90:83–103
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment 26:17–36
Cortois R, Schröder-Georgi T, Weigelt A, van der Putten WH, De Deyn GB (2016) Plant–soil feedbacks: role of plant functional group and plant traits. Journal of Ecology 104:1608–1617
Crain CM, Bertness MD (2006) Ecosystem engineering across environmental gradients: implications for conservation and management. BioScience 56:211–218
De Deyn GB (2017) Plant life history and above–belowground interactions: missing links. Oikos 126:497–507
De Vries FT, Manning P, Tallowin JRB, Mortimer SR, Pilgrim ES, Harrison KA, Hobbs PJ et al (2012) Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecology Letters 15:1230–1239
Detenbeck NE, Galatowitsch SM, Atkinson J, Ball H (1999) Evaluating perturbations and developing restoration strategies for inland wetlands in the Great Lakes basin. Wetlands 19:789–820
Diaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, Montserrat-Martí G, Grime JP, Zarrinkamer F et al (2004) The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science 15:295–304
Dieleman WIJ, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, Morgan JA, Volder A, Beier C, Dukes JS, King J, Leuzinger S, Linder S, Luo Y, Oren R, de Angelis P, Tingey D, Hoosbeek MR, Janssens IA (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology 18:2681–2693
Dietrich L, Delzon S, Hoch G, Kahmen A (2019) No role for xylem embolism or carbohydrate shortage in temperate trees during the severe 2015 drought. Journal of Ecology 107:334–349
Dykyjová D, Úlehlová B (1998) Mineral economy and cycling of minerals in wetlands. In: Westlake DF, Květ J, Szczepański A (eds) The production ecology of wetlands. Cambridge University Press, Cambridge, UK, pp 319–366
Edwards KR (2015) Effect of nutrient additions and site hydrology on belowground production and root nutrient contents in two wet grasslands. Ecological Engineering 84:325–335
Edwards KR, Picek T, Čížková H, Máchalová-Zemanová K, Stará A (2015) Nutrient addition effects on carbon fluxes in wet grasslands with either organic or mineral soil. Wetlands 35:55–68
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annual Review of Environment and Resources 30:75–115
Fischer FM, Wright AJ, Eisenhauer N, Ebeling A, Roscher C, Wagg C, Weigelt A, Weisser WW, Pillar VD (2016) Plant species richness and functional traits affect community stability after a flood event. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 371:20150276. https://doi.org/10.1098/rstb.2015.0276
Fox J, Weisberg S (2011) An {R} companion to applied regression, Second edn. Sage, Thousand Oaks CA http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. Accessed 26 May 2018
Garssen AG, Baattrup-Pedersen A, Riis T, Raven BM, Hoffman CC, Verhoeven JTA, Soons MB (2017) Effects of increased flooding on riparian vegetation: field experiments simulating climate change along five European lowland streams. Global Change Biology 23:3052–3063
Gough L, Osenberg CW, Gross KL, Collins SL (2000) Fertilization effects on species density and primary productivity in herbaceous plant communities. Oikos 89:428–439
Grigulis K, Lavorel S, Krainer U, Legay N, Baxendale C, Dumont M, Kastl E, Arnoldi C, Bardgett RD, Poly F, Pommier T, Schloter M, Tappeiner U, Bahn M, Clément JC (2013) Relative contributions of plant traits and soil microbial properties to mountain grassland ecosystem services. Journal of Ecology 101:47–57
Grime JP (1988) The C-S-R model of primary plant strategies – origins, implications and tests. In: Gottlieb LD, Jain SK (eds) Plant evolutionary biology chapman and hall. UK, London, pp 371–393
Güsewell S, Bollens U, Ryser P, Klötzli F (2003) Contrasting effects of nitrogen, phosphorus and water regime on first- and second-year growth of 16 wetland plant species. Functional Ecology 17:754–765
Hefting M, Clément JC, Dowrick D, Cosandy AC, Bernal S, Cimpian C, Tatur A et al (2004) Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67:113–134
Hejný S, Husák Š (1978) Higher plant communities. In: Dykyjová D, Květ J (eds) Pond littoral ecosystems. Springer, Berlin, pp 23–64
Hejný S, Segal S (1998) General ecology of wetlands. In: Westlake DF, Květ J, Szczepański A (eds) The production ecology of wetlands. Cambridge University Press, Cambridge, UK, pp 1–77
Hroudová Z, Zákravský P (2002) Littoral plant communities and soils, in: Květ J, Jeník J, Soukupová L (eds.), freshwater wetlands and their sustainable future. Man and the biosphere series, v. 28, Parthenon publishing, Boca Raton, Florida, USA, pp.195-210
Intergovernmental Panel on Climate Change (IPCC) (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland
Joyce CB (2014) Ecological consequences and restoration potential of abandoned wet grasslands. Ecological Engineering 66:91–102
Joyce CB, Wade PM (eds) (1998) European wet grasslands: biodiversity, management and restoration. John Wiley and Sons, Chicester, UK
Káplová M, Edwards KR, Květ J (2011) The effect of nutrient level on plant structure and production in a wet grassland: a field study. Plant Ecology 212:809–819
Kaštovská E, Edwards K, Picek T, Šantrůčková H (2015) A larger investment into exudation by competitive versus conservative plants is connected to more coupled plant–microbe N cycling. Biogeochemistry 122:47–59
Kaštovská E, Edwards K, Šantrůčková H (2017) Rhizodeposition flux of competitive versus conservative graminoid: contribution of exudates and root lysates as affected by N loading. Plant and Soil 412:331–344
Klein T, Hoch G, Yakir D, Körner C (2014) Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiology 34:981–992
Končalová H (1990) Anatomical adaptations to waterlogging in roots of wetland graminoids: limitations and drawbacks. Aquatic Botany 38:127–134
Lambers H, Chapin FS, Pons TL (1998) Plant physiological ecology, 1st edn. Springer-Verlag, New York, NY, USA
Mainiero R, Kazda M (2005) Effects of Carex rostrata on soil oxygen in relation to soil moisture. Plant and Soil 270:311–320
McJannet CL, Keddy PA, Pick FR (1995) Nitrogen and phosphorus tissue concentrations in 41 wetland plants—a comparison across habitats and functional groups. Functional Ecology 9:231–238
Menges ES, Waller DM (1983) Plant strategies in relation to elevation and light in floodplain herbs. The American Naturalist 122:454–473
Metcalf CJE, Rees M, Alexander JM, Rose K (2006) Growth-survival trade-offs and allometries in rosette-forming perennials. Functional Ecology 20:217–225
Middleton BA (1999) Wetland restoration, flood pulsing, and disturbance dynamics. John Wiley and Sons, New York, NY, USA
Minden V, Kleyer M (2015) Ecosystem multifunctionality of coastal marshes is determined by key plant traits. Journal of Vegetation Science 26:651–662
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. John Wiley and Sons, New York, NY, USA
Orwin KH, Buckland SM, Johnson D, Turnet BL, Smart S, Oakley S, Bardgett RD (2010) Linkages of plant traits to soil properties and the functioning of temperate grassland. Journal of Ecology 98:1074–1083
Pennings SC, Clark CM, Cleland EE, Collins SL, Gough L, Gross KL, Milchunas DG, Suding KN (2005) Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 110:547–555
Perelman SB, Burkart SE, León RJC (2003) The role of a native tussock grass (Paspalum quadrifarium lam.) in structuring plant communities in the flooding Pampa grasslands, Argentina. Biodiversity and Conservation 12:225–238
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environmental and Experimental Botany 46:299–312
Picek T, Kaštovská E, Edwards K, Zemanová K, Dušek J (2008) Short term effects of experimental eutrophication on carbon and nitrogen cycling in two types of wet grassland. Community Ecology 9:81–90
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1–127, <URL: http://CRAN.R-project.org/package=nlme>. Accessed 17 April 2018
Prach K (1993) Vegetational changes in a wet meadow complex, South Bohemia, Czech Republic. Folia geobotanica & phytotaxonomica 28:1–13
Prach K (2008) Vegetation changes in a wet meadow complex during the past half-century. Folia Geobotanica and Phytotaxonomica 43:119–130
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Accessed 3 March 2018
Reese AT, Lulow K, David LA, Wright JP (2018) Plant community and soil conditions individually affect soil microbial community assembly in experimental mesocosms. Ecology and Evolution 8:1196–1205
Saggar S, Hedley C, Mackay AD (1997) Partitioning and translocation of photosynthetically fixed 14C in grazed hill pastures. Biology and Fertility of Soils 25:152–158
Sala OE, Gherardi LA, Reichmann L, Jobbagy E, Peters D (2012) Legacies of precipitation fluctuations on primary production: theory and data synthesis. Philosophical Transactions of the Royal Society, B: Biological Sciences 367:3135–3144
Shipley B, De Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB (2016) Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 180:923–931
Sierra CA, Trumbore SE, Davidson EA, Vicca S, Janssens I (2015) Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. Journal of Advances in Modeling Earth Systems 7:335–356
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman, San Francisco, CA, USA
Sorrell BK, Tanner CC, Sukias JPS (2002) Effects of water depth and substrate on growth and morphology of Eleocharis sphacelata: implications for culm support and internal gas transport. Aquatic Botany 73:93–106
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton, NJ, USA
Tallowin JRB, Jefferson RG (1999) Hay production from lowland semi-natural grasslands: a review of implications for ruminant livestock systems. Grass and Forage Science 54:99–115
van der Werf A, Visser AJ, Schieving F, Lambers H (1993) Evidence for optimal partitioning of biomass and nitrogen at a range of nitrogen availabilities for a fast- and slow-growing species. Functional Ecology 7:63–74
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment 23:1237–1245
Vítková J, Dušek J, Stellner S, Moulicová L, Čížková H (2017) Effect of hummock-forming vegetation on methane emissions from a temperate sedge-grass marsh. Wetlands 37:675–686
Vitousek PM, D’ Antonio CM, Loope LL, Remánek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology 21:1–16
Wardlaw IF (1990) The control of carbon partitioning in plants. New Phytologist 116:341–381
Wedin DA, Tilman D (1990) Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84:433–441
Westlake DF (1966) The biomass and productivity of Glyceria maxima: I. Seasonal changes in biomass. Journal of Ecology 54:745–753
White AC, Rogers A, Rees M, Osborne CP (2016) How can we make plants grow faster? A source–sink perspective on growth rate. Journal of Experimental Botany 67:31–45
Wisheu IC, Keddy PA (1992) Competition and centrifugal organization of plant communities: theory and tests. Journal of Vegetation Science 3:147–156
Wu X, Liu H, Li X, Ciais P, Babst F, Guo W, Zhang C, Magliulo V, Pavelka M, Liu S, Huang Y, Wang P, Shi C, Ma Y (2018) Differentiating drought legacy effects on vegetation growth over the temperate northern hemisphere. Global Change Biology 24:504–516
Zemanová K, Čížková H, Edwards K, Šantrůčková H (2008) Soil CO2 efflux in three wet meadow ecosystems with different C and N status. Community Ecology 9:49–55
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GN (2009) Mixed effects models and extensions in ecology with R. Springer, New York, NY, USA
Acknowledgements
Funding for this study came from a grant from the Grant Agency of the Czech Republic (GAČR: 16-21743S and 526/09/1545). We thank members of the Třeboň Basin Biosphere Reserve, especially Ladislav Rektoris and Miroslav Hatle, for access to the site. Many people helped in collecting and processing the plant samples including Mirka Káplová, Daniel Vaněk, Lukaš Bareš, Ville Närhi, Marketa Applová and Pavla Staňková.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Edwards, K.R., Čížková, H. Nutrient Inputs and Hydrology Interact with Plant Functional Type in Affecting Plant Production and Nutrient Contents in a Wet Grassland. Wetlands 40, 707–719 (2020). https://doi.org/10.1007/s13157-019-01216-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01216-0