Abstract
Zooplankton are an essential component of healthy functioning lake and wetland ecosystems. Despite this, zooplankton communities within constructed treatment wetlands (CTWs) in agricultural landscapes remain unstudied. Taxa richness, total abundances and community composition were evaluated for zooplankton assemblages from three habitat types (lakes, CTWs and drainage ditches) within five intensive agricultural peat lake catchments in New Zealand. Relationships to water quality, physicochemical and biotic habitat variables were examined. Zooplankton were dominated by cladocerans, copepods, ostracods and rotifer taxa, representing a range of communities typical of lake and pond habitats. CTWs supported species otherwise absent from lake and drain habitats, increasing the overall biodiversity of the highly-modified peat lake catchments. Taxa richness of CTWs was higher than that of drains, and a few CTWs had greater diversity than several lakes. The morphological variables area and depth contributed to the greatest differences between habitats, followed by pH, inorganic nitrogen, conductivity and temperature. Correspondingly, zooplankton communities were significantly influenced by habitat area, depth and pH, as well as ammonium, phosphate, water temperature, dissolved oxygen, and macrophyte cover. Opportunities were explored for refining CTW designs to enhance zooplankton biodiversity and potentially improve treatment efficiency through increasing the complexity and diversity of CTW habitat niches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Constructed treatment wetlands (CTWs) are used globally as technologies to improve water quality (Kadlec and Knight 1996) and are effective in reducing levels of suspended solids, nitrogen and phosphorus, as well as organic matter and pathogens (Vymazal 2007; Vymazal and Kröpfelová 2008; Kadlec 2010; Dunne et al. 2012). CTWs are designed and created to emulate and enhance the natural processes and functions of wetland ecosystems involving wetland vegetation, soils, and microbial and aquatic communities (Mitsch and Gosselink 2007; Kadlec and Wallace 2008). The efficacy of CTWs depends upon the functionality, resilience and ecological integrity of the wetland ecosystems ability to acclimate to changes in hydrology, pollutant loads and water chemistry. Ecological integrity integrates physical, chemical and biological integrity; the latter refers to an ecosystem’s capacity to support and maintain a balanced, integrated, and adaptive biological system with a full range of elements, processes and biotic interactions (Karr 1996).
Biodiversity is an essential component of biological integrity. As part of this biodiversity, zooplankton communities provide a critical link for the flow of energy and nutrients between primary producers and higher trophic levels (Gray et al. 2012; Kattel 2012). Such assemblages are also important in maintaining the ecological integrity of shallow lakes and wetlands (Moss et al. 2003; Van den Broeck et al. 2015), and have been included in a number of biotic indices developed to evaluate the ecological quality and integrity of wetlands (Lougheed and Chow-Fraser 2002; Boix et al. 2005). Zooplankton communities of wastewater treatment ponds, including high rate algal ponds, have been well researched, primarily regarding removal or control of zooplankton that can limit algal production and reduce treatment efficacy (Schlüter et al. 1987; Montemezzani et al. 2015). Methods for manipulating zooplankton community composition and relative abundances within aquaculture ponds have also been extensively researched (Geiger 1983; Piasecki et al. 2004; Milstein et al. 2006). Yet, in contrast to studies of such intensively managed and controlled pond systems, no detailed studies exist of zooplankton within agricultural CTWs. Knowledge of community composition, feeding guilds and habitat preferences of zooplankton within CTWs could contribute to improved treatment system design and help to optimise the assimilation of nutrients and reduction of pathogens.
Wetlands, including swamp, marsh, fen and peat bog ecosystems, as well as numerous peat lakes, were once key landscape features in the central Waikato region of New Zealand (Shearer 1997), supporting a diverse indigenous and endemic flora and fauna (Lowe and Green 1987). However, wetlands and lakes have declined in abundance, size and ecological integrity following extensive peatland drainage, cultivation and conversion to pasture beginning in the late 1800s (Hunt 2007). Wetland extent in the Waikato has been reduced by approximately 92% from an estimated pre-human area of >356,000 ha to c. 28,000 ha. Wetland areas have been largely replaced by highly productive, intensive dairy farming (MfE and StatsNZ 2015). Many of the remaining 31 peat lakes in the Waikato region have poor water quality and frequent cyanobacterial algal blooms, resulting from elevated nutrient and sediment levels associated with this change in land use (Hamilton et al. 2010), causing loss of much of their natural character and native biodiversity (Shearer 1997; Beard 2010).
Restoration actions are currently being implemented in several Waikato peat lake catchments to reduce nutrient and sediment runoff and improve lake water quality (Waikato 2006; Peters et al. 2008). Methods include retiring areas of marginal pasture to create esplanade reserves, fencing and planting of riparian and wetland areas around the lake margins and along the banks of inlet waterways, and creation of free surface-flow CTWs to intercept inflows and improve water quality. Within such highly modified, intensive agricultural landscapes, CTWs may feasibly enhance the biodiversity of the lake catchment through provision of habitat isolated from toxic algal blooms and adverse environmental conditions ubiquitous within the lakes themselves.
In this study, we assessed the biodiversity of CTWs by examining zooplankton communities from different aquatic habitats in five shallow peat lake catchments with intensive dairy production as the predominant land use. Habitat types, comprising lakes and their associated CTWs and drainage ditches (heavily modified and/or channelised artificial streams, referred to as drains herein), were studied as part of a greater body of research examining whether CTWs are effective tools for peat lake restoration. Zooplankton community composition was predicted to differ between habitat types, driven primarily by differences in habitat size (area and depth) and complexity (emergent macrophyte cover), water quality variables, and the presence or absence of fish. Based on these factors, we predicted that the biodiversity of zooplankton in CTWs would be higher than that of drains and lower than those of lake habitats.
Our key objectives were to:
-
(i)
compare zooplankton communities (taxa richness, abundances and community composition) between CTW, lake, and drain habitat types;
-
(ii)
examine environmental mechanisms driving differences in the zooplankton communities; and
-
(iii)
discuss opportunities for refining CTW designs to enhance zooplankton biodiversity.
Methods
Study sites
The research was carried out in the central Waikato region, New Zealand (37.8 ͦ S, 175.2 ͦ E), where there are a number of shallow peat lakes located within agricultural catchments which are almost entirely used for intensive dairy production. Five shallow peat lakes were selected as study sites where CTWs have been implemented as mitigation tools for the inflows of the lakes: Kainui, Kaituna, Komakorau, Koromatua and Serpentine North. These were the only Waikato lakes with CTWs at the time the research was conducted and the CTWs were similar in age, constructed between 1999 and 2001. Lakes Kainui and Kaituna are within the Kainui restiad peat bog in the Waikato District, north of Hamilton city. Lake Koromatua is on the edge of the Rukuhia restiad peat bog, while Lake Serpentine is on the fringe of the Moanatuatua restiad peat bog, both in the Waipa District south of Hamilton city (Clarkson et al. 2004). The central Waikato has a temperate maritime climate with annual rainfall across the lake catchments (approximately 35 km from north to south) ranging from 1100 mm to 1300 mm (Dravitzki and McGregor 2011).
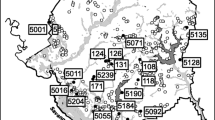
Three habitat types were sampled within each lake catchment and included five lakes, eight drains, and 27 CTWs (40 sampling locations in total). The location of the study lakes and positioning of habitat types within their catchments are provided in Fig. 1. A summary of the lake morphologies, trophic status, Trophic Level Index (TLI; Burns et al. 2000), water clarity, average concentrations of chlorophyll a (Chl a), total phosphorus (TP) and total nitrogen (TN), the number of major inflows to each lake, and the number of CTWs and their site codes is given in Table 1.
Location of studied peat lakes in the central Waikato (inset), North Island, New Zealand. Lake areas of open water and riparian margins were digitised from 2016 aerial photographs. Catchment boundaries were delineated from 2008 LiDAR (Light Detection and Ranging) data (source; Waikato Regional Council)
Field Sampling
Sites were sampled in late summer (2–8 February) 2011, following two weeks of persistent heavy rain (c. 215–235 mm across the study area), causing refilling of the CTWs after a dry spring and early summer (c. 125–135 mm over the preceding 12 weeks) (CliFlo 2017). We acknowledge that our sampling is a snapshot of the late-austral summer zooplankton community and environmental conditions, and that greater zooplankton diversity and variety of conditions would likely be encountered if seasonal or higher frequency sampling was undertaken. Single measurements of physicochemical variables were made at each site, including wetted area, depth, connectivity with the downstream lake (high = 3, medium = 2, low = 1, no connectivity = 0), as well as water temperature, dissolved oxygen concentration (DO), specific conductivity and pH, using a YSI 6000 UPG Multi Parameter Sonde (Yellow Springs Instruments, Ohio, USA). Area of the lakes was calculated from digitised aerial images (2016), and was measured manually in the field for CTWs and drains (over the 10 m reach surveyed). Sites with ‘no connectivity’ to the downstream lake occurred where the original drain outlet had been filled to create a ‘filtration’ outlet for the CTW.
Water samples for measurement of nutrients, Chl a, suspended solids and zooplankton were collected concurrently from the middle of CTWs and drains from a depth of approximately 0.3 m using a 1 L measuring jug on a 2 m pole. Samples were collected from lake habitats at a similar depth, from platforms built by duck-hunters which extend out into the lake approximately 5–10 m from the shore.
Water samples for particulate and filterable nutrients were collected in 50 ml centrifuge tubes (Greiner Bio1, Germany) and for suspended solids in opaque 1 L bottles. Filterable nutrient samples were syringe-filtered (Whatman GF/C 0.45 μm) in the field and the filter paper retained and wrapped in aluminium foil for Chl a analysis. Samples were placed on ice after collection, with nutrient and Chl a samples frozen upon return to the laboratory. Water samples for suspended solids analyses were stored in the dark at 4 °C and processed in the laboratory within three days of collection.
Biotic variables measured included percentage cover of emergent macrophytes, visual presence/absence of iron flocculants, and observed presence/absence of fish. A minimum of two G-minnow traps were set per CTW and drain over four nights of trapping. Fish were identified to species level using descriptions from McDowall (2000). The presence/absence of fish for the lakes was determined from the literature, and percentage cover of emergent macrophytes estimated for the entire lake shore using aerial photographs. Iron flocculants were measured by the presence of rust-coloured particles collected on the filter paper retained for Chl a analyses.
Zooplankton samples were collected by pouring 3 to 10 L of water through a zooplankton net (40 μm mesh) and were immediately preserved in ethanol (> 50% final concentration) before subsequent identification and enumeration in the laboratory.
Sample analyses
Chlorophyll a samples were analysed using a calibrated fluorometer following acetone extraction (Arar and Collins 1997). Filtered nutrient samples were analysed for concentrations of ammonium (NH4-N), phosphate (PO4-P), nitrate + nitrite (NO3–N + NO2-N) and nitrite (NO2–N). Concentrations of nitrate were determined by the difference between NO3-N + NO2-N and NO2-N. Analyses were carried out using an Aquakem 200 discrete analyser (Thermo Fisher) with standard colorimetric methods (Federation and American Public Health Association 2005). Limits of detection were 0.001 mg N L−1 for NO2-N, NO3–N, 0.002 mg N L−1 for NH4-N and 0.001 mg P L−1 for PO4-P. Total P and TN were determined following alkaline persulphate digestion (Federation and American Public Health Association 2005) and analysis for PO4-P and NO3-N + NO2-N, respectively, using a Lachat QuickChem® Flow Injection Analyser (FIA + 8000 Series, Zellweger Analytics, Inc.). Concentrations of total organic nitrogen (ORG-N) were calculated by subtracting the sum of NH4-N, NO3–N and NO2-N from TN.
Suspended solids samples were filtered in the laboratory through pre-combusted (550 °C for 2 h) and pre-weighed glass microfibre filters (Whatman GF/C 0.5 μm). Total suspended solids (TSS) concentrations were determined gravimetrically following drying (105 °C for a minimum of 8 h) and volatile suspended solid (VSS) concentrations were determined following subsequent ashing (550 °C for 4 h). Non-volatile suspended solids (Non-VSS) were calculated from the difference between TSS and VSS.
Zooplankton samples were enumerated in the laboratory using a dissecting microscope at c. 30× magnification until at least 300 individuals, or the whole sample, was counted. Identification was performed using a compound microscope to the lowest level practical, using standard taxonomic guides (Shiel 1995; Chapman et al. 2011).
Statistical analyses
Taxa richness for zooplankton was calculated as the total number of taxa present in each sample. Relative abundances of major zooplankton groups; cladocerans, copepods (including calanoids, cyclopoids and copepod nauplii), ostracods, rotifers and ‘others’ (including amphipods, dipterans, hemipterans, hydracarina and tardigrades) were calculated for each habitat type and compared using Kruskal-Wallis single factor analysis (Statistica Software version 8.0; Statsoft, Tulsa, OK, USA). The relative abundances of major zooplankton groups were first estimated for each site (n = 40) and then averaged across habitat types (lake n = 5, CTW n = 27, drain n = 8) to derive mean relative abundances. This method was chosen as it better incorporated the variability among the sites within each habitat type.
Multivariate analyses were undertaken using Primer 6 (version 6.1.15, Primer-E Ltd., Plymouth Marine Laboratory, Plymouth, U.K) with the PERMANOVA + add-in (version 1.0.5) to determine whether habitat differences existed between lakes, CTWs and drains (based on environmental variables), and to assess patterns of zooplankton community composition. The environmental variables included in analyses were physicochemical (mean depth, area, water temperature, DO, specific conductivity and pH), water quality-related (NH4-N, NO3-N, Org-N, PO4-P, Non-VSS and VSS) and biotic (Chl a, percentage cover of emergent macrophytes, iron flocculant and fish presence/absence). All analyses of environmental variables were based on Euclidean distance matrices (Biondini et al. 1991) performed on log(x + 1) transformed and standardised data (zero mean and unit variance). All analyses of zooplankton data were based on Bray-Curtis similarity matrices (Bray and Curtis 1957) and performed on square-root transformed data as recommended by Anderson et al. (2008).
Prior to undertaking multivariate analyses, a Pearson correlation was performed to identify any highly correlated physicochemical variables. Connectivity was excluded from this analysis as it was not applicable to lake samples, as well as TN and TP due to high correlations with Org-N and PO4-P, respectively. Individual nutrient species were selected for inclusion as they had stronger relationships with zooplankton community composition than TN and TP alone. Volume was excluded due to high correlations with area and depth measurements.
The variation in environmental variables among habitats was analysed using a single factor (habitat type) permutational multivariate analysis of variance, PERMANOVA (Anderson 2001a; Anderson et al. 2008). A Type III PERMANOVA for unbalanced designs was performed and significance was determined by 9999 unrestricted permutations of the raw data (Anderson 2001b; Anderson and ter Braak 2003). Pair-wise comparisons of group (habitat) means were completed in the case of a significant factor effect to assess between which pairs of habitat types significant differences occurred (McArdle and Anderson 2001). Variables contributing most to the variation among habitats were then identified using similarity percentage analysis (SIMPER), which calculates the average dissimilarity between all pairs of samples and assesses the relative dissimilarity contributed by each variable (Clarke 1993).
The variation in zooplankton community composition between habitat types was similarly evaluated using a single-factor Type III PERMANOVA with pair-wise comparisons of group (habitat) means completed to assess levels of significance. The taxa contributing most to differences in zooplankton community composition between habitat types were determined using the SIMPER procedure.
Non-parametric multifactor multiple regression, using the Distance-based Linear Modelling routine in Primer 6 (DistLM), was used to test for the influence of environmental habitat variables (physicochemical, water quality-related and biotic attributes) in structuring the variation in zooplankton community composition (Anderson et al. 2008). The DistLM procedure tests for significant differences in multivariate response variables to explanatory variables based on a selected distance-based measure in the form of a resemblance matrix (Anderson et al. 2008). The step-wise selection procedure based on 9999 permutations was used to select and test habitat variables with an adjusted R2 selection criterion to eliminate insignificant variables.
Results
Zooplankton community composition
Seventy-three taxa were identified from the three habitat types, including 7 cladoceran, 3 copepod, and 55 rotifer species, ostracods and 8 macroinvertebrate taxa (amphipods, dipterans, hemipterans, hydracarinas and tardigrades). Total number of taxa recorded for each habitat type was greatest from CTWs (52), followed by lakes (40), and drains (20) (S.I. Table 7 ). Lake samples had the highest, although variable, mean taxon richness (15.2, SD = 4.9) followed by CTWs (8.7, SD = 3.8) and drains (6.8, SD = 2.9; Fig. 2a). Similarly, mean zooplankton total abundance was highest in lake samples (262 animals L−1), followed by CTWs and then drain samples (70 and 46 animals L−1, respectively; Fig. 2e). Differences in mean total abundances and richness of zooplankton were not statistically significant amongst habitat types due to high within-group variability. Lakes Komakorau and Koromatua had highest taxa richness (21 and 19, respectively), while the greatest richness from CTW habitats was recorded from KN1 (entering Lake Kainui) and KR2 (entering Lake Koromatua) (both 16). The CTW KT2, adjacent to Lake Kaituna, had the lowest richness with only cyclopoid copepodites and copepod nauplii recorded from the sample despite the CTW having high lake connectivity and a large area (1080 m2).
Mean values for taxa richness and total abundance of zooplankton (a, e) and total abundances of zooplankton species most dissimilar between habitat types including; Bdelloids (b), Brachionus spp. (c), Copepod nauplii (d), Cyclopoid copepodites (f), Keratella spp. (g) and Lecane spp. (h) plotted on a log10-scaled axis for each habitat type (lake, CTW and drain)
Lake zooplankton assemblages were distinctly different from CTW and drain communities (SIMPER analysis, average Bray-Curtis dissimilarities 83.7 and 86.6, respectively). Differences were driven by the predominance of cladocerans, particularly the rotifers Brachionus calyciflorus and Keratella tropica (Fig. 2c, g), as well as Polyarthra vulgaris, Synchaeta longipes, and the cladoceran Bosmina meridionalis (Table 2), all of which were at higher abundances in lake habitats but virtually absent from the CTWs and drains. Cumulatively, these taxa, as well as high densities of copepod nauplii, accounted for 45% and 48% of the lake-CTW and lake-drain differences, respectively (Table 2).
There was a relatively subtle difference in the dominant taxa typical of drain and CTW habitats, which is reflected by an average dissimilarity of 67.9 derived from the SIMPER analysis (Table 2) and represented graphically in Fig. 2. Cumulatively, cyclopoid copepodites (10.8), copepod nauplii (9.3) and bdelloid rotifers (9.9) contributed 44.1% to the dissimilarity between CTW and drain zooplankton assemblages (Table 2). High relative abundances of Lecane spp. within CTW habitats (Fig. 2h) also contributed to 17% of the community compositional differences in comparison to drain habitats (Table 2).
Comparison of the relative abundances of the major zooplankton groups (cladocerans, copepods, ostracods and rotifers) for each habitat type supported the finer-scale differences between zooplankton assemblages determined by the SIMPER analysis. Cladocerans and calanoid copepods were significantly more abundant in lake habitats compared with CTWs and drains (Table 3). Copepod nauplii and cyclopoid copepodids were common within each habitat (Fig. 2d, f), but differed in their relative abundances (Table 3). Rotifers were also significantly more abundant in lake and CTW habitats compared with drains (Table 3), due to the relatively high abundances of bdelloids and Lecane spp. within CTW habitats (Fig. 2b, h).
Environmental variables
Lake habitats were significantly larger and more alkaline than CTWs and drains, and both lake shore and CTW habitats had significantly greater depths than drains (Table 4). Mean values of physicochemical, biotic and water quality variables were not significantly different between habitat types due to high variability within habitats.
Based on PERMANOVA, habitat type accounted for a significant proportion of the variation in biotic and abiotic environmental variables across all sites (Fpseudo = 4.78, P = 0.0001). Pairwise comparisons indicated significant differences between each habitat type (Lake, Drain t = 2.72; Lake, CTW t = 2.10; Drain, CTW t = 2.07, P < 0.01). The morphological variables area and depth contributed to the greatest dissimilarity between habitat types, followed by pH, inorganic-N and conductivity (SIMPER results, Table 5.) Water temperature was also important, contributing to 6.8% of the dissimilarity between lake and CTW habitats, and 7.7% between lake and drain habitats.
Habitat variables influencing zooplankton communities
Habitat type was determined by PERMANOVA to account for a significant proportion of the variation in zooplankton community composition (Fpseudo = 3.59, P = 0.0001). Pairwise comparisons indicated significant differences between each habitat type based on zooplankton community composition (Lake-Drain t = 2.19, P < 0.01; Lake-CTW t = 2.21, P < 0.01; Drain-CTW t = 1.40, P < 0.05).
The DistLM procedure assigned the greatest proportion of the variation in zooplankton community composition among habitat types to area (22.1%) followed by pH (16.7%) and NH4-N (10.4%; Table 6). Area was a key driver of the difference between lakes and CTWs, as well as between lakes and drains based on SIMPER analyses (Table 5), while differences in pH were significant between lakes and CTWs. The DistLM marginal tests also determined water temperature and DO were significant drivers of the variation in community composition between lake habitats and CTWs, as well as between lakes and drains (Table 6).
The DistLM analysis indicated conductivity and depth were significant explanatory variables, reflecting differences between CTWs and drains, and supporting previous analyses (Table 5). PO4-P was a significant explanatory variable in marginal tests due to elevated concentrations in CTWs (mean = 0.24 mg N L−1) compared to lakes and drains (0.06 and 0.01 mg N L−1, respectively). Finally, NH4-N concentration, iron flocculant and emergent macrophyte cover, each of which were elevated in drains, were also identified as significant by the DistLM marginal tests (Table 6), supporting the SIMPER results (Table 5). Mean NH4-N concentrations from drains (0.22 mg N L−1) were twice as high as those from CTWs (0.11 mg N L−1) and more than five-fold greater than lake concentrations (0.04 mg N L−1; Table 5).
Discussion
Zooplankton diversity
Our results suggest agricultural CTWs support greater zooplankton diversity than drain habitats and can increase the overall biodiversity of highly modified peat lake catchments. For example, within the catchment of Lake Kainui, 15 taxa were recorded from the lake itself, five additional taxa were recorded from drains, and a further 17 taxa from CTW habitats. Collectively, CTWs had the highest total diversity (54 taxa) followed by lakes (40) and then drains (20) (S.I. Table 7). Mean zooplankton taxa richness for CTWs (c. 9) was intermediate between lake (15) and drain (6) habitats, although no significant differences were apparent due to high variability within habitat types.
The diversity of zooplankton from the habitats in our study is comparable to that from freshwater ecosystems elsewhere. For example, zooplankton taxa richness for our CTW habitats was similar to floodplain ponds of Truman Lake, a reservoir in Missouri (Medley and Havel 2007), midsummer zooplankton assemblages from wetlands in the mid-west USA (Beaver et al. 1999), and small (area < 1 ha) shallow lakes in southeastern Wisconsin, USA (Dodson et al. 2005). The lower taxa richness of drain habitats in this study was similar to that of agriculturally impacted wetlands in Wisconsin (mean 3.8) studied by Dodson and Lillie (2001), while richness from lakes Komakorau (21) and Koromatua (19) was similar to eutrophic shallow lake ecosystems in the northern hemisphere (Søndergaard et al. 2005), as well as a number of lakes in the North Island of New Zealand (Duggan et al. 2001).
Notably, two of the CTWs (KN1 and KR2) had greater taxa richness (both 16) than lakes Kainui (15), Kaituna (12) and Serpentine South (9), despite having considerably smaller areas (0.2 and 0.02 ha compared to 25, 15, and 5 ha, respectively). Søndergaard et al. (2005) similarly found a weak relationship between zooplankton taxa richness and habitat size following an investigation of almost 800 Danish shallow lakes (median depth 1.5 m), ranging from 0.01 to 4200 ha, going on to suggest that ponds and small lakes are important biodiversity components in agricultural landscapes. CTWs KN1 and KR2 were relatively complex habitats, with deep (> 1 m) and shallow (< 0.5 m) zones, areas of open water and moderate macrophyte cover; thus, greater zooplankton diversity may be expected owing to a greater range of potential habitats and niches (Lucena-Moya and Duggan 2011).
Zooplankton assemblages & functional attributes
CTW-zooplankton assemblages differed from lake and drain communities owing to relatively few cladocerans and a predominance of rotifers, primarily comprising large numbers of bdelloid rotifers and Lecane species. Bdelloid rotifers are primarily benthic and are able to survive extended dry periods via anhydrobiosis (Crowe et al. 1992), making them well adapted to CTW habitats in artificially drained agricultural peat lake catchments where water levels can fluctuate widely. Like most rotifers, bdelloids are suspension feeders and thrive on dead organic matter and bacteria (Ricci 1984). Along with excess sediment and nutrients, pathogenic bacteria have been identified as one of New Zealand’s three major water quality problems (MfE and StatsNZ 2017). Runoff from agricultural catchments is known to transport pathogenic bacteria including Escherichia coli, Salmonella, Campylobacter and Shigella, as well as the pathogenic protozoans Cryptosporidium and Giardia (Hooda et al. 2000; Jamieson et al. 2002; Ballantine and Davies-Colley 2014). Bacterivorous zooplankton have been shown to reduce pathogenic bacteria from water (Schallenberg et al. 2005) and are likely to play an important role in the treatment efficiency of CTWs in agricultural landscapes. Therefore, the prevalence of these species in the CTWs of this study is encouraging.
While zooplankton such as bdelloids feed directly on bacteria, metazooplankton including calanoid and cyclopoid copepods as well as large cladocerans such as Ceriodaphnia dubia (Galbraith and Burns 2010), consume bacteria indirectly via predation on ciliates (Hansen 2000). Bacterivorous ciliates are important for reducing bacterial densities in effluent from sewage treatment plants (Curds et al. 1968; Madoni 2003) and may also suppress viruses (Pinheiro et al. 2007). The calanoid copepod Calamoecia lucasi was abundant in Lake Koromatua and present in CTW KR2 and lakes Kainui, Komakorau, and Serpentine North, while C. dubia was present in CTWs KN5 and KT5 as well as Lake Kainui. Furthermore, early copepodid instars (copepod nauplii and cyclopoid copepodites), which feed preferentially on ciliates (Hansen 2000), were prevalent in CTW habitats, comprising approximately 45% of relative abundances. The presence of these zooplankton species in the CTWs of this study is promising as they may contribute to reducing pathogenic bacterial communities entering the downstream lakes. Wilcock et al. (2012) likewise suggest that enhancing communities of bacterivorous microorganisms is likely to increase the efficacy of agricultural wetland treatment systems through consumption of harmful bacteria.
Drain-zooplankton assemblages differed from CTWs and were driven by a lack of rotifers and relatively large numbers of ostracods, cyclopoid copepodites and mosquito larvae. Drain habitats generally supported lower zooplankton diversity and abundances, exempting the drain site of Lake Kainui (KND). KND had low macrophyte cover compared to other drain habitats as a result of being cleared in the preceding spring, which may have contributed to greater zooplankton taxa richness (11) and higher abundances (247 animals L−1).
Lake-zooplankton assemblages were distinctly different from CTW and drain communities, due to greater abundances of cladocerans and pelagic rotifer species. Cladoceran zooplankton play a vital role in energy transfer and food web dynamics (Kattel 2012) and were particularly abundant in lake habitats, comprising a quarter of the zooplankton community. The cladoceran Bosmina meridionalis was one of the dominant species from our lake samples, second only to the rotifer Keratella tropica, each species being commonly abundant in eutrophic New Zealand lakes (Duggan et al. 2002). Interestingly, two species more typical of lakes with low trophic state, Polyarthra vulgaris and Synchaeta longipes, were abundant in lakes Koromatua and Serpentine North, respectively, despite the lakes being highly eutrophic.
Habitat characteristics
Each habitat type supported significantly different zooplankton communities (refer to results of PERMANOVA and pair-wise comparisons), including between drains and CTWs. Habitat morphologies including surface area, water depth and macrophyte cover were most strongly associated with variations in zooplankton composition, whilst significant water quality parameters included pH, conductivity, iron flocculant and concentrations of PO4-P and inorganic-N. Drain habitats were smaller and shallower than CTWs, with dense macrophyte cover, high concentrations of NH4-N and NO3-N, and high frequency of iron flocculant occurrence. CTW habitats were intermediate in size between lakes and drains, relatively deep and with high Chl a, Org-N and VSS concentrations, indicative of elevated phytoplankton biomass. Levels of PO4-P were also highest in CTWs, particularly in those with low pH. Lakes were naturally the largest habitats and moderately deep with warmer water temperatures, high DO and neutral pH. Similar to CTW habitats, lakes had elevated Chl a, Org-N and VSS concentrations due to high phytoplankton densities, which in turn supported greater zooplankton abundances.
As evidenced by the results from this study, dense macrophyte cover in small, shallow watercourses suppresses phytoplankton growth and can cause stagnant, reducing conditions, elevating NH4-N levels. Nonetheless, while excessive macrophyte growth can have negative impacts on stream communities (Collier et al. 1999), macrophytes improve the structural complexity of freshwater habitats and strongly influence community structure and diversity (Warfe and Barmuta 2006; Lucena-Moya and Duggan 2011).
Macrophytes are an important refuge against predation for pelagic cladocerans, as described by Timms and Moss (1984) following observations of different water clarity between connected shallow wetland ecosystems in the Norfolk Broads. The size of macrophyte beds is also important, particularly for populations of horizontally migrating cladocerans such as Ceriodaphnia and Bosmina species. Lauridsen et al. (1996) suggest numerous small refuges (c. 2 m diameter) are likely to support higher densities of these species rather than single large refuges (> 10 m diameter), which are more typically dominated by macrophyte-associated, non-migrating cladocerans such as Chydorus and Simocephalus species. Each of the aforementioned cladoceran species occurred in a number of the lake and CTW habitats in our study; thus, it is possible similar dynamics may occur in Waikato peat lake ecosystems. Furthermore, the structural complexity of different macrophytes provides habitat niches favoured by different zooplankton taxa (Lucena-Moya and Duggan 2011). Thus, the variety of emergent, submerged and floating macrophytes within a wetland ecosystem will influence zooplankton biodiversity and biomass.
Finally, many of the CTWs in this study were designed to capture coarse sediment to reduce infilling of the shallow peat lakes downstream. While deposited sediment was not measured in this study, the effects of sedimentation, particularly in cultivated catchments, requires consideration as populations of most zooplankton taxa can persist over long periods through egg banks (Hairston 1996). Gleason et al. (2003) investigated the effects of sediment loads from intensive agricultural activities on the emergence of zooplankton from wetland soil egg banks and found burial by sediment as little as 0.5 cm deep reduced invertebrate emergence by 99%. Duggan et al. (2002) also found limited diversity in lakes with high sediment loads. Designing CTWs with distinct areas of open water and variable depths, by incorporating sedimentation forebays as well as open-water habitats isolated from sediment inputs, will help to minimise any adverse sedimentation effects on zooplankton communities and promote greater diversity.
Conclusions
CTWs can improve the overall biodiversity of highly-modified peat lake catchments, by supporting zooplankton species otherwise absent from lake and drain habitats. Zooplankton taxa richness and abundances were broadly higher from CTWs than drain habitats, and a few CTWs supported greater diversity than several lakes. The results from our research suggest CTWs afford dual benefits for peat lake restoration within intensive agricultural landscapes through provision of habitat for zooplankton communities as well as water quality improvements.
To further enhance zooplankton communities, we propose creating CTWs with variable depths and areas deeper than existing drains, with larger areas of open water and moderate to low levels of diverse macrophyte cover. Opportunities exist to manipulate the influence of macrophyte beds through careful design, construction, plant selection and maintenance to support targeted zooplankton species which can sustain grazing on phytoplankton and improve water quality treatment (Schriver et al. 1995). Just as recent research has extended our knowledge of zooplankton population dynamics within high rate algal ponds in support of improved wastewater treatment (Montemezzani et al. 2016, 2017), expanding our understanding of the lifecycles and habitat requirements of bacterivorous zooplankton and ciliates in wetlands could additionally inform CTW designs to improve operational efficiency whilst concurrently supporting greater zooplankton biodiversity.
References
Anderson MJ (2001a) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46
Anderson MJ (2001b) Permutation tests for univariate or multivariate analysis of variance and regression. Canadian Journal of Fisheries and Aquatic Sciences 58:626–639
Anderson MJ, ter Braak C (2003) Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation 73:85–113
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for Primer: Guide to software and statistical methods. PRIMER-E, Plymouth
Arar EJ, Collins GB (1997) In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence: Method 445.0 Method 445.0, Revision 1.2 edition. U.S. Environmental Protection Agency, Cincinnati, Ohio
Ballantine DJ, Davies-Colley RJ (2014) Water quality trends in New Zealand rivers: 1989–2009. Environmental Monitoring and Assessment 186:1939–1950
Beard CM (2010) Wetlands and lowland floodplains. In: Collier KJ, Hamilton DP, Vant WN, Howard-Williams C (eds) The Waters of the Waikato. Environment Waikato, Centre for Biodiversity and Ecology Research, Hamilton, pp 265–279
Beaver JR, Miller-Lemke AM, Acton JK (1999) Midsummer zooplankton assemblages in four types of wetlands in the Upper Midwest, USA. Hydrobiologia 380:209–220
Biondini ME, Mielke PW, Redente EF (1991) Permutation Techniques Based on Euclidean Analysis Spaces: A New and Powerful Statistical Method for Ecological Research. In: Feoli E, Orloci L (eds) Computer Assisted Vegetation Analysis. Kluwer Academic Publishers, Dordrecht, pp 221–240
Boix D, Gascón S, Sala J, Martinoy M, Gifre J, Quintana XD (2005) A new index of water quality assessment in Mediterranean wetlands based on crustacean and insect assemblages: the case of Catalunya (NE Iberian peninsula). Aquatic Conservation: Marine and Freshwater Ecosystems 15:635–651
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs 27:326–349
Burns NM, Bryers G, Bowman EJ (2000) Protocols for Monitoring Trophic Levels of New Zealand Lakes and Reservoirs. Ministry for the Environment, Wellington, p 122
Chapman MA, Lewis MH, Winterbourn MJ (2011) Guide to freshwater Crustacea of New Zealand. New Zealand Freshwater Sciences Society, Christchurch
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117–143
Clarkson BR, Schipper LA, Lehmann A (2004) Vegetation and peat characteristics in the development of lowland restiad peat bogs, North Island, New Zealand. Wetlands 24:133–151
CliFlo (2017) NIWA's National Climate Database on the Web. National Institute of Water and Atmospheric Research, Hamilton
Collier KJ, Champion PD, Croker GF (1999) Patch- and reach-scale dynamics of a macrophyte-invertebrate system in a New Zealand lowland stream. Hydrobiologia 392:89–97
Crowe JH, Hoekstra FA, Crowe LM (1992) Anhydrobiosis. Annual Review of Physiology 54:579–599
Curds C, Cockburn A, Vandyke JM (1968) An experimental study of the role of the ciliated protozoa in the activated sludge process. Water pollution control 67:312–329
Dodson SI, Lillie RA (2001) Zooplankton communities of restored depressional wetlands in Wisconsin, USA. Wetlands 21:292–300
Dodson SI, Richard AL, Will-Wolf S (2005) Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecological Applications 15:1191–1198
Dravitzki S, McGregor J (2011) Extreme precipitation of the Waikato region, New Zealand. International Journal of Climatology 31:1803–1812
Duggan IC, Green JD, Shiel RJ (2001) Distribution of rotifers in North Island, New Zealand, and their potential use as bioindicators of lake trophic state. Hydrobiologia 446-447:155–164
Duggan IC, Green JD, Shiel RJ (2002) Distribution of rotifer assemblages in North Island, New Zealand, lakes: relationships to environmental and historical factors. Freshwater Biology 47:195–206
Dunne EJ, Coveney MF, Marzolf ER, Hoge VR, Conrow R, Naleway R, Lowe EF, Battoe LE (2012) Efficacy of a large-scale constructed wetland to remove phosphorus and suspended solids from Lake Apopka, Florida. Ecological Engineering 42:90–100
Federation WE, American Public Health Association (2005) Standard Methods for the Examination of Water and Wastewater 21st edition. American Public Health Association (APHA), Washington, DC
Galbraith LM, Burns CW (2010) Drivers of ciliate and phytoplankton community structure across a range of water bodies in southern New Zealand. Journal of Plankton Research 32:327–339
Geiger JG (1983) A review of pond zooplankton production and fertilization for the culture of larval and fingerling striped bass. Aquaculture 35:353–369
Gleason RA, Euliss NH, Hubbard DE, Duffy WG (2003) Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil egg and seed banks. Wetlands 23:26–34
Gray DK, Arnott SE, Shead JA, Derry AM (2012) The recovery of acid-damaged zooplankton communities in Canadian Lakes: the relative importance of abiotic, biotic and spatial variables. Freshwater Biology 57:741–758
Hairston NG (1996) Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography 41:1087–1092
Hamilton DP, Vant WN, Neilson KA (2010) Lowland lakes. In: Collier KJ, Hamilton DP, Vant WN, Howard-Williams C (eds) The Waters of the Waikato. Environment Waikato, Centre for Biodiversity and Ecology Research, Hamilton, pp 245–264
Hansen A (2000) Response of ciliates and Cryptomonas to the spring cohort of a cyclopoid copepod in a shallow hypereutrophic lake. Journal of Plankton Research 22:185–203
Hooda PS, Edwards AC, Anderson HA, Miller A (2000) A review of water quality concerns in livestock farming areas. Science of The Total Environment 250:143–167
Hunt J (2007) Wetlands of New Zealand - A bitter-sweet story. Random House, Auckland
Jamieson RC, Gordon RJ, Sharples KE, Stratton GW, Madani A (2002) Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: A review. Canadian Biosystems Engineering / Le Genie des biosystems au Canada 44:1.1–1.9
Kadlec RH (2010) Nitrate dynamics in event-driven wetlands. Ecological Engineering 36:503–516
Kadlec RH, Knight RL (1996) Treatment Wetlands. Lewis Publishers, Boca Raton
Kadlec RH, Wallace S (2008) Treatment Wetlands, 2nd edn. CRC Press, Boca Raton
Karr JR (1996) Ecological integrity and ecological health are not the same. In: Schulze P (ed) Engineering within Ecological Constraints. National Academy Press, Washington, DC, pp 97–109
Kattel GR (2012) Can we improve management practice of floodplain lakes using cladoceran zooplankton? River Research and Applications 28:1113–1120
Lauridsen T, Pedersen LJ, Jeppesen E, Sønergaard M (1996) The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. Journal of Plankton Research 18:2283–2294
Lougheed VL, Chow-Fraser P (2002) Development and use of a zooplankton index of wetland quality in the Laurentian Great Lakes basin. Ecological Applications 12:474–486
Lowe DJ, Green JD (1987) Origins and development of the lakes. In: Viner AB (ed) Inland waters of New Zealand. Department of Scientific and Industrial Research, Wellington, pp 1–64
Lucena-Moya P, Duggan IC (2011) Macrophyte architecture affects the abundance and diversity of littoral microfauna. Aquatic Ecology 45:279–287
Madoni P (2003) Protozoa as Indicators of Wastewater Treatment Efficiency. In: Mara D, Horan NJ (eds) The Handbook of water and wastewater microbiology. Academic Press, Elsevier, Amsterdam, pp 361–371
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 82:290–297
McDowall RM (2000) The Reed field guide to New Zealand freshwater fishes. Reed Books, Auckland
Medley KA, Havel JE (2007) Hydrology and local environmental factors influencing zooplankton communities in floodplain ponds. Wetlands 27:864–872
MfE and StatsNZ (2015) New Zealand’s Environmental Reporting Series: Environment Aotearoa 2015. Available from www.mfe.govt.nz and www.stats.govt.nz, Wellington, New Zealand
MfE and StatsNZ (2017) New Zealand’s Environmental Reporting Series: Our fresh water 2017. Available from www.mfe.govt.nz and www.stats.govt.nz, Wellington, New Zealand, p 98
Milstein A, Valdenberg A, Harpaz S (2006) Fish larvae–zooplankton relationships in microcosm simulations of earthen nursery ponds. I. Freshwater system. Aquaculture International 14:231–246
Mitsch WJ, Gosselink JG (2007) Wetlands 4th edition. Wiley, Hoboken
Montemezzani V, Duggan IC, Hogg ID, Craggs RJ (2015) A review of potential methods for zooplankton control in wastewater treatment High Rate Algal Ponds and algal production raceways. Algal Research 11:211–226
Montemezzani V, Duggan IC, Hogg ID, Craggs RJ (2016) Zooplankton community influence on seasonal performance and microalgal dominance in wastewater treatment High Rate Algal Ponds. Algal Research 17:168–184
Montemezzani V, Duggan IC, Hogg ID, Craggs RJ (2017) Assessment of potential zooplankton control treatments for wastewater treatment High Rate Algal Ponds. Algal Research 24(Part A):40–63
Moss B, Stephen D, Alvarez C, Becares E, Bund WVD, Collings SE (2003) The determination of ecological status in shallow lakes — a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquatic Conservation: Marine and Freshwater Ecosystems 13:507–549
Peters M, Neilson K, Speirs D, Kaufler G, Roxburgh T, MacKay B, Fergie S, McDonald A, Hayes A, Thompson K (2008) Guidelines for landowners in peat lake catchment. NZ Landcare Trust, Hamilton
Piasecki W, Goodwin AE, Eiras JC, Nowak BF (2004) Importance of Copepoda in freshwater aquaculture. Zoological Studies 43:193–205
Pinheiro MDO, Power ME, Butler BJ, Dayeh VR, Slawson R, Lee LEJ, Lynn DH, Bols NC (2007) Use of tetrahymena thermophila to study the role of protozoa in inactivation of viruses in water. Applied and Environmental Microbiology 73:643–649
Ricci C (1984) Culturing of some bdelloid rotifers. Hydrobiologia 112:45–51
Schallenberg M, Bremer PJ, Henkel S, Launhardt A, Burns CW (2005) Survival of Campylobacter jejuni in Water: Effect of Grazing by the Freshwater Crustacean Daphnia carinata (Cladocera). Applied and Environmental Microbiology 71:5085–5088
Schlüter M, Groeneweg J, Soeder CJ (1987) Impact of rotifer grazing on population dynamics of green microalgae in high-rate ponds. Water Research 21:1293–1297
Schriver P, Bøgestrand J, Jeppesen E, Søndergaard M (1995) Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33:255–270
Shearer JC (1997) Natural and anthropogenic influences on peat development in Waikato and Hauraki Plains restiad bogs. Journal of the Royal Society of New Zealand 27:295–313
Shiel RJ (1995) A guide to the identification of rotifers, cladocerans and copepods from Australian inland waters. Co-operative Research Centre for Freshwater Ecology. Murray-Darling Freshwater Research Centre, Albury
Søndergaard M, Jeppesen E, Jensen JP (2005) Pond or lake: does it make any difference? Archiv Fur Hydrobiologie 162:143–165
Timms RM, Moss B (1984) Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnology and Oceanography 29:472–486
Van den Broeck M, Waterkeyn A, Rhazi L, Grillas P, Brendonck L (2015) Assessing the ecological integrity of endorheic wetlands, with focus on Mediterranean temporary ponds. Ecological Indicators 54:1–11
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Science of The Total Environment 380:48–65
Vymazal J, Kröpfelová L (2008) Removal of organics in constructed wetlands with horizontal sub-surface flow: A review of the field experience. Sci Science of The Total Environment 407:3911–3922
Waikato RC (2006) For Peat's Sake: Good management practices for Waikato peat farmers. Waikato Regional Council, Hamilton
Warfe DM, Barmuta LA (2006) Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia 150:141–154
Wilcock RJ, Müller K, van Assema GB, Bellingham MA, Ovenden R (2012) Attenuation of nitrogen, phosphorus and E. Coli inputs from pasture runoff to surface waters by a farm wetland: The importance of wetland shape and residence time. Water Air Soil Pollut 223:499–509
Acknowledgments
The authors acknowledge funding support from the New Zealand Ministry of Business, Innovation and Employment (UOWX1503; Enhancing the health and resilience of New Zealand lakes) and the Waikato Regional Council. We thank Michael Pingram and an anonymous reviewer for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 91 kb)
Rights and permissions
About this article
Cite this article
Eivers, R.S., Duggan, I.C., Hamilton, D.P. et al. Constructed treatment wetlands provide habitat for zooplankton communities in agricultural peat lake catchments. Wetlands 38, 95–108 (2018). https://doi.org/10.1007/s13157-017-0959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-017-0959-4