Abstract
We assessed area and habitat heterogeneity effects on avian richness and composition in bofedales that differed in size and microhabitat diversity. We analyzed data collected in 2 seasons and 24 bofedales using General Linear Models, Ordinary Least Square models to establish the relationship of predictor variables on richness and Akaike Information Criterion for model selection. We evaluate composition classifying species into groups using Bray Curtis ordination, followed by Multiple Response Permutation Procedure to test for differences among groups, and Indicator Species Analysis to identify species. Bofedales differed in richness (F = 5.1, p < 0.001) and microhabitat diversity (F = 23.4, p < 0.001), but no seasonal differences emerged (p > 0.05). The best model indicates that 54% of variance in richness was explained by area and microhabitat diversity, however, a tendency to decrease in microhabitat diversity as area increases, suggests a relatively more important role of area. Results are supported by composition, as microhabitats not only differed pairwise (T = −94.14, A = 0.601, p < 0.001) and had significant indicator species (p < 0.05), but because its differential contribution to richness, as some microhabitats were more speciose than others. As such, few species-rich microhabitats may contribute more to richness than many species-poor ones which is not predicted by the habitat heterogeneity hypothesis. Disentangling the influence of area and habitat heterogeneity on species richness is important to establish conservation priorities that ensure bofedales integrity under imminent climate change.
Resumen
Evaluamos el efecto del área y la heterogeneidad del hábitat en la riqueza y composición de aves en bofedales que difieren en tamaño y diversidad de microhábitats. Los datos recopilados en 2 estaciones y 24 bofedales fueron analizados usando Modelos Generales Lineales, Modelos de Mínimos Cuadrados Ordinarios para establecer la relación entre las variables predictivas y la riqueza, y el Criterio de Información de Akaike para seleccionar los modelos. Evaluamos la composición de especies clasificándolas en grupos con el Ordenamiento de Bray Curtis, seguido por el Análisis de Permutación de Respuesta Múltiple para detectar diferencias entre los grupos, y el Análisis de Especies Indicadoras para identificar las especies. Los bofedales difieren en riqueza (F = 5.1, p < 0.001) y diversidad de microhábitats (F = 23.4, p < 0.001), pero no hallamos diferencias estacionales (p > 0.05). El modelo seleccionado indica que el área y la diversidad de microhábitats explican 54% de la varianza en la riqueza, sin embargo, encontramos una tendencia inversa entre la diversidad de microhábitats y el área, la cual sugiere un papel relativamente más importante del área en la riqueza de especies. Nuestros resultados son respaldados por los datos de composición, ya que los microhábitats no sólo fueron diferentes en comparaciones pareadas (T = −94.14, A = 0.601, p < 0.001) y estuvieron representados significativamente por especies indicadoras (p < 0.05), sino que contribuyeron diferencialmente con la riqueza. Así, pocos microhábitats ricos en especies contribuirían más a la riqueza que varios microhábitats pobres en especies lo cual no concuerda con las predicciones de la hipótesis de heterogeneidad del hábitat. Determinar la influencia que el área y la heterogeneidad tienen en la riqueza de especies es importante para establecer prioridades de conservación que garanticen la integridad de los bofedales ante el inminente cambio climático.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number and identity of species present at a site are influenced by the interaction of factors operating at different spatial and temporal scales (Ricklefs and Schluter 1993; Huston 1999). At a regional scale, species richness and community composition are likely influenced by speciation rates, biogeographic factors, and/or climate, while at a local scale, physical and ecological factors such as habitat structure, micro-environmental conditions, and interactions (e.g., predation, competition, mutualism, etc.) play a prominent role in determining species richness and composition (Vuilleumier 1970; Terborgh 1977; Angermeier and Winston 1998; Rahbek 2005).
The scale dependence of species richness is represented by the species–area relationship (SAR; Arrhenius 1921; Gleason 1925). SAR has been studied in the context of two not mutually exclusive hypotheses: the ‘area per se’ (Preston 1960, 1962), derived from the island biogeography equilibrium theory (MacArthur and Wilson 1967) and the ‘habitat heterogeneity hypothesis’ (Williams 1964), developed within the context of niche theory (Hutchinson 1957). In the former, as area and isolation increases, the probability of encountering more species decreases through their effects on colonization and extinction rates (even in a uniform environment); in the latter, as area increases, more habitat types are encountered, allowing more species to coexist due to niche partitioning (MacArthur and MacArthur 1961; Holt 2009; Stein et al. 2014). As such, the relationship between area and habitat heterogeneity is usually positive, however, it could also be negative when greater microhabitat diversity reduces the amount of suitable area available for each species and, after some threshold, richness decreases (‘area-heterogeneity trade off’, Kadmon and Allouche 2007, Allouche et al. 2012, Bar-Massada and Wood 2014).
The notion that increasing environmental heterogeneity promotes a larger number of species with different ecological requirements has been widely acknowledged (e.g., Ricklefs and Lovette 1999; Allouche et al. 2012; Bar-Massada and Wood 2014; Stein et al. 2014), but its relative contribution has proven difficult to discern because area and habitat heterogeneity are strongly correlated at large spatial scales (Simberloff 1976; Kohn and Walsh 1994; Ricklefs and Lovette 1999).
The effects of area and habitat heterogeneity on local species richness and composition are particularly relevant for models that predict shifts in the geographic range and distribution of species. Shifts in distribution of species may affect overall richness and ecosystem function particularly under different scenarios of climate change, (e.g., Thomas and Lennon 1999; Graham et al. 2011; Young 2012; Herzog et al. 2012). Predicting patterns of species richness in models projections linking conservation efforts that maximize biodiversity (Margules and Pressey 2000; Brooks et al. 2006) and ecosystem function (Tilman et al. 1997; Chapin et al. 1998; Cadotte et al. 2011; but see Schwartz et al. 2000) have become a fundamental aspect in conservation. However, accuracy of models requires basic knowledge of spatial patterns of species richness and composition, which for most tropical ecosystems is far from being accomplished, even for some well-known groups of organisms such as birds (Herzog and Kattan 2011).
In the high Andes, the complex topography and elevational gradients provide a variety of local conditions likely influencing species components (Benham and Witt 2016). Cushion bogs (locally known as bofedales) are a high Andean wetland system characterized by water flowing pools and rivulets that depend on glacier melt and precipitation for constant water flow, surrounded by cushion vegetation (Weberbauer 1936; Squeo et al. 2006; Ruthsatz 2012). Cushions are thick layers of vegetation growing over slowly accumulating organic matter in various stages of decomposition due to low temperature and oxygen available at high elevations. Through time, cushions have the capacity for insulation and water retention providing important ecosystem services such as carbon and nitrogen sinks, sources of methane, regulators of permafrost and hydrological cycles (Keddy 2010; Vuille 2013). Bofedales are one of the most productive life-support systems, characterized by a distinct and diverse flora and fauna, but at the same time they constitute a fragile and threatened ecosystem markedly sensitive to human disturbances. Major threats to bofedales include unsustainable land use (e.g., overgrazing, peat mining; Foote and Krogman 2006), urban expansion (Mitsch and Gosselink 1993), and climate change (Bury et al. 2013; IPCC 2014). Since studies in the bofedal ecosystem are poorly documented (but see Tellería et al. 2006; Gibbons 2012) our main goal was to document bird species richness and composition in relation to bofedal area and habitat heterogeneity (microhabitat diversity). We basically ask the following two questions: (1) How do the number of species and the composition of bird assemblages vary in bofedales that differ in size, distance from other bofedales (isolation), and microhabitat diversity? and (2) what variables (i.e., area, microhabitat diversity) best predict avian richness in bofedales?
Methods
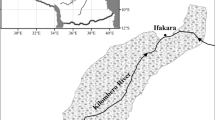
We studied 24 bofedales located in the Huancavelica and Ayacucho Departments (Fig. 1, Table 1) in the Central Andean Puna region (Olson et al. 2001). In the high Andes, climatic conditions vary depending on elevation, sun exposure and other local factors, but in general, daily temperatures are extremely oligothermic, while yearly variation in mean temperature is small (Vuille et al. 2008; Vuille 2013). On the other hand, the amount of precipitation defines a wet season, with 60% to 95% of total annual rainfall occurring from December to March, and a dry season with 0% to 40% rainfall from April to November. Data were collected during four 12 – day visits in November – December 2010 and 2012; and April – May 2013 and 2014. Criteria for bofedal selection included sites above 4300 m (to avoid species from montane or non-Puna ecosystems), isolated by mountain ridges (to reduce connectivity), groundwater or glacial origin (i.e., microtrophic), and water presence throughout the year (i.e., hydromorphic). We avoided potential regional effects by restricting the study to the central part of a precipitation gradient that diminishes from east to west and corresponds to changes in bofedales plant species composition (Valencia et al. 2013). All bofedales in the region are under the influence of linear infrastructure projects (two underground gas pipelines run from east to west), numerous access roads, grazing pressure from sheep, alpaca, and llamas since pastoralism has dominated the region over millennia (Browman 1974; Villarroel et al. 2014).
Geographical location of bofedales (B1-B24) in Ayacucho (dark shaded) and Huancavelica (light shaded) in the Central Andes of Peru. All bofedales were above 4300 m, surrounded by mountain ridges, and with water present throughout the year (See Table 1 for specifics)
On each visit, we conducted bird censuses in two non-consecutive days, from 7:45 AM to approximately 10:45 AM, along a line transect that surrounded each bofedal. Two trained surveyors walked in opposite directions at constant speed (~ 0.40 km/min, Servat et al. 2013). We identified and counted all individuals in the water-saturated and humid and sub-humid zones of the bofedal (sensu Valencia et al. 2013) and recorded the microhabitat(s) used by foraging birds (e.g., cushion vegetation, pools/shores, bare areas, etc.) Birds flying (or inactive) were not assigned to any microhabitat. We reduced variation in detectability due to (1) weather conditions, by not sampling during inclement weather, (2) temporal differences, by visiting bofedales in different days within and among visits, and (3) observer differences, by having the same team of observers trained together to standardize census ability, and by randomly assigning observers to sites.
We estimated area by georeferencing 20–30 points (Garmin GPSmap 60CSx) along the perimeter of each bofedal using Google Earth Pro (www.google.com/earth). We averaged the elevation data taken at georeferenced points to obtain a single measure for each bofedal. Isolation was estimated by measuring the linear distance from the border of each bofedal to the border of its nearest neighbor.
Bofedales have aquatic and terrestrial microhabitats with micro-invertebrates and plants suitable as food resources for foraging birds that included: (1) Cushion vegetation, composed mainly of Distichia muscoides and Plantago tubulosa; (2) Water pools, with plant species such as Potamogeton strictus and a few species of Ranunculus (Valencia et al. 2013), offering large concentrations of plankton for aquatic birds. Water pools are surrounded by shores that vary in substrate type from sieve mud, silt, or gravel, used by shorebirds and waders; (3) Bare expanses of soil, some of which may be dry vernal pools that are searched by ground-feeding birds; (4) Rocky outcrops from landslides that absorb and radiate heat (Weberbauer 1936) providing microsites for lithophilous plants, ferns, and tall-stalk herbs that attract pollinators, frugivorous and seed eating birds; 5) Tussock-forming grasses or tall perennial grasses growing in bunches or clumps (e.g., species of Festuca, Poa, Calamagrostis, etc.); 6) Short lawn-like vegetation, composed of graminoids (grasses and grass-like forms) and forbs (non-graminoid and broad leaved herbaceous plants) that may include degraded vegetation; and 7) Shrubs, scattered plants up to 30 cm height (mostly species of Asteraceae and Ericaceae) found in marginal areas of the bofedal.
We measured habitat heterogeneity as microhabitat diversity, a measure that combines two components: the number of microhabitat types and its cover (%) at each bofedal. To estimate components we took panoramic photographs from the same locations (two high elevation sites) in each bofedal per season, using a Cannon Powershot SX30IS camera with regular lens (i.e., 360o horizontal and 45o vertical fields of view). We drew contour lines around water bodies, tussock, bare areas, rocky outcrops, and cushion vegetation in the photographs; counted all microhabitat types and estimated the mean cover (%) of each microhabitat per bofedal per season. Data was used to calculate microhabitat diversity using Shannon-Wiener diversity index (H′) (Shannon 1948 in Morris et al. 2014).
Data Analysis
We estimated sampling effort, defined as the cumulative number of species observed at each bofedal by season, to assess the completeness and representativeness of bird surveys. We used the species accumulation curves function (specaccum) in the ‘vegan’ Package (Oksanen et al. 2012) within the CRAN platform (R Development Core Team 2006). We obtained the mean and variance of bird species richness per bofedal per season after randomization of the original matrix (999 times), with the number of species randomly taken for each sample (Colwell et al. 2012).
To test for differences in species richness, area, isolation, microhabitat diversity and cover, between seasons (2), and bofedales (24) we used General Linear Models (GLM). We explored significant results with Tukey’s Honest Significant Difference (HSD) post hoc tests (Sokal and Rohlf 2012). All data used in the analyses were checked for normality (Wilk-Shapiro test) and homogeneity of variances (Levene’s test). Data were transformed to logn (bird richness and microhabitat diversity) or arcsine (microhabitat cover) to meet normality assumptions.
Descriptions of the species-area relationship have been mathematically represented by power and logarithmic functions (Connor and McCoy 1979, Rosenzweig 1995, Lennon et al. 2001). However, since power functions imply a constant increase in species richness with increasing area (whereas logarithmic functions imply a constant absolute increase) (White et al. 2006), we considered a linearized power function (LogS = C + LogA) for multimodel inference. To assess collinearity among predictor variables (i.e., bofedal area, isolation, and microhabitat diversity), we estimate the Variance Inflation Factor (VIF), which measures how much the variance of an estimated regression coefficient is increased due to collinearity. We used as threshold for variable removal VIF values >3 (Zuur et al. 2010) in the Companion to Applied Regression Package (CAR, Fox and Weisberg 2011) within the CRAN platform (R Development Core Team 2006). To estimate the relationship between bird species richness and predictor variables, we used Ordinary Least Square (OLS) models. All candidate models were ranked using Akaike Information Criterion corrected for small sample sizes (AICc, Bolker et al. 2009) in the Multimodel Inference Package (MuMIn, Bartoń 2016) within the CRAN platform (R Development Core Team 2006). We chose the dredge function to explore the differences between the AICc values of the best model (i.e., model with the lowest AICc) and other candidate models (ΔAICc). Models with AICc differences <2 were considered supported by the data (Bolker et al. 2009; Richards 2015). All analyses were run in R Software (R Development Core Team 2006), except where indicated.
Species composition was explored using Bray Curtis ordination (PC ORD Version 6, McCunne and Mefford 2011) to obtain a spatial configuration of species in different groups (microhabitats) within and across bofedales. To do so, we built a matrix of bird species (rows) by microhabitats (columns) for each bofedal, with the relative use of each microhabitat filling the cells of the matrix. In all matrices, we relativized data by row (species), and selected Sørensen (Bray Curtis) distance (i.e., the average of pairwise dissimilarities in microhabitat use between bofedales), and used the original Bray Curtis method for end-point selection.
To determine whether bird species composition in microhabitats within and among bofedales differed significantly, we used the species groups obtained from Bray Curtis ordination of each bofedal and perform Multiple Response Permutation Procedure (MRPP). MRPP uses classified bird groups to test the null hypothesis of no differences in use within and among microhabitats across bofedales providing measures of the degree of separation among microhabitats (T), within-microhabitat agreement (A), and P values (after 900 runs of the original matrix, using a random starting configuration) (McCunne and Grace 2002). To identify the species that best characterize each microhabitat, we used Indicator Species Analysis (ISA) (PC ORD Version 6, McCunne and Mefford 2011). ISA provides an indicator value (IV) for each species based on its frequency of occurrence in each microhabitat across bofedales (IV reaches 100% when the species uses the same microhabitat in all bofedales where it is present), and a P value after comparing the observed with the predicted association (after a randomization procedure with 900 runs of the original matrix) between species and microhabitats to determine its statistical significance.
Results
We found 52 bird species in 24 bofedales that varied in area from 2.1 to 10.7 ha (Mean = 8.0 ± 2.43) (Table 1, Appendix 1). Most birds in bofedales (86%) had narrow to medium size geographic distribution (< 500,000 to 1′500,000 km2) and 78% had an elevational range of distribution of 1000 to 5500 m. (Appendix 1).
The cumulative number of bird species in bofedales tends to reach an asymptote in both the dry and wet seasons (Fig. 2). Seasonal variation in species richness was not significant (p > 0.05), despite more species being detected during the dry season (49) than during the wet season (39) (Fig. 2, Appendix 1). Spatial differences emerged when comparing richness across bofedales (GLM Fbofedales = 5.1, df = 23, 3; p < 0.001) (Fig. 3), with significant variation due to the “species-rich” bofedales B9, B19 and B22; and the “species-poor” B1, B5 and B18 (Fig. 3), as indicated by post-hoc Tukey test.
Species richness/ha logarithmically transformed (LN) in 24 bofedales (B1-B24) surveyed in the dry (discontinuous line and white circles) and wet season (continuous line and black circles). General Linear Model (GLM) ANOVA (F) values for comparisons among seasons and bofedales are provided. Lowercase fonts (a, b) indicate bofedales that significantly contribute with GLM differences
Microhabitat diversity differed across bofedales (FH’ = 23.4, df = 23, p < 0.001, n = 48), independent of season (p > 0.05). Our results revealed that microhabitat diversity was significant and inversely related to bofedal area, although the relation had a low predictive value (R2 = 0.13, p = 0.01, n = 48). Comparisons of microhabitat cover (%) across bofedales differed significantly in shrubs (F = 185.5, df = 23, p < 0.001), tussock (F = 381.8, df = 23, p < 0.001), rocky outcrops (F = 35.3, df = 23, p < 0.001), and bare areas (F = 16.8, df = 23, p < 0.001), but not for cushion vegetation and water pools/shores (p > 0.05). Seasonal differences only emerged for cushion vegetation (F = 13.9, n = 47, p < 0.001) and water pools (F = 28.5, n = 47, p < 0.001), as during the wet season water increases at the expense of cushion vegetation (conversely, in the dry season cushion vegetation and shores increase).
Predictor variables (bofedal area, isolation, elevation and microhabitat diversity) were not collinear (VIF < 2), so we included all in the OLS model to explain bird species richness in bofedales. Model selection (from the 16 OLS models by season obtained using 4 variables, Appendix 2), reveals that the combined effect of area and microhabitat diversity explained 54% of the variation in species richness in the dry season, while in the wet season, 38% of the variation in species richness was predicted by bofedal area (Table 2). The effect of isolation when combined with area only explained 43% (Table 2).
Nearly 45% of all bird species associated with bofedales were observed in 1 or 2 foraging microhabitats while 55% were found in more than three (Appendix 1). Ordination of species within and among microhabitats in the 24 bofedales studied followed five general patterns that differed in the amount of variation explained by the two first axes (Table 3). The first pattern shows bird species separated in groups associated to tussock vegetation from those in bare areas along axis x (B5, B6, B7, B15, B20, B22; Table 2). Subsequent patterns show birds species associated with pools/shores separated from species in bare areas along axis x (B8, B12, B13; Table 2); shrubs along axis y (B1, B4; Table 3); rocky outcrops along axis y (B3, B9, B11, B14, B17, B19, B21, B24; Table 2); and in cushion vegetation along axis y (B2, B10, B16, B18, B23; Table 3).
Classification of bird species by microhabitat was supported by MRPP analysis, as significant differences in pairwise comparisons between microhabitats and random-corrected within-microhabitat agreement were found (T = −94.14, A = 0.601, p < 0.000). Relative comparisons of significant A values shows that bird assemblages in cushion vegetation shared less species with rocky outcrops (A = 0.31, Table 4). In the same manner, assemblages associated with pools/shores shared less species with short vegetation (A = 0.27, Table 4), suggesting less homogeneity in species composition.
Microhabitats were also characterized by the presence of indicator species. ISA reveals that 37 bird species were significant indicators of microhabitats (p < 0.05, Table 5) from which 17 were present in the same microhabitat across bofedales more than 30% of the time (Table 5). As such, Diuca speculifera and Lessonia oreas were significant indicator species in cushion vegetation, while Chloephaga melanoptera, Phegornis mitchellii and Plegadis ridgwayii were significant indicators of pools and shores of bofedales (Table 5). Other species such as Upucerthia validirostris and Oreotrochilus melanogaster in rocky outcrops, Gallinago andina in tussock and Colaptes rupicola and G. cunicularia in short vegetation, had high values as indicator species (the only exception being bare areas, with no indicators) (Table 5).
Discussion
Avian richness in bofedales varied with some of them being consistently rich or consistently poor independent of season. Bofedal area and microhabitat diversity were the best predictors of species richness, although elucidating the direct and indirect effects of area on species richness is still debated (Tews et al. 2004 and references therein). Species richness could be influenced by area directly, as large areas may have higher colonization rates and support larger populations providing less vulnerability to stochastic extinction (area per se hypothesis); or indirectly, through its effect on habitat heterogeneity, often presumed to increase in direct relation to area (habitat heterogeneity hypothesis, e.g., Kohn and Walsh 1994, Allouche et al. 2012; Bar-Massada and Wood 2014, Stein et al. 2014). Our results however, show a tendency for a decrease in microhabitat diversity as area increases, suggesting a relatively more important role of area per se on species richness in the bofedal system
The overriding effect of area on richness of bofedales is further supported by our results on bird species composition. Our findings revealed that sets of species consistently (and significantly) characterized cushion vegetation, pools and shores, rocky outcrops, shrubs, tussock and short vegetation (the exception being bare areas, used opportunistically by different species) (Table 2). Nonetheless, microhabitats contributed differently to species richness as some had fewer associated species (e.g., tussock, scrubs) than others (e.g., cushion vegetation, pools/shores) (Table 3), suggesting that few species-rich microhabitats (i.e., “homogenous”) may contribute more to increase bird richness (e.g., key habitat types, Davidar 2001), than many species-poor microhabitats (i.e., “heterogeneous”), a result not supported by the habitat heterogeneity hypothesis. Moreover, bird species associated with bofedales may differ in their foraging requirements, and thus species may respond to the presence of suitable foraging habitats. For instance, some species that are restricted to forage in one or two microhabitats (e.g., Phegornis mitchellii, Diuca speculifera) could favor homogeneous bofedales (with less microhabitats) as their preferred microhabitat may be better represented, while other species that use different foraging microhabitats (e.g., Muscisaxicola cinereus) may prefer heterogeneous bofedales as these provide more suitable microhabitats to forage (e.g., Riffell et al. 2001).
Our results do not provide support for isolation effects, as the distances between bofedales may not be large enough to drive a response on species richness (Watling and Donnelly 2006). However, our measures of isolation, the linear distance to nearest bofedal, may not necessarily reflect isolation, as bofedales in this study were surrounded by mountains and a complex topography that may effectively isolate them (Table 1). High dispersal capabilities of birds could also override area effects (Ricklefs and Lovette 1999) although data on dispersal is lacking, a possible indication of its role could be determined by the lack of endemics associated with bofedales in the region of study. Further studies should focus on species with different dispersal capabilities and levels of restriction to the system including bofedales from other Andean regions.
Field studies provide key information to identify gaps in existing knowledge for accurate projections that maximize biodiversity and avoid misleading conservation efforts. Disentangling the role of area and habitat heterogeneity in promoting species richness is important to understand which conditions help explain the coexistence of species in threatened ecosystems such as bofedales. In the face of a rapidly changing climate, the ability to make informed decisions is extremely urgent to maintain the integrity of the bofedal system.
References
Allouche O, Kalyuzhny O, Moreno-Rueda G, Pizarro M, Kadmon R (2012) Area–heterogeneity tradeoff and the diversity of ecological communities. Proceedings of the National Academy of Sciences of the United States of America 109:17,495–17,500.
Angermeier PL, Winston MR (1998) Local vs. regional influences on local diversity in stream fish communities of Virginia. Ecology 79:911–927
Arrhenius O (1921) Species and area. Journal of Ecology 9:95–99
Bar-Massada A, Wood EM (2014) The richness–heterogeneity relationship differs between heterogeneity measures within and among habitats. Ecography 37:528–535
Bartoń K (2016) MuMIn: Multi-Model Inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn. Accessed 17 Jan 2017
Benham PM, Witt CC (2016) The dual role of Andean topography in primary divergence: functional and neutral variation among populations of the hummingbird, Metallura tyrianthina. BMC Evolutionary Biology 16:22. doi:10.1186/s12862-016-0595-2
BirdLife International (2016) IUCN Red List for birds. http://www.birdlife.org. Accessed 25 May 2016.
Bury J, Mark BG, Mark C, Kenneth RAY, McKenzie JM, Barrie AF, Polk MH (2013) New geographies of water and climate change in Peru: Coupled natural and social transformations in the Santa River watershed. Annals of the Association of American Geographers 103:363–374
Brooks TM, Mittermeier RA, da Fonseca GAB (2006) Global biodiversity conservation priorities. Science 313:58–61
Browman D (1974) Pastoral nomadism in the Andes. Current Anthropology 15:188–196
Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T, Maechler M, et al. (2009) gplots: Various R programming tools for plotting data. R package version 2.7.4.
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48:1079–1087
Chapin FS, Sala OE, Burke IC, Grime JP, Hooper DU, Lauenroth WK, Lombard A, Mooney HA, Mosier AR, Naeem S, Pacala SW, Roy J, Steffen WL, Tilman D (1998) Ecosystem consequences of changing biodiversity. Bioscience 48:45–52
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. Journal of Plant Ecology 1:3–21
Connor EF, McCoy ED (1979) The statistics and biology of the species-area telationship. Am Nat 113(6):791–833
Del Hoyo J, Sargatal E, Christie J, de Juana E (2016) Handbook of the Birds of the World Alive. http://www.hbw.com/node/56369. Accessed 25 May 2016
eBird (2016) eBird: An online database of bird distribution and abundance [web application]. eBird, Cornell Lab of Ornithology, Ithaca. http://www.ebird.org. Accessed 25 May 2016
Foote L, Krogman N (2006) Wetlands in Canada’s western boreal forest: Agents of change. The Forestry Chronicle 82:825–833
Fox J and Weisberg S (2011) An R companion to applied regression, second edition. Sage, Thousand Oaks
Gibbons R (2012) Bird ecology and conservation in Peru’s high Andean peat lands. Ph.D. Dissertation. Louisiana State University College of Science, Department of Biological Sciences and Museum of Natural Science. http://etd.lsu.edu/docs/available/etd-04102012-135844
Gleason HA (1925) Species and area. Ecology 6:66–74
Graham CH, Loiselle BA, Velásquez-Tibatá J, Cuesta F (2011) Species distribution modeling and the challenge of predicting future distributions. In: Herzog SK, Martinez R, Jørgensen PM, Tiessen H (eds) Climate change and biodiversity in the tropical Andes. IAI-SCOPE, São José dos Campos, pp 295–310
Herzog SK, Martinez R, Jørgensen PM, Tiessen H (2012) Climatic change and biodiversity in the tropical Andes. Inter-American institute for global change research (IAI) and scientific committee on problems of the environment (SCOPE). MacArthur Foundation, Chicago
Herzog SK, Kattan GH (2011) Patterns of diversity and endemism in the birds of the tropical Andes. In: Herzog SK, Martinez R, Jørgensen PM, Tiessen H (eds) Climate change and biodiversity in the tropical Andes. Paris: Inter-American institute for global change research (IAI) and scientific committee on problems of the environment (SCOPE). Chicago, MacArthur Foundation, pp 245–259
Holt RD (2009) Bringing the Hutchinsonian niche into the twenty-first century: Ecological and evolutionary perspectives. Proceedings of the National Academy of Sciences, USA 106:19,659–19,665
Hutchinson GE (1957) Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22:415–427
Huston MA (1999) Local processes and regional patterns: appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86:393–401
Intergovernmental Panel on Climate Change (2014) Climate Change 2014: synthesis report. contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change (Core Writing Team, R.K. Pachauri and L.A. Meyer (Eds.)). Geneva, p 151
Kadmon RA, Allouche O (2007) Integrating the effects of area, isolation and habitat heterogeneity on species diversity: A unification of island biogeography and niche theory. American Naturalist 170:443–454
Keddy P (2010) Wetland Ecology: Principles and Conservation. Cambridge University Press, Cambridge, p 1–497
Kohn DD, Walsh DM (1994) Plant species richness–the effect of island size and habitat diversity. Journal of Ecology 82:367–377
Lennon JL, Koleff P, Greenwood JJD, Gaston KJ (2001) The geographical structure of British bird distributions: diversity, spatial turnover and scale. J Anim Ecol 70(6):966–979
MacArthur RH, MacArthur JW (1961) On bird species diversity. Ecology 42:594–598
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton, Monographs in population biology
McCunne B, Grace JB (2002) Analysis of ecological communities; MjM Software, Gleneden Beach, Oregon, www.pcord.com, p 304
McCunne B, Mefford MJ (2011) PC-ORD. multivariate analysis of ecological data. Version 6. MjM Software, Gleneden Beach, Oregon.
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:249–253
Mitsch WJ, Gosselink JG (1993) Wetlands. Wiley
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, Rillig MC (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecology and Evolution 4:3514–3524
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51:933–938
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, Solymos P, Stevens H, Wagner H (2012) Package ‘vegan’. Community Ecology Package (Version 2.0–6)
Preston FW (1960) Time and space and the variation of species. Ecology 41:611–627
Preston FW (1962) The canonical distribution of commonness and rarity: part I. Ecology 43:185–215
R Development Core Team (2006) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. ISBN: 3-900,051-07-0
Rahbek C (2005) The role of spatial scale and the perception of large-scale species–richness patterns. Ecological Letters:224–239
Remsen JV, Areta I, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Pérez-Emán J, Robbins MB, Stiles FG, Stotz DF, Zimmer KJ. Version (2016) A classification of the bird species of South America. American Ornithologists’ Union. (2017) http://www.museum.lsu.edu/~Remsen/SACCBaseline.html. Accessed 25 May 2016.
Richards SA (2015) Likelihood and model selection. In: Fox GA, Negrete-Yankelevich S, Sosa VJ (eds) Ecological statistics: Contemporary theory and application. Oxford University Press, Oxford, pp 58–80
Ricklefs RE, Schluter D (1993) Species diversity in ecological communities: Historical and geographical perspectives. University of Chicago Press, Chicago, p 414
Ricklefs RE, Lovette IJ (1999) The role of island area per se and habitat diversity in the species-area relationship of four lesser Antillean faunal groups. Journal of Animal Ecology 68:1142–1160
Riffell SK, Keas BE, Burton TM (2001) Area and habitat relationships of birds in Great Lakes coastal wet meadows. Wetlands 21(4):492–507
Rosenzweig ML (1995) Species diversity in space and time. Cambridge University Press. IK, USA
Ruthsatz B (2012) Vegetación y ecología de los bofedales Altoandinos de Bolivia. Phytocoenologia 42:133–179
Schwartz MW, Brigham CA, Hoeksema JD, Lyons KG, Mills MH, van Mantgem PJ (2000) Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122:297–305
Servat G, Alcocer R, Larico M, Olarte M, Hurtado N (2013) Richness and abundance of birds in bofedales within the area of influence of the PERU LNG pipeline in Abra Apacheta and Pampas–Palmitos Basin. In: Alonso A, Dallmeier F, Servat G (eds) Monitoring biodiversity: lessons from a Trans-Andean megaproject, pp 154–164
Shannon CE (1948) A mathematical theory of communication. The Bell System Technical Journal 27(379–423):623–656
Squeo FA, Warner BG, Aravena R, Espinoza D (2006) Bofedales: high altitude peatlands of the central Andes. Revista Chilena de Historia Natural 79:245–255
Simberloff D (1976) Experimental zoogeography of islands: Effects of island size. Ecology 57:629–648
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research. 4th edition. W. H. Freeman and Co., New York, p 937
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecological Letters 17:866–880
Tellería J, Venero JL, Santos T (2006) Conserving birdlife of Peruvian highland bogs: effects of patch size and habitat quality on species richness and bird numbers. Ardeola 53:271–283
Terborgh J (1977) Bird species diversity on an Andean elevational gradient. Ecology 58:1007–1019
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31(1):79–92
Thomas CD, Lennon LL (1999) Birds extend their ranges northwards. Nature 399:213
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Valencia N, Cano A, Delgado A, Trinidad H, Gonzales P (2013) Plant composition and coverage of bofedales in an East-West macro transect in the Central Andes of Peru. In: Alonso A, Dallmeier F, Servat G (eds) Monitoring biodiversity: lessons from a Trans-Andean megaproject. Smithsonian Press, pp 64–79
Villarroel EK, Mollinedo PL, Domic AI, Capriles JM, and Espinoza C (2014) Local management of Andean wetlands in Sajama National Park, Bolivia: Persistence of the collective system in increasingly Family-oriented arrangements. Mt Res Dev 34(4):356–368
Vuilleumier F (1970) Insular biogeography in continental regions I: The northern Andes of South America. The American Naturalist 104:373–388
Vuille M (2013) Climate change and water resources in the tropical Andes. Inter-American Development Bank
Vuille MF, Wagnon P, Juen I, Kaser G, Mark BG, Bradley RS (2008) Climate change and tropical Andean glaciers: Past, present and future. Earth Science Reviews 89:79–96
Watling JI, Donnelly MA (2006) Fragments as Islands: a Synthesis of Faunal Responses to Habitat Patchiness. Conservation Biology 20:1016–1025
Weberbauer A (1936) Phytogeography of the Peruvian Andes. In McBride JF (ed.) Flora of Peru. Field Museum of Natural History, Botanic Series Publications 351:13
Williams CB (1964) Patterns in the balance of nature. Academic Press, London
White EP, Adler PB, Lauenroth WK, Gill RA, Greenberg D, Kaufman DM, Rassweiler A, Rusak JA, Smith MD, Steinbeck JR, Waide RB, Yao J (2006) A comparison of the species-time relationship across ecosystems and taxonomic groups. Oikos 112(1):185–195
Young KR (2012) Introduction to Andean geographies. In: Herzog SK, Martinez R, Jørgensen PM, Tiessen H (eds) Climatic change and biodiversity in the tropical Andes. Inter-American institute for global change research (IAI) and Scientific committee on problems of the environment (SCOPE). Chicago, MacArthur Foundation, pp 128–140
Zuur AF, Leno EN, Ephick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1:3–14
Acknowledgements
This study is a contribution of the Biodiversity Monitoring and Assessment Program (BMAP) from the Center of Conservation and Sustainability of the Smithsonian Conservation Biology Institute and PERU-LNG. We are thankful to all BMAP personnel in Lima, Ayacucho, and Washington DC, for their support with field logistics throughout the study, and T. Erwin for reviewing an early version of the manuscript. We specially thank past and present administrators of the program for their contribution at different stages of the project. Authorizations to conduct fieldwork were issued by the Direccion General de Flora y Fauna Silvestre (RD 405–2012-AG-DGFFS-GDEFFS). This is contribution No. 43 of the Peru Biodiversity Program of the Smithsonian Conservation Biology Institute.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Servat, G.P., Alcocer, R., Larico, M.V. et al. The Effects of Area and Habitat Heterogeneity on Bird Richness and Composition in High Elevation Wetlands (“Bofedales”) of the Central Andes of Peru. Wetlands 38, 1133–1145 (2018). https://doi.org/10.1007/s13157-017-0919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-017-0919-z