Abstract
Fluctuations in size and turnover of centralized, aggregative breeding locations are attributable to both natural and anthropogenic causes, and distinguishing between these sources is critical for successful conservation and management of colonially breeding animals. We used a 40-year data set to examine the relative importance of colony variables to colony dynamics of endangered Wood Storks (Mycteria americana) within the United States. Larger colonies were less prone to abandonment and those of greater longevity were more likely to re-colonize, suggesting size and previous history have intrinsic value to Wood Storks. Colonies with a higher degree of physical connection to the mainland were more likely to be abandoned, probably because the isolation reduces access for mammalian nest predators. Proximity to human activity was positively related to the probability of re-colonization, indicating either that Wood Storks and humans are attracted to similar ecological features, or that there may be some positive benefit from nesting near human activities. Local rainfall in the 12 months prior to nest initiation was positively related to re-colonization rates and negatively related to extinction rates, suggesting that colony-site effects on persistence are mediated by annual weather patterns. Our findings present means to prioritize conservation efforts for colonial nesting waterbirds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many animals, centralized breeding areas are crucial to life history and demography, with well-known examples from colonially nesting birds (Danchin and Wagner 1997), baleen whales (Corkeron and Connor 1999), nesting aquatic turtles (McClenachan et al. 2006), and calving ungulates (Fryxell et al. 1988). Such sites usually provide unusual resources such as refuge from predators or superior food for parents or young. These concentrations of breeding animals can also result in strong feedbacks to local community structure and nutrient budgets (Frederick and Powell 1994; Bouchard and Bjorndal 2000; Naiman et al. 2002).
Breeding refuge site characteristics have long been recognized as central to conservation efforts, to the extent that management or conservation of refuge site characteristics can change source-sink dynamics (Dutton et al. 2005; Troëng and Rankin 2005). The notion of stable, long term breeding sites also fits well with a land acquisition approach to conservation. In practice, breeding or central aggregation sites often have some natural variations in turnover (Erwin et al. 1998; Buckley and Buckley 2000), or population size (Ainley et al. 2004) due to weather, fluctuating local food supply (Sydeman et al. 2006), vegetative succession (Loeb et al. 1992) or density dependent degradation of the breeding site (Ganter and Cooke 1998; Loye and Carroll 1998; Shirai 2013). However, such inter-annual fluctuations in aggregation size or site use can also be driven by anthropogenic factors, including human disturbance (Wilson et al. 1991; Bouton et al. 2005), competition for food or addition of food by humans (Tasker et al. 2000; Murray 2009), introduced or anthropogenically supported nest predators (McChesney and Tershy 1998; Crooks and Soulé 1999), contamination (Bustnes et al. 2005) or changes in land use. When managing centralized breeding sites, it is therefore crucial to understand the role of both natural and anthropogenic influences in order to interpret trend information, develop expectations for temporal responses to management actions, and prioritize sites for conservation and management.
Wood Storks (Mycteria americana) characteristically breed in nesting colonies (cf 50 – 3000 pairs, Coulter et al. 1999) and there is considerable variability in colony persistence (Frederick and Meyer 2008), with some colonies lasting up to 40 years and others lasting only one or two. There have also been geographical shifts over decades in the centroid of the breeding range (Ogden 1994; Brooks and Dean 2008), possibly related to anthropogenic land use actions. Because of their endangered designation, breeding by this species has been monitored intensively for about 40 years throughout its range in the United States, which presents an opportunity to ask whether colony site dynamic is consistently affected by changes of colony site attributes. Although the status of Wood Storks have been changed from endangered to threatened in June 2014, the population is still considered vulnerable to extinction (Borkhataria et al. 2012) and protection status remains very similar.
Wood Storks feed almost exclusively on fishes and invertebrates in shallow wetlands with high prey concentrations (Kahl 1964). Dense concentrations of prey (usually fish) occur where prior and current water conditions allow both the growth of a robust fish population, and concentration/entrapment of those prey (Gawlik 2002). Because these conditions are often ephemeral in space and time, Wood Storks usually need large expanses of hydrologically dynamic wetlands to find and exploit prey concentrations, especially during the breeding season when energetic requirements increase dramatically (Kahl 1964; Ogden 1994). Interruptions of food supply during breeding can lead to mass abandonments of nests and young (Ramo and Busto 1992; Ogden 1994; Borkhataria 2009). Foraging habitat is therefore a critical resource for reproduction in this species, and there is reasonable evidence that population trajectories of Wood Storks are directly linked to foraging habitat quality through effects on several life stages (Borkhataria et al. 2012).
The attributes of the colony sites themselves may also strongly influence choice of colony location in Wood Storks. Like many colonial nesting birds, Wood Storks have no behavioral defense against mammalian predators (e.g., Procyon lotor) that can climb trees (Coulter and Bryan 1995). One hypothesized antipredator strategy is to nest in trees that are in standing water, or are located on islands. This ensures that potential mammalian predators are vulnerable to crocodilians that often congregate under wading bird colonies (Burtner 2011; Nell et al. 2016). A prediction arising from this relationship is that colonies completely surrounded by water will be more persistent than those with partial connection to the mainland.
Breeding site persistence may also be influenced by proximity of a nearby breeding site. A large colony can either splinter off, or absorb animals from nearby colonies (Oro 2003; Ainley et al. 2004). Conversely, Wood Storks nesting in small, unsuccessful colonies might be drawn in future years to nearby large successful colonies (Kim et al. 2009). Thus nearby large colonies might function to either increase or decrease the size of a nearby smaller colony.
Wood Stork colonies may also be directly or indirectly influenced by human activities. On one hand, colonies have been found to initiate and persist near residential areas in Florida (Rodgers and Schwikert 1997). However, Bouton et al. (2005) found that close approach by humans predisposed nesting Wood Storks in Brazil to abandonment. Non-nesting Wood Storks flushed at relatively long distances compared with other waterbirds (Rodgers and Schwikert 2002), a finding in keeping with the large body size of Wood Storks (Møller 2008). Beyond this information, the compatibility of Wood Stork nesting with various types of human disturbance remains poorly understood.
We predicted that stork colonies in locations with relatively low levels of human disturbance, less development in local foraging areas, tree rather than shrub substrate, clear separation from the mainland by continuous open water, and a recent history of large numbers of nests would have lower probabilities of abandonment and higher probabilities of becoming active following temporary abandonment. We also predicted that colony sites with a nearby larger colony would be more prone to be abandoned than those without a large nearby colony. To test these predictions we used a 40-year, region-wide record of nesting to model colony dynamics in relation to site attributes at multiple spatial scales.
Methods
Colony Data
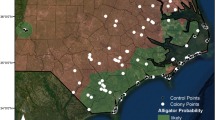
Stork colonies have been actively searched for and documented in the southeastern United States during the past 40 years because of their designation as endangered. The vast majority of records of nesting have originated from annual aerial surveys throughout the range (Brooks and Dean 2008; Frederick and Meyer 2008; Murphy and Coker 2008; Winn et al. 2008). Typically those surveys have been conducted from fixed-wing or rotor aircraft flying at altitudes of 150–250 m. Areas of nesting within colony vegetation are typically discrete and rarely exceed 400 m in diameter, and since Wood Storks nest in the tops of trees, they are highly visible from an aerial platform. We collated historical information on Wood Stork colony activity (i.e. active or inactive in any given year) from 1970 to 2010 in the southeastern United States. Each year, colonies were classified as active (more than two nests with eggs or young seen), inactive, or not surveyed. We included any colony with two or more consecutively known records (including at least one active year) in our analyses, totaling 208 colonies (=2880 colony-years; Fig. 1).
Changes in Surrounding Land Use
We quantified habitat surrounding colonies, and asked whether change in land use was associated with colony extinction and re-colonization rates. Because of inconsistencies between regional land use databases within the larger U.S. breeding range of Wood Storks, we used data only for all Florida colonies (73 colonies) in this analysis. We quantified land use with classifications in the Florida Land Use Cover and Classification System (FLUCCS), a vector-based layer derived from aerial photos with 1–3 m resolution. We calculated percent cover of each of forested wetland (FW), non-forested wetland (NFW), agriculture (AG), grassland (GR), developed area (DE), and upland forest (UF) within the 25-km buffer. We only used FLUCCS layers from 1997, 2000, 2004, to 2007 because of inconsistencies in land use classifications within the FLUCCS data in other years. We used 25 km radius as the mean buffer distance surrounding each colony, based on mean plus one standard deviation distances of Wood Stork feeding flights during the breeding season in Georgia and Florida (Bryan and Coulter 1987; Bryan et al. 1995; Gaines et al. 1998; J. Lauritsen and K. Meyer, unpublished data). We then calculated % net change in FW, NFW, AG, GR, DE, and UF from 1997 to 2007. These collapsed categories were those most relevant to changes in Wood Stork foraging and breeding habitat, changes in agricultural practices, and as measures of nearby developmental pressure.
However, we found no evidence that land use changed significantly in area during the 1997–2007 period. No land use categories within the 25 km buffer that changed more than 1.86 %. We concluded that there was not enough variation in these attributes to test for biologically significant relationships with occupancy. Therefore, only colony site and hydrology attributes were used in the analyses.
Colony Site Attributes
We characterized colony site attributes both from historical satellite images and from site visits. During May to August 2010, we characterized colony site attributes via aerial surveys of colonies, using both fixed wing and helicopter platforms. We recorded nesting substrate type, apparent changes in nesting substrate condition compared to the earlier active periods, the degree to which a colony was connected to the mainland, and sources of human disturbance within 500 m of the colony site that could be observed from the aircraft. We categorized nesting substrate type (NST) as either “trees only” (>3 m height) or “shrubs dominant” (<3 m). Changes to the condition of the nesting substrate (NSCC) included obvious deterioration or loss of nesting habitat due to logging, acute weather (e.g., hurricane or major storm effect), tree disease, or tree poisoning due to accumulation of guano (i.e. 2 groups: “unchanged” or “changed”). We assigned each site one of three values representative of its degree of connectivity to the mainland (ISLA): “1” = isolated islands with no connections to mainland, “2” = less than two-thirds circumference of the nesting location connected with land through rooted vegetation or floating mat, and “3” greater than two-thirds circumference of nesting area connected to mainland. We categorized each colony based on its proximity to potential source of human disturbance (DIST) using the following criteria: < 100 m, 100–200 m, 200–300 m, 300–400 m, 400–500 m, and no development within 500 m. Evidence of human disturbance included signs of logging, paved roads, houses or other structures, intensive agriculture, or recreational activities (airboat trails, boat launch, walking trails, tracks of off-road vehicles). To represent our hypotheses (e.g. increasing distance to human disturbance decreases the likelihood of colony abandonment) both ISLA and DIST were treated as ordinal variables such that incorporating them in the modeling process only requires one additional parameter for each (assuming a common intercept).
To explore whether history of colony activity influenced colony dynamics, we used numbers of years since the first active record as a measure of longevity (LONG). If the colony activity sequence had fewer than 4 consecutive years of confirmed inactive or unknown status between active records, we considered the colony to have a continuous history. However, if any colony activity sequence had four or more consecutive years of confirmed inactive or unknown status between active records, we considered the colony to have a discontinuous history, and the sequence then was considered ended. Any new activity at this colony after this sequence was treated as the start of a new colony history interval. We also used the number of nesting pairs in year i to evaluate whether the size of a colony was associated with abandonment in year i + 1. In some years, colonies were noted only as “active” with no colony size information. In these cases we used the mean colony size for the given colony from all active years.
To measure the effect of having nearby colonies on colony dynamics, we modeled the probability of abandonment and the probability of becoming active following temporary abandonment as functions of the size and distance of its nearest neighbor, which we expressed as:
where S i is the effect of colony j on colony i; α is a scaling parameter where 1/α is defined as the average migration distance of the species (25 km; mean plus one standard deviation of the feeding flight distances of Wood Storks); d ij is the distance from colony i to the nearest colony j. A j is number of breeding pairs of the nearest colony j. S i is a simplification of an incidence function measure (Moilanen and Nieminen 2002) in which we are only concerned with the degree of connectivity between the focal colony and its nearest neighboring colony.
Hydrology
Stork breeding is known to be highly responsive to local hydrology, which is in turn related to local rainfall (Kahl 1964; Coulter et al. 1999). Our interest was in understanding the influence of colony site attributes on colony dynamics, therefore we controlled for the influence of local hydrology on colony dynamics by using precipitation as an indicator of surface water conditions in local wetlands. This provides a consistent measure for the extended study area, and precipitation and water level are closely associated in shallow wetland systems. Based on nearest recording stations to individual colonies (mean = 33 km, range = 2–80 km), we obtained monthly precipitation data from 23 weather stations across the southeastern United States from the National Climatic Data Center.
We created three indices representing total precipitation occurring during 12 and 36 months prior to nest initiation, and 5 months after nest initiation. Total precipitation in each period was indexed to longer term rainfall patterns by subtracting the long term mean for that period, and dividing by the standard deviation of that mean, expressed below:
Since there is a latitudinal cline in Wood Stork nest initiation date (Rodgers and Schwikert 1997), we defined timing of typical nest initiation for four general regions: south Florida (south of Lake Okeechobee; December), central Florida (south of 29 ° 20′ N; January), north Florida and Georgia (north of 29 ° 20′ N; February), and South and North Carolina (March).
Data Analysis
Following Erwin et al. (1998) we modeled Wood Stork colony site dynamics as a conditional first-order markov process in which an active colony in year t can either become abandoned or remain active in year t + 1, whereas an abandoned colony in year t can become active (re-colonized) or continue to be abandoned in year t + 1. If a colony site was not visited at all during a specific year, the data for that occasion provided no information to the modeling process. Since active colonies were not systematically observed prior to their discovery, we make no inferences about the initial colonization process of Wood Stork colonies, only for the probability of becoming active following temporary abandonment, or re-colonization.
In the surveys examined for this study, biologists visited colonies with known coordinates. Active stork nests are large and conspicuous, and the probability of detecting them at a colony site is extremely high. For example, for a subset of 15 colonies located in the Everglades, visited 2–6 times per season over 26 years (=390 colony-years), the probability of detecting an active colony was 1.0.
Modeling first-order markov processes such as Wood Stork colony dynamics can be done using a variety of available statistical software packages (Therneau 2015). For its convenience, especially when incorporating temporally varying site covariates (e.g. precipitation and colony longevity), we modeled Wood Stork colony dynamics by simplifying the multi-season occupancy model available in program MARK 6.1 (White and Burnham 1999) to a single observation per year and by fixing the probability of detecting an active colony equal to 1.0.
We constructed 84 a priori models representing predictions about the relationships between 10 site covariates and colony site dynamics (Table 1). We used Akaike’s Information Criterion corrected for small sample sizes (AICc) for model selection and ranked models according to AIC scores and weights (w i ). We considered models with ∆AIC c < 2, as having similar fit to the data and chose the ‘best model’ among them based on the model that had the fewest numbers of parameters (Burnham and Anderson 2002). We assessed the direction and significance of slope coefficients of the best fit model to test our predictions and to identify which processes had the most effect on Wood Stork colony site dynamics.
Results
Wood Storks colony longevity ranges from 1 to 41 years with the average of 9.36 (SE = 0.61). The overall abandon rate across years and colonies was 0.19 (SE = 0.01) and re-colonization rate was 0.21 (SE = 0.01). The global model was best supported by the data (w i = 0.91, Table 2). This model included 9 of the 10 original variables (the precipitation covariates, P1 and P2, were correlated and thus never included in the same model), six of which had significant effects on colony dynamics in the same direction as our predictions (Table 1). Colony size in the previous year, degree of isolation from the mainland, and nesting substrate type were negatively correlated with the probability of colony abandonment. Small colonies, those dominated by shrubs (<3 m), and those most connected to the mainland were associated with the highest abandonment rates. Longevity did not influence the probability of a colony becoming abandoned but was positively related to re-colonization. Temporarily abandoned colonies that had previously existed for more than 15 years had a significantly higher probability of becoming active again compared to those with shorter histories. Surprisingly, proximity to sources of human disturbance had a significant positive relationship with the probability of re-colonization and had no significant relationship with the probability of abandonment, which was contrary to our predictions (Table 1).
Precipitation during the 12 months prior to nest initiation had a negative relationship with abandonment and was positively related to re-colonization. Although the effect was not significant, colonies with a larger Wood Stork colony nearby also had a stronger tendency to become abandoned and were less likely to be re-colonized compared to colony sites without a large colony nearby.
Discussion
Longevity was a strong factor positively influencing re-colonization, even when all other colony site effects were taken into account. This effect of previous occurrence history therefore seems to have intrinsic value for predicting the willingness of Wood Storks to re-occupy colonies. It is unclear whether Wood Storks are coming back to these colonies because longevity is associated with some unmeasured site attributes, or if temporal stability itself has value for breeding birds. Previous colony activity status has been found to be an important predictor of future occurrence in both Gray Herons (Ardea cinerea) and Purple Herons (Ardea purpurea, Barbraud et al. 2003), and Wood Storks have been noted as being moderately philopatric (Frederick and Ogden 1997). It is possible that Wood Storks have a better chance of re-locating previous mates at the beginning of the nesting season at more stable colonies, or that longer lived colonies provide Wood Storks with the opportunity to enhance breeding success through social dominance or knowledge of local food sources. Such mechanisms could increase return rates of adults through enhanced lifetime reproductive success (Ganter and Cooke 1998; Bled et al. 2011). Our results indicate that older colonies should receive conservation and management priority because of their temporal stability and attractiveness.
We also found a negative relationship between colony size and abandonment, suggesting that larger colonies are more likely to persist, all other factors being equal. This trend has also been found for other colonial species (Barbraud et al. 2003; Lombard et al. 2010) and may have to do with a variety of social and individual risk processes (Delgado et al. 2011). However, Wood Stork colony size has become smaller in the United States through time (Brooks and Dean 2008), so the effects of colony size and longevity on colony dynamics may be confounded by this temporal trend. It is therefore not clear if the recent trends toward smaller colonies can be directly translated into a trend towards higher abandonment rates. However, it seems likely that colonies in the largest size categories should be in a higher conservation priority category than the smallest size categories.
Among the colony-site variables, proximity of human activity, degree of island isolation and nesting substrate type were highly correlated with colony site dynamics. Colonies with greater connection to the mainland were much more likely to go extinct. In Egypt, Little Tern (Sterna albifrons) colony occupancy was highest on intermediate sized islands (Eason et al. 2012), due to mammalian presence on larger islands. Previous studies of Wood Stork colonies are consistent in predicting that without the protection of water, mammalian predators are likely to gain access to Wood Stork nests and cause abandonment (Rodgers 1987; Coulter and Bryan 1995). Our analysis indicates that this mechanism results in an important effect at the scale of the range-wide population, and indicates that colonies on true islands have much greater persistence and conservation value than those with some connection to mainland. This suggests both that true islands should rank most highly in protection and conservation planning, and that management to remove encroaching floating vegetative mats can greatly reduce chances of colony abandonment.
Colonies closer to evidence of human activities were less likely to become extinct and were more likely to be colonized compared to colonies with more distant apparent sources of disturbance. Note that we did not measure disturbance directly, and many kinds of effects may be conflated by our use of distance to human activity as a proxy for disturbance. Further, close proximity was defined as being within 500 m. This threshold includes distances that are considerably larger than recommended setback distances from colonies for this species (cf 180–250 m, Rodgers and Schwikert 2002).
Two very different interpretations of this overlap between human and stork activities are possible. One is that Wood Storks are not particularly sensitive to the effects of human disturbance and nesting may be compatible with a number of different kinds of human land uses in the vicinity of colonies. Indeed, many of the colonies in close proximity to housing, roads and active waterways are long lived (>10 year) and productive. However, it is also possible that both humans and Wood Storks are merely attracted to similar environmental features, creating an apparent association between sources of human disturbance and nesting suitability. Humans and Wood Storks show a preference for high densities of lakes and watercourses, and both avoid commercial pine plantations and large areas of oligotrophic wetlands. It is also possible that proximity of human activities may add some level of ecological disturbance that is beneficial for Wood Stork foraging (e.g., presence of open habitat, ditches, and watercourses) or nesting (local reduction in, or protection from nest predators). We suggest 1) that this result does not necessarily imply neutral or positive effects of human land use on Wood Stork nesting, and 2) studies should be undertaken to examine the mechanisms of possible positive effects, and to further test the hypothesis that Wood Storks and humans prefer the same landscape features.
Previous studies have shown that Wood Stork breeding success and productivity were associated with hydrological conditions (Herring and Gawlik 2011), since particular sequences of water levels may be required to produce high standing stocks of prey (Ruetz et al. 2005; Dorn and Trexler 2007) and to make prey available through the concentrating action of falling surface water (Kushlan and Frohring 1986; Ogden et al. 1987; Coulter and Bryan 1995). In this study, colonies with wetter conditions 12 months before nest initiation had higher occurrence probabilities. Bryan and Robinette (2008) found that pre-breeding season rainfall had a moderate positive relationship with breeding success for colonies in coastal Georgia, but not for inland colonies. Similarly, Ogden (1994) found numbers of Wood Stork nests in the Everglades were associated with higher annual marsh water levels in the months prior to nesting. While wetter conditions may not always benefit Wood Storks (Ogden 1994), the hydrological relationship we found seems consistent with previous work, and suggests that any effects of colony site attributes are co-dependent upon recent rainfall regimes.
Colonies of birds are usually non-randomly placed with respect to resources, and there is emerging evidence that they are non-random with respect to one another. Barbraud et al. (2003) suggested reciprocal relationships between areas with higher Purple Heron colony extinction rates and those with higher re-colonization in the Camargue of France. Kajzer et al. (2012) found a negative relationship between colony occurrence and distance to nearest population. Orlowski and Czapulak (2007) reported that Rook (Corvus frugilegus) colony extinction was negatively related to colony size, and positively dependent upon size of surrounding colonies. While smaller colonies of Ivory Gulls (Pagophila eburnea) were more likely to go extinct, Robertson et al. (2007) found no evidence of local rescue effects from nearby larger colonies. We did not find significant relationships between the proximity of large nearby colonies and Wood Stork colony dynamics, suggesting that the proximity of sites to other large nearby colonies may be a less important driver of Wood Stork colony dynamics compared to other site attributes. The balance between splitting of large colonies and growth by absorption of small ones is probably driven by multiple ecological and social factors (Danchin and Wagner 1997; Russell and Rosales 2010; Delgado et al. 2011).
Finally, we attempted to incorporate an array of landscape scale variables to explain the colony dynamics. These landscape variables were developed both from an understanding of the nesting and feeding ecology of the species and formed explicit hypotheses about how Wood Storks might react to specific kinds of land use change such as human encroachment and conversion of wetland forests to open wetlands. However, these potential variables were constrained by existing data to a large degree as land use layers among years and regions are either lacking or inconsistent in classifications, thus limiting our ability to explore the relationship. Our data from limited time and region (i.e., 1997–2007 in Florida) showed that there was not enough variation in these variables to test for ecological relationships with occupancy. Nevertheless, landscape components should be considered for regional conservation and management plans to make sure population level success.
In this paper we have identified large size, longevity, trees as nesting substrate, proximity to human activity, and lack of colony connections with the mainland, as attributes that positively affect the persistence of Wood Stork colony sites. These relationships provide managers with new, directly applicable criteria for prioritizing colonies for high conservation or management action. Colony site dynamics were also strongly influenced by prior rainfall. This is consistent with previous studies and is supported by an understanding of the mechanisms by which wetland prey are produced and made available to Wood Storks. Geographic and temporal patterns of rainfall in the southeastern United States are strongly susceptible to global change forcing functions (Seager et al. 2009; Li et al. 2011; Misra et al. 2011), suggesting a mechanism by which Wood Stork nesting preferences will be directly or indirectly affected by global change.
References
Ainley DG, Ribic CA, Ballard G, Heath S, Gaffney I, Karl BJ, Barton KJ, Wilson PR, Webb S (2004) Geographic structure of Adelie Penguin populations: overlap in colony-specific foraging areas. Ecological Monographs 74:159–178
Barbraud C, Nichols JD, Hines JE, Hafner H (2003) Estimating rates of local extinction and colonization in colonial species and an extension to the metapopulation and community levels. Oikos 101:113–126
Bled F, Royle JA, Cam E (2011) Assessing hypotheses about nesting site occupancy dynamics. Ecology 92:938–951
Borkhataria RR (2009) Modeling population viability and habitat suitability for the endangered wood stork (Mycteria americana) in the southeastern United States. Dissertation, University of Florida, Gainesville
Borkhataria RR, Frederick PC, Keller RA, Collazo JA (2012) Temporal variation in local wetland hydrology influences postdispersal survival of juvenile Wood Storks (Mycteria americana). The Auk 129:438–448
Bouchard S, Bjorndal KA (2000) Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81:2305–2313
Bouton SN, Frederick PC, Rocha CD, Barbosa Dos Santos AT, Bouton TC (2005) Effects of tourist disturbance on Wood Stork nesting success and breeding behavior in the Brazilian Pantanal. Waterbirds 28:487–497
Brooks WB, Dean T (2008) Measuring the biological status of the U. S. breeding population of Wood Storks. Waterbirds 31:50–62
Bryan AL Jr, Coulter MC (1987) Foraging flight characteristics of Wood Storks in east-central Georgia, USA. Colonial Waterbirds 10:157–161
Bryan AL Jr, Robinette JR (2008) Breeding success of Wood Storks nesting in Georgia and South Carolina. Waterbirds 31:19–24
Bryan AL Jr, Coulter MC, Pennycuick CJ (1995) Foraging strategies and energetic costs of foraging flights by breeding wood storks. The Condor 97:133–140
Buckley PA, Buckley FG (2000) Patterns of colony-site use and disuse in saltmarsh-nesting Common and Roseate Terns. Journal of Field Ornithology 71:356–369
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Verlag, New York
Burtner BF (2011) Symbiosis between long-legged wading birds (Ciconiiformes) and alligators (Alligator mississippiensis)? Testing the “nest protector” hypothesis. Thesis, University of Florida, Gainesville
Bustnes JO, Miland O, Fjeld M, Erikstad KE, Skaare JU (2005) Relationships between ecological variables and four organochlorine pollutants in an artic glaucous gull (Larus hyperboreus) population. Environmental Pollution 136:175–185
Corkeron PJ, Connor R (1999) Why do baleen whales migrate? Marine Mammal Science 15:1228–1245
Coulter MC, Bryan AL Jr (1995) Factors affecting reproductive success of Wood Storks (Mycteria americana) in east-central Georgia. The Auk:237–243
Coulter MC, Rodgers JA Jr, Ogden JC, Depkin FC (1999) Wood Stork (Mycteria americana). The Birds of North America:24
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented landscape. Nature 400:563–566
Danchin E, Wagner RH (1997) The evolution of coloniality: the emergence of new perspectives. Trends in Ecology and Evolution 12:342–347
Delgado MM, Ratikainen II, Kokko H (2011) Inertia: the discrepancy between individual and common good in dispersal and prospecting behaviour. Biological Reviews 86:717–732
Dorn NJ, Trexler JC (2007) Crayfish assemblage shifts in a large drought-prone wetland: the roles of hydrology and competition. Freshwater Biology 52:2399–2411
Dutton DL, Dutton PH, Chaloupka M, Bouton RH (2005) Increase of a Caribbean leatherback turtle Dermochelys coriacea nesting population linked to long-term nest protection. Biological Conservation 126:186–194
Eason P, Rabea B, Attum O (2012) Island shape, size, and isolation affect nest-site selection by little terns. Journal of Field Ornithology 83:372–380
Erwin RM, Nichols JD, Eyler TB, Stotts DB, Truitt BR (1998) Modeling colony-site dynamics: a case study of gull-billed terns (Sterna nilotica) in coastal Virginia. The Auk:970–978
Frederick PC, Meyer KD (2008) Longevity and size of Wood Stork (Mycteria americana) colonies in Florida as guides for an effective monitoring strategy in the southeastern United States. Waterbirds 31:12–18
Frederick PC, Powell GVN III (1994) Nutrient transport by wading birds in the Everglades. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach
Fryxell JM, Greever J, Sinclair ARE (1988) Why are migratory ungulates so abundant? American Naturalist 131:781–798
Gaines KF, Bryan AL Jr, Dixon PM, Harris MJ (1998) Foraging habitat use by Wood Storks nesting in the coastal zone of Georgia, USA. Colonial Waterbirds 21:43–52
Ganter B, Cooke F (1998) Colonial nesters in a deteriorating habitat: site fidelity and colony dynamics of lesser snow geese. The Auk 115:642–652
Gawlik DE (2002) The effects of prey availability on the numerical response of wading birds. Ecological Monographs 72:329–346
Herring HK, Gawlik DE (2011) Resource selection functions for Wood Stork foraging habitat in the southern Everglades. Waterbirds 34:133–142
Kahl MP (1964) Food ecology of the wood stork (Mycteria americana) in Florida. Ecological Monographs 34:98–117
Kajzer J, Lenda M, Kosmicki A, Bobrek R, Kowalczyk T, Martyka R, Skorka P (2012) Patch occupancy and abundance of local populations in landscapes differing in degree of habitat fragmentation: a case study of the colonial Black-headed Gull, Chroicocephalus ridibundus. Journal of Biogeography 39:371–381
Kim S-Y, Torres R, Drummond H (2009) Simultaneous positive and negative density-dependent dispersal in a colonial bird species. Ecology 90:230–239
Kushlan JA, Frohring PC (1986) The history of the southern Florida Wood Stork population. The Wilson Bulletin:368–386
Li W, Li L, Fu R, Deng L, Wang H (2011) Changes to the north atlantic subtropical high and its role in the intensification of summer rainfall variability in the southeastern United States. Journal of Climate 24:1499–1506
Loeb SC, Pepper WD, Doyle AT (1992) Habitat characteristics of active and abandoned Red-cockaded Woodpecker colonies. Southern Journal of Applied Forestry 16:120–125
Lombard CD, Collazo JA, McNair DB (2010) Nest and chick survival and colony-site dynamics of least terns in the U. S. Virgin Islands. The Condor 112:56–64
Loye JE, Carroll SP (1998) Ectoparasite behavior and its effects on avian nest site selection. Annals of the Entomological Society of America 91:159–163
McChesney GJ, Tershy BR (1998) History and status of introduced mammals and impacts to breeding seabirds on the California Channel and northwestern Baja California Islands. Colonial Waterbirds 21:335–347
McClenachan L, Jackson JBC, Newman MJH (2006) Conservation implications of historic sea turtle nesting beach loss. Frontiers in Ecology and the Environment 4:290–296
Misra V, Carlson E, Craig RK, Enfield D, Kirtman B, Landing W, Lee S-K, Letson D, Marks F, Obeysekera J, Powell M, Shin S-l (2011) Climate scenarios: a Florida-Centric view. Florida Climate Change Task Force, Available online at http://floridaclimate.org/whitepapers/
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83:1131–1145
Møller AP (2008) Flight distance and population trends in European breeding birds. Behavioral Ecology 19:1095–1102
Murphy TM, Coker JW (2008) A twenty-six year history of Wood Stork nesting in South Carolina. Waterbirds 31:3–7
Murray NM (2009) Foraging behavior and success of Australian White Ibis (Threskiornis mollucca) in an urban environment. Notornis 56:201–205
Naiman RJ, Bilby RE, Schindler DE, Helfield JM (2002) Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5:399–417
Nell LA, Frederick PC, Mazzotti FJ, Vliet KA, Brandt LA (2016) Presence of breeding birds improves body condition for a crocodilian nest protector. PloS One 11, e0149572
Ogden JC (1994) A comparison of wading bird nesting colony dynamics (1931–1946 and 1974–1989) as an indication of ecosystem conditions in the southern Everglades. In: Davis S, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 533–570
Ogden JC, McCrimmon DA Jr, Bancroft GT, Patty BW (1987) Breeding populations of the wood stork in the southeastern United States. The Condor:752–759
Orlowski G, Czapulak A (2007) Different extinction risks of the breeding colonies of Rooks Corvus frugilegus in rural and urban areas of SW Poland. Acta Ornithologica 42:145–155
Oro D (2003) Managing seabird metapopulations in the Mediterranean: constraints and challenges. Scientia Marina 67:13–22
Ramo C, Busto B (1992) Nesting failure of the wood stork in a neotropical wetland. The Condor 94:777–781
Robertson GJ, Gilchrist HG, Mallory ML (2007) Colony dynamics and persistence of Ivory Gull breeding in Canada. Avian Conservation and Ecology 2, Art 8, [online] URL: http://www.ace-eco.org/vol2/iss2/art8/
Rodgers JA Jr (1987) On the antipredator advantages of coloniality: a word of caution. The Wilson Bulletin 99:269–271
Rodgers JA Jr, Schwikert ST (1997) Breeding success and chronology of Wood Storks Mycteria americana in northern and central Florida, USA. Ibis 139:76–91
Rodgers JA Jr, Schwikert ST (2002) Buffer zone distances to protect foraging and loafing waterbirds from disturbance by personal watercraft and outboard powered boats. Conservation Biology 16:216–224
Ruetz CR, Trexler JC, Jordan F, Loftus WF, Perry SA (2005) Population dynamics of wetland fishes: spatio-temporal patterns synchronized by hydrological disturbance? Journal of Animal Ecology 74:322–332
Russell GJ, Rosales A (2010) Sociability leads to instability. Theoretical Ecology 3:3–12
Seager R, Tzanova A, Nakamura J (2009) Drought in the southeastern United States: causes, variability over the last millennium, and the potential for future hydroclimatic change. Journal of Climate 22:5021–5045
Shirai T (2013) Colony development and density-dependent processes in breeding Grey Herons. International Journal of Zoology. doi:10.1155/2013/404065
Sydeman WJ, Bradley RW, Warzybok P, Abraham CL, Jahncke J, Hyrenbach KD, Kousky V, Hipfner JM, Ohman MD (2006) Planktivorous auklet Ptychoramphus aleuticus responses to ocean climate, 2005: Unusual atmospheric blocking?. Geophysical Research Letters 33 doi:10.1029/2006GL026736
Tasker ML, Camphuysen CJ, Cooper J, Garthe S, Montevecchi WA, Blaber SJM (2000) The effects of fishing on marine birds. ICES Journal of Marine Science 57:531–547
Therneau TM (2015) A Package for Survival Analysis in S. version 2.38, http://CRAN.R-project.org/package=survival
Troëng S, Rankin E (2005) Long term conservation efforts contribute to positive green turtle Chelonia mydas nesting trend at Tortuguero, Costa Rica. Biological Conservation 121:111–116
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird study 46:120–139
Wilson RP, Culik B, Danfeld R, Adelung D (1991) People in Antarctica--how much do Adélie Penguins Pygoscelis adeliae care? Polar Biology 11:363–370
Winn B, Swan D, Ozier J, Harris MJ (2008) Wood Stork nesting in Georgia: 1992–2005. Waterbirds 31:8–11
Acknowledgments
We are indebted to all the biologists (many retired) who helped us with buried records, notebooks and recollections. This paper is dedicated to the memory of John Ogden, who studied and conserved Wood Storks throughout his professional life. We are grateful to National Fish and Wildlife Foundation (2007-0017-000), Georgia Department of Natural Resources (00076801; 00076802), South Florida Water Management District (4500014203), and Felburn Foundation (00073200) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, JS., Reichert, B.E., Frederick, P.C. et al. Breeding Site Longevity and Site Characteristics Have Intrinsic Value for Predicting Persistence of Colonies of an Endangered Bird. Wetlands 36, 639–647 (2016). https://doi.org/10.1007/s13157-016-0774-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-016-0774-3