Abstract
The coastal wetlands of northeastern Florida Bay are seasonally-inundated dwarf mangrove habitat and serve as a primary foraging ground for wading birds nesting in Florida Bay. A common paradigm in pulse-inundated wetlands is that prey base fishes increase in abundance while the wetland is flooded and then become highly concentrated in deeper water refuges as water levels recede, becoming highly available to wading birds whose nesting success depends on these concentrations. Although widely accepted, the relationship between water levels, prey availability and nesting success has rarely been quantified. I examine this paradigm using Roseate Spoonbills that nest on the islands in northeastern Florida Bay and forage on the mainland. Spoonbill nesting success and water levels on their foraging grounds have been monitored since 1987 and prey base fishes have been systematically sampled at as many as 10 known spoonbill foraging sites since 1990. Results demonstrated that the relationship between water level and prey abundance was not linear but rather there is likely a threshold, or series of thresholds, in water level that result in concentrated prey. Furthermore, the study indicates that spoonbills require water level-induced prey concentrations in order to have enough food available to successfully raise young.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gawlik (2002) best articulated a widely accepted and well-studied paradigm regarding the function of ephemeral wetlands in determining nesting success of wading birds. In short, during high water periods prey species are relatively less susceptible to predation and their populations grow exponentially and during low water periods, prey are concentrated into lower elevation habitats that provide refuge when ephemeral wetlands are dry. Wading birds exploit the concentrations and time nesting and nest location so that there is a readily available food source for the rapidly growing and energetically demanding young and that this availability must be sustained through the entire nesting cycle. While most components of this paradigm have been demonstrated empirically (Kahl 1964; Higer and Kolipinski 1967; Kushlan 1976a, 1978, 1980; Ogden et al. 1980; Loftus and Kushlan 1987; Powell 1987; Frederick and Collopy 1989; Bancroft et al. 1994; Frederick and Spalding 1994; Loftus and Eklund 1994; DeAngelis et al. 1997; Lorenz 2000; Gawlik 2002; Herring et al. 2011) the connection between water level/prey availability and nesting success has been somewhat elusive. Because nesting sites and foraging locations are spatially distant and the location of foraging sites changes temporally as a patch of concentrated prey is depleted, it is difficult to determine prey abundance at specific sites where a particular pair of successfully nesting birds foraged. Furthermore, there could be nesting failure unrelated to food availability (e.g. predation, disease, weather, human disturbance etc.).
Another challenge in demonstrating this connection is that the relationship between low water levels and prey availability is likely to be non-linear. For example, high fish concentrations can arise when water levels are relatively high due to oxygen and thermal stress that drive prey from wetlands into deeper water (e.g. Frederick and Loftus 1993). Conversely, prey availability can be low when water levels are low due to depletion of prey from predation and/or effects of overcrowding (e.g. Gawlik 2002) or hydrologically-limited productivity (e.g. Lorenz 2000). Although the linearity of the relationships between water level, prey availability and nesting success are investigated here, the analyses also focus on the concept of a Prey Concentration Threshold (PCT). I propose that prey concentrations do not adhere to a strictly-linear relationship with water level, rather, there is some water depth (the PCT) at which prey will abandon the ephemeral wetlands and move to deep water refuges prior to the wetlands drying out entirely. When water levels drop to this point, there is short-lived pulse in prey concentrations as all the prey flee the drying wetland en masse. These concentrated prey are then are quickly depleted through predation and other mortality factors.
I address this paradigm using 22 years of Roseate Spoonbill (Platalea ajaja) nesting data from colonies on islands in northeastern Florida Bay (NEFB) and water level and prey (demersal fish) data from multiple foraging sites located in mainland mangrove wetlands specifically to 1) test the linearity of the relationships between water level, prey availability and nesting success, 2) investigate the concept of the PCT, and 3) to investigate whether prey availability has a direct impact on nesting success in a wading bird species.
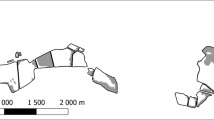
Roseate Spoonbills were extirpated from Florida by the early 1900’s due to overhunting to provide feathers to the fashion industry (Allen 1942). Legal protection resulted in population recovery and by the late 1970’s the population had recovered to more than 1200 nests in Florida Bay (Powell et al. 1989), more than half of which were located in extreme northeastern corner of Florida Bay (Fig. 1; Lorenz et al. 2002). In 1984, the completion and operation of a series of canals and pumps (known as the South Dade Conveyance System or SDCS) had a profound impact on the way fresh water flowed into NEFB. Prior to the SDCS, most of the fresh water flowed from the Everglades into Florida Bay via Taylor Slough and associated creeks to the east (Fig. 2). The SDCS diverted water away from Taylor Slough and into the C-111 canal (Fig. 2), fundamentally altering the hydrology of Taylor Slough and NEFB (Kotun and Renshaw, this issue). Since completion of the SDCS, notable changes have been observed in the flora and fauna of Florida Bay, particularly in the northeastern region (Lorenz, this issue) and spoonbill numbers in NEFB have been drastically reduced, with <50 pairs in 2008–09 (Fig. 1; Lorenz and Dyer 2010).
Methods
Wetland Site Description
Spoonbills nesting in NEFB primarily forage in the seasonal ephemeral mangrove wetlands north of the Bay from Taylor Slough eastward to Turkey Point (Fig. 2; Bjork and Powell 1994; Lorenz et al. 2002; Lorenz unpublished satellite tracking data). The mainland wetlands of NEFB are dwarf mangrove habitat, characterized by a centralized creek (“creek” sub-habitat) that contains water throughout the year that is surrounded by expansive shallow flats (“flats” sub-habitat) that are ephemerally inundated (Fig. 2). Vegetation consists of widely spaced (0.5–5.0 m) dwarf red mangrove (Rhizophora mangle) trees (0.5–2.0 m tall) with varying amounts of herbaceous vegetation between individual trees. Seasonal growth of Eleocharis cellulosa, Utricularia spp. and Chara hornimani is common and the substrate is flocculent, unconsolidated, carbonate marl (Browder et al. 1994).
There is a characteristic seasonal pattern to the water level fluctuations on these wetlands, with high water levels that inundate the ephemeral wetlands during the wet season (June–Nov) and low water during the dry season (Dec–May) that exposes the ephemeral wetland and results in only the central creeks being inundated (Lorenz 1999). The principle drivers of this long-term cycle are the thermal expansion and contraction of the Gulf of Mexico (Marmar 1954; Holmquist et al. 1989) and wet season/dry season rainfall patterns (Duever et al. 1994). Because the onset of the rainy season provides a natural break in the cycle (Lorenz and Serafy 2006), “hydroyear” is defined from June 1 to May 31. Wind-driven tides can increase or decrease water levels (up to 40 cm) on the wetlands very quickly and those conditions can be maintained until cessation of the wind event (Holmquist et al. 1989). Upstream water management practices, such as pulse releases from the C-111 canal (Fig. 2), can also result in rapid increases in water levels (Kotun and Renshaw, this issue; Lorenz, this issue) that may endure for several days. The southern Biscayne Bay wetlands are generally unaffected by these pulse releases as water flow through the C-111 canal is blocked near US Highway 1 (US1; Fig. 2), so the majority of water released through the C-111 flows southward from the canal on the west side of US-1 toward Florida Bay (Kotun and Renshaw, this issue; Lorenz, this issue). Finally, diurnal tides affect water levels on the wetlands of southern Biscayne Bay, although the amplitude is relatively small (9–15 cm; Lorenz 1999); there are no diurnal tides on the NEFB wetlands (Lorenz 1999).
Data Collection
Water Level Records
Water level recorders were placed at ten known spoonbill foraging locations in the NEFB wetlands and west of southern Biscayne Bay (Fig. 2). Hydrostations recorded depth (hourly) relative to the elevation of the flats (i.e., a reading of 0 cm on the recorder indicated that the flats were completely dry while the creek was flooded). Establishment of these sites was staggered through time but are identified as long-term (established prior to 1992) mid-term (established in early 2000s) and short-term (established after 2005; dates of the establishment of sites are presented in the ESM 1). Prior to 2000, data were collected using a Telog® 2108 potentiometric recorder with a float and pulley design. After 2000, telemetered hydrostations (Remote Data Inc., using Hydrolab® pressure sensors to record water depth on a remotely-accessible Campbell® data recorder) were established at each site in addition to the Telog® recorders, thereby creating redundancy in water level data collection. Gaps in the data were filled by using regression models between nearby hydrostations (see ESM 1 for further details).
Prey Fish Sampling
Drop traps were used to collect fish according to the methodology of Lorenz et al. (1997). Three 9-m2 traps were used in each sub-habitat (creek and flats) at each site. Each trap surrounded an individual dwarf mangrove tree, thereby sampling both prop root habitat and the open area between trees (Fig. 2). Trees were selected for sampling such that each site had a similar array of tree sizes with roughly equivalent prop root density sampled between sites. Traps were set, left in place overnight and deployed the following day within 2 h after sunrise. Fish were cleared from the trap using rotenone. Traps remained in place until the following day and any fish found floating within the trap were added to the sample and their weights were estimated from length-weight regressions generated from fishes from the initial collection (Lorenz et al. 1997). Sample collections were targeted for June, September, and monthly from November through April, however, logistical, economical and climatological problems prevented complete sampling at some sites (presented in ESM 1). The majority of fish collections were made during the dry season and transitional periods so that the impact of fluctuations in water level could be assessed.
Although the drop traps were specifically designed to catch the small demersal fishes that are the primary prey items of spoonbills (Lorenz et al. 1997), incidental collections of larger fishes did occur. In some cases, a single large individual weighed more than the entire sample of smaller fishes. Length-frequency distributions indicated that all fish found on the flats were <6.5 cm TL (total length). The flats made up the majority of the habitat, indicating that fish larger than 6.5 cm TL were not an integral part of the demersal fish community. Based on this observation, all fish ≥6.5 cm TL (3.2 % of total fish collected) were omitted from analyses. The elimination of these large fish limits the data to prey that spoonbills are likely to capture, as spoonbills’ principle diet is fish up to approximately 5 cm (Dumas 2000).
Spoonbill Nesting Colony Surveys
Spoonbills typically nest in Florida Bay between November and April (Powell et al. 1989). During this period, nest production was estimated by repeated visits to a given colony on a 7–10 day cycle. Up to 65 nests were marked with uniquely numbered nest tags during the late incubation period. An estimate of the mean hatch day was made based on chick size and morphology when they were first observed. At approximately 21 day, chicks begin to move out of the nest and spend their time in adjacent trees (Allen 1942; Dumas 2000; Lorenz et al. 2002) and surveys must be discontinued for the safety of the chicks (susceptible to falling out of the trees when disturbed). Chicks that made it to 21 day (from here referred to as the nestling period) were considered successful even though some mortality does occur after they leave the nest.
Spoonbill nest success surveys were performed at Tern Key (historically the largest colony in NEFB) during every nesting cycle from 1987–88 to 2006–07 except for 1993–94. Beginning in 2007–08, the Tern Key colony failed to form so several smaller colonies near Tern Key were surveyed in 2007–08 and 2008–09. No individual nest data were available for the years 1988–89, 1991–92, 1992–93 and 1994–95 (for various reasons), however, summary statistics for mean hatch date and mean nest production were available. In most years a small number of spoonbills will nest a second time but in 1998–99, and from 2001–02 to 2005–06, the second nesting effort was sizable (almost as large or larger than the first nesting). These second nestings were surveyed using the above techniques as well, and treated the same as the first nestings.
Data Analysis
Prey Availability
Average density and biomass of fish were calculated for each sub-habitat (creeks and flats) at each site. The mean number of prey/trap from the sub-habitat with the largest number of prey collected was considered the estimate of available prey. The direct use of prey availability (i.e., abundance or biomass m−2) is confounded by the fact that each of the sites has a central creek that drains different sized watersheds and sites with larger drainage basins tended to have higher concentrations of fishes than smaller drainage basins. Concentration events would not be isolated to just the drainage in which our sites are located but would be spread over a region that site represents. If the simple estimate of fish density were used than sites with smaller basins would be masked by those with larger ones and it would appear that concentration events never occurred at the sites with smaller basins. In order to standardize the size of the catchment area we relativized each sample to the maximum abundance and biomass for each site. This created an index (on a 0-to-1 scale) for each site, hereby referred to as the fish density availability index or DAI and the biomass availability index or BAI.
Prey Concentration Threshold
The mean and standard deviation for all DAI estimates were calculated. All samples collected with a DAI > mean +1 SD were considered to be from a fish concentration event. June or September samples experiencing a concentration event were removed from the estimate of the PCT because the events were likely to be the result of thermal or oxygen stress rather than water level. The tidal sites of southern Biscayne Bay (MB, BS, CS and TP) were also problematic to estimating the PCT. This is because it takes up to 2 h to deploy all six traps and water level was only collected on an hourly basis so the actual depth at the time of trap deployment is unknown. As a result, concentration events at these sites were also removed from estimating the PCT. For the remaining concentration event samples, the daily mean water level was calculated for the date of the samples. The PCT was defined as the maximum depth at which a concentration event occurred.
Total Colony Nestling Period
The nestling period for each nesting cycle was defined as the period from 2 day before the first monitored nest hatched until 2 day after the last monitored nest had chicks reach 21 day post-hatch. The nestling period had to be estimated for years for which only the mean hatch date was available (Table 1). The mean difference in days from the first hatch date to the mean hatch date was 9 day and from the mean hatch date until the last chick reached 21 day was 40 day (Table 1). For years with only the mean hatch date available, the first hatch date and the date the last chick reached 21 day were estimated by subtracting 9 day and adding 40 day, respectively.
Mean Water Depth During the Nestling Period
For years that individual nests were monitored, the mean water level for the 21 day post-hatch was calculated for each nest from the long-term water level recording stations. The 4 years without individual nest data could not be included in calculating mean water depth for individual nests but were used to calculate mean depth for the entire nestling period.
Mean DAI and BAI for the Nestling Period
All fish samples that fell within the nestling period (Table 2) were used to calculate mean DAI and BAI for each nesting cycle. The number of samples collected during each cycle was highly variable with more samples collected as the study went on and fish sampling sites were added. Also, there were different sites used to estimate the DAI and BAI for each cycle, but bias caused by intra-site variation was removed by scaling by the maximum for each site (0 to 1 scale).
Statistical Analyses
Regressions were used to compare the linearity of water level with prey availability indices, water level with nest production and prey availability indices with nest production. Analysis of variance (ANOVA) was used to compare water levels with nest success (successful = a nest that produces ≥1 chick) and water levels with nest production (chicks/nest or c/n) for individual nests. The difference between DAI and BAI for failed and successful nesting cycles were also tested using ANOVA.
Results
Negative relationships were detected between mean water level and both mean nest production (r 2 = 0.41, p < 0.001) and nest success (r 2 = 0.31, p < 0.001; Fig. 3). ANOVA between the number of chicks produced and the mean water level for the nestling period of each individual nest were significant (F4,700 = 39.20, p < 0.001). Individual nests produced between 0 and 4 c/n and for each incremental increase in production water level was significantly lower (Fig. 4). All assumption for regression models and ANOVA were met.
I observed marked inter- and intra-annual variation in water level and corresponding prey abundance and biomass throughout this study’s 811 prey sampling events (results of individual collection are presented in the ESM 1). Regression models between water level and fish concentrations were not statistically significant confirming that the relationship between water level and prey availability was non-linear (Fig. 5). The mean and standard deviation of the DAI for these samples was 0.182 and 0.187 respectively. There were 40 samples with a DAI greater than the mean plus one standard deviation (0.369) and qualified for use in estimating the PCT (Table 3). The deepest water level that these concentration samples were collected in was 13.15 cm (collected at JB in April 2000) thereby defined as the PCT.
Regressions of the relationship between chick production and prey availability indices had mixed results (Fig. 6). There was a significant linear relationship between DAI and chick production (r 2 = 0.34, p < 0.001) but not between BAI and chick production (r 2 = 0.12, p = 0.11). ANOVA of DAI and BAI between failed (average <1 c/n) and successful (average >1 c/n) nesting cycles were significant (DAI: F(1,20) = 7.78, p < 0.05; BAI: F(1,20) = 4.62, p < 0.05), with successful nesting cycles having a significantly higher degree of available prey (Fig. 7). All assumptions for regression models and ANOVA were met.
Discussion
Results presented here suggest that prey do not concentrate linearly with decreasing water depth, rather, there is a depth threshold at which fish first become concentrated. In the mangrove NEFB wetlands this appears to occur when water levels drop below ~13 cm on the ephemeral wetland surface (i.e., the PCT). Previous studies have suggested these concentrated prey are rapidly depleted, primarily through predation (e.g., Kahl 1964; Master 1992; Gawlik 2002). These data also indicate that concentration events can occur at water levels as low as 5 cm below the wetland surface and at numerous depths in between (Table 3). As water levels continue to decline below the PCT, prey that survive the initial concentration event become re-concentrated at lower water, resulting in sequential concentration events at the same location (based on local topography). The concept of thresholds that concentrate fish explains, at least in part, why there is not a linear relationship between water level and fish prey availability. Kahl (1964) presented data that support the concept of a water level threshold for concentrating prey. He indicated that a 6 cm drop in water levels at a Wood Stork (Mycteria american) foraging site increased the density of prey fish from 50 m−2 to ~2,000 m−2. After dropping another 6 cm, the density remained about the same (2,200 fish m−2). This suggests that, at some level between the first and second recession events fish were forced to leave the adjacent wetland.
The regression results relating water level with nesting success concur with numerous other studies that, in southern Florida, nesting wading birds have a greater degree of nesting success at lower water levels (Kahl 1964; Frederick and Collopy 1989; Powell et al. 1989; Bancroft et al. 1994; Frederick and Spalding 1994; Hoffman et al. 1994; Ogden 1994; Lorenz et al. 2002). The estimation of the PCT at about 13 cm augments the results of the ANOVA of water level and nest production (Fig. 4) since failed nests had a mean water level and standard error above the PCT. Nests producing 1 c/n also had a mean just above the PCT but the standard error that spans below the PCT. Nests that produced 2, 3 and 4 c/n were foraging under conditions where the mean water level and standard error were below the PCT, and each incremental increase in productivity had significantly lower water level.
There was a linear relationship between DAI and nest production but not BAI and nest production (Fig. 6). Lorenz and Serafy (2006) documented that, at these sampling locations, the assemblage of fishes present is a better determinant of biomass than the density of fish present. Furthermore, they documented that salinity was the major determinant of the community structure with communities from lower salinity environments having larger biomass. Although it is intuitive that higher biomass should be more important than the total density of available fish for determining nest productivity, it is the density of fish that determines whether there is a concentration event or not. The fact that fish are concentrated at these sites may suggest that fish further upstream in lower salinity environments may be concentrated as well. These would have higher biomass and would also be readily exploited by nesting spoonbills. Thus, the fact that fish are concentrated may be a better indicator that foraging conditions are better throughout the landscape than the biomass that is available at these particular locations and times.
The ANOVA of DAI and BAI between failed and successful nesting cycles demonstrated that prey were more available during nesting cycles that resulted in an average of greater than 1 c/n than those that produced less than 1 c/n (Fig. 7). These results, in addition to the DAI regression model (Fig. 6) support the relationship between available prey on the primary foraging grounds with the ability of spoonbills nesting on islands in NEFB to raise chicks through the critical 21 day post hatch period.
Studies that relate water level to nesting success express or imply that this is the result of prey becoming more available to wading birds at lower water levels (Ogden et al. 1980; Powell 1987; Frederick and Collopy 1989; Frederick and Spalding 1994; Ogden 1994) however, few studies present any prey availability data. Conversely, many studies demonstrate that wading birds forage more successfully in areas where fish have been concentrated (Kahl 1964; Kushlan 1976b; Master 1992; Gawlik 2002), but rarely can this foraging success be related back to the success or failure of a specific colony or population although it is commonly inferred. Previous studies have demonstrated that spoonbills nesting in NEFB primarily forage in the wetlands where I measured water levels and collected prey samples (Bjork and Powell 1994; Lorenz et al. 2002; Lorenz unpublished satellite tacking data). By surveying spoonbill colonies so as to know nest production and identify the nestling period and by using numerical indices of prey collected on their primary foraging grounds during the nestling phase, links between lower water levels, greater prey availability and higher nest production were demonstrated.
Lorenz et al. (2002) demonstrated that, prior to anthropogenic alterations to the foraging habitats of Florida Bay, spoonbills produced an average of 2.25 c/n, resulting in an exponential increase in the number of spoonbill nests in Florida Bay. Since the completion of the SDCS in 1984, the average production has been 0.98 c/n (Table 1). De la Court and Aguilera (1997) indicated that Eurasian Spoonbills (Platalea leucordia) exhibit nesting fidelity to their natal colony location and that there is only a small degree of gene flow between discrete nesting populations. Similarly, I have found that Roseate Spoonbills in Florida likely occur in discrete nesting populations that are largely insular when it comes to immigration and emigration (unpublished banding and tracking data). It appears that the conditions that result in a production rate of <1 c/n are not able to sustain NEFB’s population thereby explaining the striking decline in nest numbers (Fig. 1).
Results indicate that if water management practices result in a reversal of the dry down process such that PCT is exceeded during the nestling period, prey will disperse and become unavailable to higher trophic levels. The high energetic demands of rapidly growing wading bird chicks (e.g. Kahl 1964) suggest that nesting attempts will likely fail if prey are unavailable for even a relatively brief period (2–3 day). Such reversals have occurred with regularity since the completion of the SDCS (Lorenz 2000), but in the last decade water management practices began to take into account environmental impacts and efforts were made to avoid such reversals. Spoonbill nesting success has been higher since this has happened (Lorenz and Dyer 2010).
Kotun and Renshaw (this issue) indicate that current operation of the SDCS have lowered wet season and increased dry season water levels in Taylor Slough and that this had similar hydrologic repercussions throughout NEFB. Data presented here indicate these conditions should result in lower prey production during the wet season and less prey availability during the nesting season. Therefore, the water management practices of recent decades likely had a significant role in the depressed nesting success and the declining population of spoonbills in Florida Bay. Given that numerous other species have been similarly affected (Lorenz, this issue) and that spoonbills are an indicator of ecosystem integrity for Florida Bay and the southern Everglades (Lorenz et al. 2009), the current efforts to restore natural flows are necessary and justified.
In conclusion, this study demonstrated that the relationship between water level and prey abundance was not linear but rather there is likely a threshold, or series of thresholds, in water level that result in prey concentrations. Furthermore, the study indicates that spoonbills require water level-induced concentrated prey in order to have enough food available to successfully raise young.
References
Allen RP (1942) The roseate spoonbill. Dover Publications Inc., New York
Bancroft GT, Strong AM, Sawicki RJ, Hoffman W, Jewell SD (1994) Relationships among wading bird foraging patterns, colony locations, and hydrology in the Everglades. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 615–658
Bjork RD, Powell GVN (1994) Relationships between hydrologic conditions and quality and quantity of foraging habitat for Roseate Spoonbills and other wading birds in the C-111 basin. Final report to the South Florida Research Center, Everglades National Park, Homestead, Florida
Browder JA, Gleason PJ, Swift DR (1994) Periphyton in the Everglades: spatial variation, environmental correlates and ecological implications. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 379–418
De la Court C, Aguilera E (1997) Dispersal and migration in Eurasian spoonbills. Ardea 85:193–202
DeAngelis DL, Loftus WF, Texler JC, Ulanowicz RE (1997) Modeling fish dynamics and effects of stress in a hydrologically pulsed ecosystem. J Aquat Ecosyst Stress Recover 6:1–13
Duever MJ, Meeder JF, Meeder LC, McCollom JM (1994) The climate of south Florida and its role in shaping the Everglades ecosystem. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 225–248
Dumas J (2000) Roseate Spoonbill (Ajaia ajaja). In: Poole A, Gill F (eds) The birds of North America, No. 490. The Birds of North America, Inc, Philadelphia
Frederick PC, Collopy MW (1989) Nesting success of five ciconiiform species in relation to water conditions in the Florida Everglades. Auk 106:625–634
Frederick PC, Loftus WF (1993) Responses of marsh fishes and breeding wading birds to low temperatures: a possible behavioral link between predator and prey. Estuaries 16:198–215
Frederick PC, Spalding MG (1994) Factors affecting reproductive success in wading birds. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 659–692
Gawlik DE (2002) The effects of prey availability on the numerical response of wading birds. Ecol Monogr 72:329–346
Herring G, Cook MI, Gawlik DE, Call EM (2011) Food availability is expressed through physiological stress indicators in nestling white ibis: a food supplementation experiment. Funct Ecol 25:682–690
Higer AL, Kolipinski MC (1967) Pull-up trap: a quantitative device for sampling shallow water animals. Ecology 48:1008–1009
Hoffman W, Bancroft GT, Sawicki RJ (1994) Foraging habitat of wading birds in the water conservation areas of the Everglades. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 585–614
Holmquist JG, Powell GVN, Sogard SM (1989) Sediment, water level and water temperature characteristics of Florida Bay’s grass-covered mud banks. Bull Mar Sci 44:348–364
Kahl MP (1964) Food ecology of the wood stork (Mycteria american) in Florida. Ecol Monogr 34:97–117
Kushlan JA (1976a) Environmental stability and fish community diversity. Ecology 57:821–825
Kushlan JA (1976b) Wading bird predation in a seasonally fluctuating pond. Auk 93:464–476
Kushlan JA (1978) Feeding ecology of wading birds. In: Sprunt A, Ogden JC, Winckler S (eds) Wading birds: research report no. 7 of the National Audubon Society. National Audubon Society, New York, pp 249–299
Kushlan JA (1980) Population fluctuations in Everglades fishes. Copeia 1980:870–874
Loftus WF, Eklund AM (1994) Long-term dynamics off an Everglades small-fish assemblage. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp 461–483
Loftus WF, Kushlan JA (1987) Freshwater fishes of southern Florida. Bull Florida State Mus Biol Sci 31:137–344
Lorenz JJ (1999) The response of fishes to physicochemical changes in the mangroves of northeast Florida Bay. Estuaries 22:500–517
Lorenz JJ (2000) Impacts of water management on roseate spoonbills and their piscine prey in the coastal wetlands of Florida Bay. Dissertation, University of Miami
Lorenz JJ, Dyer K (2010) Roseate spoonbill nesting in Florida Bay annual report 2009–10. South Florida Wading Bird Rep 16:13–16
Lorenz JJ, Serafy JE (2006) Changes in the demersal fish community in response to altered salinity patterns in an estuarine coastal wetland: implications for Everglades and Florida Bay restoration efforts. Hydrobiologia 569:401–422
Lorenz JJ, McIvor CC, Powell GVN, Frederich PC (1997) A drop net and removable walkway for sampling fishes over wetland surfaces. Wetlands 17:346–359
Lorenz JJ, Ogden JC, Bjork RD, Powell GVN (2002) Nesting patterns of roseate spoonbills in Florida Bay 1935–1999: implications of landscape scale anthropogenic impacts. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay and the coral reefs of the Florida Keys: an ecosystem sourcebook. CRC Press, Boca Raton, pp 555–598
Lorenz JJ, Langan-Mulrooney B, Frezza PE, Harvey RG, Mazzotti FJ (2009) Roseate spoonbill reproduction as an indicator for restoration of the Everglades and the Everglades estuaries. Ecol Indic 9S:S96–S107
Marmar HA (1954) Tides and sea level in the Gulf of Mexico. In: Galstoff PS (ed) Gulf of Mexico, its origin, waters, and marine life. U.S. Fishery Bulletin 89, Washington, pp 101–118
Master TL (1992) Composition, structure, and dynamics of mixed species foraging aggregations in a southern New Jersey salt marsh. Colon Waterbirds 15:66–74
Ogden JC (1994) A comparison of wading bird nesting colony dynamics (1931–1946 and 1974–1989) as an indication of ecosystem condition in the southern Everglades. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie press, Delray Beach, pp 533–570
Ogden JC, Kale HK, Nesbitt SA (1980) The influence of annual variation in rainfall and water levels on nesting by Florida populations of wading birds. Trans Linnaean Soc 9:115–126
Powell GVN (1987) Habitat use by wading birds in a subtropical estuary: implications of hydrography. Auk 104:740–749
Powell GVN, Bjork RD, Ogden JC, Paul RT, Powell AH, Robertson WB (1989) Population trends in some Florida Bay wading birds. Wilson Bull 101:436–457
Acknowledgments
I would like to thank Shawn E. Liston (National Audubon Society) for intellectual contributions as well for the final review. Peter C. Frederick (University of Florida) Dale E. Gawlik (Florida Atlantic University) and the late John C. Ogden also contributed intellectually. I would also like to express gratitude to the myriad dedicated and hard working staff members at TSC that participated in the collection of these data over the last 20 years.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 4397 kb)
Rights and permissions
About this article
Cite this article
Lorenz, J.J. The Relationship Between Water Level, Prey Availability and Reproductive Success in Roseate Spoonbills Foraging in a Seasonally-Flooded Wetland While Nesting in Florida Bay. Wetlands 34 (Suppl 1), 201–211 (2014). https://doi.org/10.1007/s13157-012-0364-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-012-0364-y