Abstract
We examined the genetic population structure of Spartina alterniflora in Jamaica Bay, Queens, NY and the surrounding area in order to assist the ongoing restoration of Jamaica Bay. AMOVA (Analysis of Molecular Variance) indicated that population differences accounted for 15% of molecular variance (Φ PT = 0.15, p = 0.001). Observed heterozygosity (Ho) ranged from 0.61 to 0.73 among populations. A Mantel test indicated a weak and non-significant correlation between pairwise Φ PT and geographic distance matrices (r = 0.34, p = 0.12). A PCA revealed no obvious grouping pattern for sampled populations. Based on these data, we determined that the studied populations contained similar genetic variability to other populations in the New York vicinity and to those of the entire region. It seems likely that collection of germplasm from within the region will prove sufficient in maintaining overall genetic variation in restoration plantings. Given the small amount of genetic structure among populations within Jamaica Bay, however, it would be prudent to collect widely within the target marsh. We also recommend the practice of propagating plugs of S. alterniflora from wild seed, as opposed to using vegetative cuttings, when creating planting stock, in order to maximize genetic diversity in restored marshes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large scale wetland creation and restoration efforts in the United States have been implemented to improve water quality, mitigate the loss of wildlife habitat, and offset losses from continuing deterioration of existing wetlands (Mitsch and Gosselink 2000). Spartina alterniflora Loisel. (smooth cordgrass) acts as an ecosystem engineer in restoration of tidal wetlands. Restoration of S. alterniflora wetlands has successfully stabilized dredge material, reduced shoreline erosion, and restored loss of wetland value, while promoting ecosystem development (Craft et al. 1999).
There has recently been increased interest in the planting of local genotypes for restoration projects in general (Lesica and Allendorf 1999; Perkins et al. 2002; Hufford and Mazer 2003; McKay et al. 2005) and for projects utilizing S. alterniflora in particular (Jones 2003). Specific genotypes of S. alterniflora vary in their growth habits and responses to environmental conditions. Seliskar et al. (2002) planted S. alterniflora from three geographic regions in a newly created salt marsh and compared performance among genotypes. They determined that above- and below-ground biomass, root and rhizome distribution, canopy height, stem density, and carbohydrate reserves were genotype-specific. They further showed that these genotypic differences translated into differentiation of ecosystem influence. Proffitt et al. (2003) planted populations of five genetically distinct genets in wetland plots. Colonization, growth, and clonal morphology all differed with respect to genotype, but were also influenced by elevation. At the same study site, Proffitt et al. (2005) determined that phenotypic and ecotypic variation of S. alterniflora permitted significantly different levels of light penetration into the canopy and influenced the degree of suppression or facilitation of recruitment for several other plant species. They suggest that habitat heterogeneity is dependent on S. alterniflora genotypic diversity and hypothesize that an increase in the genotypic diversity will lead to an increase in the number and diversity of interactions with other species. Because S. alterniflora genotype influences phenotype, and because traits such as germination response (Seneca 1974), phenology (Somers and Grant 1981), and ecosystem responses (Seliskar et al. 2002) correlate with geographic origin, maintenance of local genetic structure via the planting of local propagules should be routine in S. alterniflora marsh restoration.
In order to assess the full importance and practicality of using local propagule sources in marsh restoration, it is first necessary to determine local population structure of S. alterniflora. This will facilitate source tracking of genetic material and initiate formulation of a picture of gene flow. We undertook the present study to determine the genetic population structure of S. alterniflora in Jamaica Bay, Queens, NY and the surrounding area, specifically to assist the ongoing restoration of the salt marsh islands in Jamaica Bay by the U.S. Army Corps of Engineers and to determine the appropriate definition of ‘local’ propagules for marsh restoration. We used a set of polymorphic simple sequence repeat molecular markers (microsatellites) to determine whether any pre-existing genetic patterns were evident within and among marshes in the study area. Evidence of pre-existing genetic patterns may indicate potential for locally adapted populations, as well as barriers to gene flow, and would suggest geographic limitations to propagule collection, pending careful assessment of the impact of such genetic variation on performance.
Methods
Natural Populations Sampled
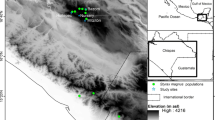
We obtained vegetative samples from marshes in New York (NY), New Jersey (NJ), Connecticut (CT), and Rhode Island (RI) (Fig. 1), encompassing the range of S. alterniflora that is expected to provide propagules for restoration in the NY City area. This includes four of the marsh islands within Jamaica Bay itself. Samples were collected from marshes in Narragansett, RI (N = 6), Barn Island, Stonington, CT (N = 6), Joco Island, Jamaica Bay, NY (N = 10), Big Egg Island, Jamaica Bay, NY (N = 9), Elders Point, Jamaica Bay, NY (N = 9), Floyd Bennett Field, Jamaica Bay, NY (N = 5), Cheesequake Sate Park, Mattawan, NJ (N = 9), and Cattus Island Park, Toms River, NJ (N = 6). We collected samples a minimum of 5 m apart and across the longest transect of each marsh whenever possible. We transported all samples to Rutgers University (New Brunswick, NJ) for DNA extraction from fresh leaf tissue.
Molecular Assay for Genetic Structure Analysis
We extracted DNA from all samples using the GenElute™ Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s specifications. We then amplified template DNA by PCR, according to the protocol described by Schuelke (2000), using microsatellite loci characterized for S. alterniflora (Blum et al. 2004; Sloop et al. 2006). Conditions of the PCR amplification were an initial heating of 94°C (5 min), followed by 30 cycles of 94°C (30 s)/56°C (45 s)/72°C (45 s), then 8 cycles of 94°C (30 s)/53°C (45 s)/72°C (45 s), and a final extension at 72°C for 10 min. Each PCR reaction included the attachment of a FAM, NED, PET, or VIC florescent label. We genotyped PCR products on an ABI 3130xl genetic analyzer (Applied Biosystems, Foster City, CA), using a LIZ 500 size standard. We identified and binned alleles using GeneMapper 3.7 software (Applied Biosystems, Foster City, CA).
Genetic Structure of Populations
We scored the following microsatellite loci for 56 sampled individuals from the eight populations: SPAR.06, SPAR.07, SPAR.08, SPAR.11, SPAR.15, SPAR.16, SPAR.19, SPAR.20, SPAR.22, SPAR.23, and SPAR.26 (Blum et al. 2004; Sloop et al. 2006). We then analyzed the resulting allelic data in genalex ver. 6 (Peakall and Smouse 2006). Heterozygosity is a widespread and biologically useful measure of genetic diversity in diploid species, including S. alterniflora, since each individual is either homozygous or heterozygous at a given locus. However, Hedrick (2005) notes that since heterozygosity measures have an upper limit of 1, it can be difficult to differentiate between populations for highly variable loci, including microsatellites, when heterozygosity is above 0.8. To correct for small and variable sample sizes we calculated a bias corrected effective number of alleles (n *e , Nielsen et al. 2003) in each population for comparative purposes. While heterozygosity is a traditional measure of genetic diversity in population genetics, Jost (2008) has shown that the effective number of alleles (here n *e ) has standard numeric behavior and is a more useful diversity measure. We conducted an Analysis of Molecular Variance (AMOVA, Excoffier et al. 1992) to quantify population structure (Φ PT), where Φ PT is a measure of population differentiation, calculated as the proportion of the variance among populations relative to the total variance, specifically \( {\Phi_{\rm{PT}}} = {{{{\hbox{V}}_{\rm{AP}}}} \mathord{\left/{\vphantom {{{{\hbox{V}}_{\rm{AP}}}} {\left( {{{\hbox{V}}_{\rm{AP}}} + {{\hbox{V}}_{\rm{WP}}}} \right)}}} \right.} {\left( {{{\hbox{V}}_{\rm{AP}}} + {{\hbox{V}}_{\rm{WP}}}} \right)}} \), where VAP is the variance among populations and VWP is the variance within populations. We used 999 permutations of the dataset to test for significance. We also generated a geographic distance matrix for the sampled populations and then compared it with the pairwise Φ PT matrix using a Mantel Test (Smouse et al. 1986). Significant similarity between a pairwise Φ PT matrix and geographic distance matrix is seen as evidence of isolation by distance, or limitation of gene flow by geographic separation. Finally, we conducted a Principal Coordinates Analysis (PCA) in SAS version 9.1 to determine whether observed patterns in the molecular data support the partitioning of the samples into specific groupings.
Results
The 11 microsatellite primer pairs yielded a total of 139 alleles and amplified between seven and 27 alleles per locus, with an average of 12.6 alleles per locus. Observed heterozygosity (Ho) ranged from 0.61 to 0.73, expected heterozygosity (He) from 0.46 to 0.75, and the effective number of alleles (n *e ) ranged from 2.06 to 6.78 (Table 1). Ho was highest at Barn Islands, CT and lowest at Narragansett, RI. n *e was highest at Floyd Bennett Field, NY and lowest at Cattus Island, NJ.
Spartina alterniflora is known to reproduce vegetatively via rhizomes (Callaway and Josselyn 1992; Proffitt et al. 2003). Nevertheless, since all sampled individuals represent unique genotypes and all sampled marshes exhibit relatively high measures of heterozygosity, sexual reproduction clearly remains an important factor in maintenance of marsh genetic variability and structure. Although S. alterniflora has been observed to have lower heterozygosity in the northern part of its natural range (Blum et al. 2007), no correlation between heterozygosity and geography was noted within the limited sampling range of this study.
The AMOVA indicated that the majority (85%) of molecular variance was found within populations. The distribution of microsatellite variation resulted in a Φ PT value of 0.15 (p = 0.001). These results are consistent with those from other wind pollinated, outcrossing grass species lacking major barriers to gene flow (e.g., Peakall et al. 1995; Kubik et al. 2001). We also performed a hierarchical AMOVA, in which we defined three regions: populations north of, within, and south of Jamaica Bay. We found no inter-regional variance, indicating that the molecular variance characterizes population differences, but shows no regional grouping within our study area. The Mantel test indicated no credible correlation between the Φ PT matrix and geographic distance matrix (r = 0.34, p = 0.12), again suggesting that populations showed no compelling geographic structure that might be compatible with isolation by distance. Spatial separation of S. alterniflora populations along a coastal continuum does not appear to be the dominating factor in determining microsatellite genetic structure over the limited latitudinal range of this study. The PCA (Fig. 2, Table 2) also revealed no obvious grouping pattern for the sampled populations.
Discussion
Genetic Structure of Populations Within the New York Metropolitan Area
Genotypic makeup of a S. alterniflora marsh is dictated by gene flow both within and among marshes. Although there is some molecular variance among marsh populations, the failure of the PCA to cluster genotypes from the same marsh may indicate that genetic exchange between marshes is common but not organized spatially. It seems likely that opportunities exist for genetic exchange between populations within the study area. This is not unexpected, considering that S. alterniflora is predominantly wind pollinated and that its seeds are dispersed over long distances by water and waterfowl (Vivian-Smith and Stiles 1994; Grevstad 2005).
There was one outlying set of genotypes from the Big Egg Marsh in Jamaica Bay, but since these samples were collected adjacent to several samples without distinct genotypes on the same marsh island, they do not seem to constitute a distinct population. Big Egg Marsh has previously been restored from propagules that were presumably collected from within the region (Dr. George W. Frame, Gateway National Recreation Area, personal communication), but the specific details of sample collection and propagation are unknown. It is possible that these outliers reflect genotypes introduced in that previous restoration. Unfortunately, since we do not have samples of the propagules used in previous restorations, we cannot evaluate this possibility.
Propagation Method
One of the most interesting findings of this study has been the lack of clonality observed among individuals sampled. Every sample tested showed a unique microsatellite genotype, even though some samples were collected less than 10 m apart, suggesting that the average size of a clonal patch was well below 10 m × 10 m. Our results are similar to those of Travis and Hester (2005), who showed that the maximum diameter of clonal patches in a Louisiana marsh could be as low as 13.9 m and that along some transects, the average distance between genotypes was less than 2 m. This level of sample diversity indicates that vegetative propagation of S. alterniflora is counterbalanced by seedling recruitment, in accord with a study of S. alterniflora clonal diversity in a Georgian salt marsh (Richards et al. 2004). Proffitt et al. (2005) and Seliskar et al. (2002) showed that genotypic diversity in S. alterniflora results in marsh habitat heterogeneity and increased overall ecosystem biodiversity. Our results thus support a strategy of planting genotypically diverse S. alterniflora plugs, derived from wild-collected seed, for marsh restoration, as advocated by Proffitt et al. (2003) and Travis et al. (2006), especially for isolated sites. Seed-derived plugs will provide substantially more diversity than vegetative propagules. As Travis et al. (2004) noted, this will have the added benefit of avoiding the threat of inbreeding and enhancing the ability to adapt to environmental disturbances.
What are ‘Local’ Propagules?
This study was conducted to answer the question: How should one define ‘local’ propagules in marsh restoration efforts? This question is important because source germplasm has become a topic of scrutiny for restoration efforts (e.g., Lesica and Allendorf 1999). If the goal of marsh restoration includes maintenance of the genetic structure of the pre-existing landscape, it is preferable to obtain genetically diverse propagules locally. The decision to maintain genetic structure is generally motivated by a concern for local adaptation, balanced by the need to allow for normal levels of gene flow, while avoiding maladapted genotypes (McKay et al. 2005). The term ’local’ only makes sense in relationship to some larger spatial delineation. In the particular context of Jamaica Bay, it is appropriate to define ‘local’, relative to S. alterniflora genetic structure along the eastern seaboard.
The most comprehensive delineation of S. alterniflora molecular genetic structure to date utilized Bayesian and AMOVA analysis of microsatellite variation and chloroplast haplotyping to subdivide the species into several broad regions: New England, North Mid-Atlantic, South Mid-Atlantic, South Atlantic and Gulf Coast, although precise boundaries between regions were not explicitly defined (Blum et al. 2007). Previous molecular studies of S. alterniflora indicated that the species does exhibit genetic structure over its entire range, compatible with isolation by distance (O’Brien and Freshwater 1999; Blum et al. 2007). Blum et al. (2007), in particular, showed that nearly all sampling sites along the eastern seaboard were significantly different from one another and that pairwise F ST values (analogous to our Φ PT estimates) were high, generally between 0.5 and 0.85. Interestingly, however, the New England and North Mid-Atlantic regions were exceptions, exhibiting low pairwise F ST values (0.04–0.1) and insignificant population differentiation.
The North Mid-Atlantic region includes Jamaica Bay, Queens, NY. While the significant Φ PT value of 0.15 indicates some level of divergence among marshes, the Mantel and PCA analyses showed that there was no clear pattern to that divergence that would suggest any specific boundaries between marsh populations and certainly no evidence of isolation by distance, at least within the limited geographic confines of this study. We would nevertheless suggest erring on the side of caution when collecting propagules for restoration. Because Jamaica Bay marshes contain comparable genetic diversity (Ho = 0.67–0.72, n *e = 3.47–6.78) to that of our entire sampled range (Ho = 0.67, n *e = 3.58), because Φ PT is significant (0.15, p = 0.001), and because wild collection of S. alterniflora has been mandated for the Jamaica Bay restoration, it would be prudent to sample widely from within Jamaica Bay to obtain propagules for the restoration. If collection within Jamaica Bay were not possible, for logistical reasons, collection from within the entire sampling range of this study would probably be reasonable. The larger regional results (Blum et al. 2007), however, suggest that source material for Jamaica Bay should not be collected from beyond the North Mid-Atlantic region.
In collecting source material for restoration, it is also appropriate to note that genetic structure, measured with neutral markers (such as microsatellites), does not necessarily reflect adaptive traits. It remains to be determined whether the observed genetic structure of S. alterniflora populations is merely the result of the usual balance between genetic drift and gene flow, or whether it is, at least in part, an indicator of biogeographically divergent adaptation. To address that issue over the entire range of S. alterniflora, Travis and Grace (2010) transplanted plants from 23 sites (from Maine to Texas) to a mudflat at the Sabine National Wildlife Refuge in Louisiana. They determined that S. alterniflora exhibited adaptations on a regional scale and recommended that donor materials be obtained from a maximum of 300 km distance from restoration sites. The critical next steps in this line of enquiry would be to conduct additional common garden experiments, reciprocal plantings, and adaptability studies within regions, and even (perhaps) within marshes, to determine the spatial scale over which adaptational differences are evident in S. alterniflora marshes.
In conclusion, S. alterniflora propagules could be defined as ‘local’, and should preserve the genetic integrity of the pre-existing landscape, as long as they originate from within the same region (sensu Blum et al. 2007) as the target marsh, but are no further than 300 km from the target marsh (Travis et al. 2006; Travis and Grace 2010), and contain ample genetic diversity. In the absence of a direct assessment of local adaptation for each restoration site and source, this strategy will maximize the chance that a restored marsh will play its intended ecological role.
References
Blum MJ et al (2004) Characterization of microsatellite loci in Spartina species (Poaceae). Molecular Ecology Notes 4:39–42
Blum MJ et al (2007) Geographic structure, genetic diversity and source tracking of Spartina alterniflora. Journal of Biogeography 34:2055–2069
Callaway JC, Josselyn MN (1992) The introduction and spread of smooth cordgrass (Spartina alterniflora) in South San Francisco Bay. Estuaries 15:218–226
Craft C et al (1999) Twenty-five years of ecosystem development of constructed Spartina alterniflora (Loisel) marshes. Ecological Applications 9:1405–1419
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Grevstad FS (2005) Simulating control strategies for a spatially structured weed invasion: Spartina alterniflora (Loisel) in Pacific Coast estuaries. Biological Invasions 7:665–677
Hedrick PW (2005) Genetics of Populations, 3rd edn. Jones and Bartlett, Sudbury
Hufford KM, Mazer SJ (2003) Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends in Ecology and Evolution 18:147–155
Jones TA (2003) The restoration gene pool concept: beyond the native versus non-native debate. Restoration Ecology 2:281–290
Jost L (2008) GST and its relatives do not measure differentiation. Molecular Ecology 17:4015–4026
Kubik C et al (2001) Genetic diversity in seven perennial ryegrass (Lolium perenne L.) cultivars based on SSR markers. Crop Science 41:1565–1572
Lesica P, Allendorf FW (1999) Ecological genetics and the restoration of plant communities: mix or match? Restoration Ecology 7:42–50
McKay JK et al (2005) “How local is local?”—A review of practical and conceptual issues in the genetics of restoration. Restoration Ecology 13:432–440
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. Wiley, New York
Nielsen R, Tarpy DR, Reeve HK (2003) Estimating effective paternity number in social insects and the effective number of alleles in a population. Molecular Ecology 12:3157–3164
O’Brien DL, Freshwater DW (1999) Genetic diversity within tall form Spartina alterniflora Loisel. along the Atlantic and Gulf Coasts of the United States. Wetlands 19:352–358
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6:288–295
Peakall R, Smouse PE, Huffs DR (1995) Evolutionary implications of allozyme and RAPD variation in diploid populations of dioecious buffalograss Buchloë dactyloides. Molecular Ecology 4:135–147
Perkins EJ et al (2002) Development of amplified fragment length polymorphism markers for Spartina alterniflora. Aquatic Botany 74:85–95
Proffitt CE et al (2005) Spartina alterniflora genotype influences facilitation and suppression of high marsh species colonizing an early successional marsh. Journal of Ecology 93:404–416
Proffitt CE, Travis SE, Edwards KR (2003) Genotype and elevation influence Spartina alterniflora colonization and growth in a created salt marsh. Ecological Applications 13:180–192
Richards CL et al (2004) Unexpectedly high clonal diversity of two salt marsh perennials across a severe environmental gradient. Ecology Letters 7:1155–1162
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology 18:233–234
Seliskar DM et al (2002) The regulation of ecosystem functions by ecotypic variation in the dominant plant: a Spartina alterniflora salt-marsh case study. Journal of Ecology 90:1–11
Seneca ED (1974) Germination and seedling response of Atlantic and Gulf coasts populations of Spartina alterniflora. American Journal of Botany 61:947–956
Sloop CM et al (2006) Characterization of 24 additional microsatellite loci in Spartina species (Poaceae). Conservation Genetics 6:1049–1052
Smouse PE, Long JC, Sokal RR (1986) Multiple regression and correlation extension of the Mantel test of matrix correspondence. Systematic Zoology 82:561–573
Somers GF, Grant D (1981) Influence of seed source upon phenology of flowering of Spartina alterniflora Loisel. and the likelihood of cross pollination. American Journal of Botany 68:6–9
Travis SE, Hester MW (2005) A space-for-time substitution reveals the long-term decline in genotypic diversity of a widespread salt marsh plant, Spartina alterniflora, over a span of 1500 years. Journal of Ecology 93:417–430
Travis SE, Grace JB (2010) Predicting performance for ecological restoration: a case study using Spartina alterniflora. Ecological Applications 20:192–204
Travis SE, Proffitt CE, Ritland K (2004) Population structure and inbreeding vary with successional stage in created Spartina alterniflora marshes. Ecological Applications 14:1189–1202
Travis SE, Proffitt CE, Edwards KR (2006) Genetic considerations for the restoration of smooth cordgrass (Spartina alterniflora) within its native range: U.S. Geological Survey Open-File Report 2006–1305
Vivian-Smith G, Stiles EW (1994) Dispersal of salt marsh seeds on the feet and feathers of waterfowl. Wetlands 14:316–319
Acknowledgments
Funding for this project was provided by the U.S. Army Corps of Engineers, U.S. Department of Agriculture Natural Resources Conservation Service (USDA NRCS), Rutgers Center for Turfgrass Science, New Jersey Agricultural Experiment Station, and Rutgers University School of Environmental and Biological Sciences. Field collection was aided by Joseph Lampert, Christine Poulsen, and the USDA Plant Materials Center, Cape May, NJ. Figure 1 was drawn by Drew Siglin. We thank Dr. Rikke Bagger Jørgensen of Risø National Laboratory in Denmark and two anonymous reviewers for helpful suggestions on previous versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s13157-010-0086-y
Rights and permissions
About this article
Cite this article
Novy, A., Smouse, P.E., Hartman, J.M. et al. Genetic Variation of Spartina alterniflora in the New York Metropolitan Area and Its Relevance for Marsh Restoration. Wetlands 30, 603–608 (2010). https://doi.org/10.1007/s13157-010-0046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-010-0046-6