Abstract
In the Kavakköy region located at Southwest of Konya (central Turkey), four Quaternary travertine mounds and two recent travertine deposition sites are roughly aligned along the Seydişehir Fault Zone. Water temperature of recent travertine sites is about 39 and 19 °C. Six different facies were determined from the Kavakköy Travertine: crystalline crust travertine, paper-thin raft travertine, coated bubble travertine, pisoid, lithoclast travertine and shrub travertine facies. Sedimentological, morphologic and geochemical characteristics of these facies point toward depositions in slope and depressional depositional systems. Rare element content and isotopic values of different facies are distributed on diagrams as two separate clusters. After comparing with two recent travertines, it is hypothesized that they are most probably related to the temperatures of travertine formation water. High δ13C contents and calculated δ13C values, using Panichi and Tongiorgi’s (in: Proceedings of the 2nd UN symposium on the development and use of geothermal resources, San Francisco, CA, 20–29 May 1975, pp. 815–825, 1976) equation, imply that parent water was charged with CO2 from a deep origin. Significant differences in the δ13C and δ18O isotopic values of recent travertines at two different sites have been interpreted as being related to water circulation path and source of CO2. The travertine precipitated by water with a temperature of 19 °C has comparatively short and shallow fluid flow paths and low (normal) heat flow and has CO2 isotopic signatures, indicating a comparatively large quantity of CO2 contribution from decarbonation of limestone. In contrast, the travertine formed by relatively hot water (39 °C) had been conductively heated during percolating through thick Paleozoic–Cenozoic sequence and has incorporated a comparatively large quantity of CO2 derived from mantle sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Travertine is a continental carbonate rock deposited around the springs having water rich in Ca and bicarbonate (Guo and Riding 1998). Pentecost (2005) classified fresh water carbonates into two groups”: “thermogene carbonates”, which are formed from hot water and “meteogene carbonates”, which are formed from cold water. Many authors have accepted that travertine is derived from hot water (Chafetz and Lawrence 1994; Altunel 1996; Guo and Riding 1998; Fouke et al. 2000; Özkul et al. 2002, 2013; Koşun 2012). Travertine is hard, crystalline, generally laminated and less porous carbonates. On the other hand, tufa is cold water carbonate. It is generally highly porous and contains higher plant and animal remains (Pedley 1990; Ford and Pedley 1996; Guo and Riding 1998). Travertine deposits are commonly associated with normal or transtensional faults (Altunel 1996; Hancock et al. 1999; Brogi and Capezzuoli 2009; Özkul et al. 2013) as they are in this study. The depositional architecture, geochemical characteristics and precipitation of travertine are mainly controlled by (i) mechanical factors such as the pCO2 of the spring water when discharged at its vent (Herman and Lorah 1987; Lorah and Herman 1988; Chafetz and Lawrence 1994; Barilaro 2012); (ii) the area and depth of water on the discharge apron (Dandurand et al. 1982; Barilaro 2012); (iii) the amount of water agitation and turbulence (Jacobson and Usdowski 1975; Lorah and Herman 1988; Barilaro 2012); (iv) aeration, jet-flow, and low-pressure effects (Zhang et al. 2001; Barilaro 2012), increasing temperature and evaporation and biological factors such as photosynthetic CO2 uptake and other physiological activities of plants, bacteria living in hot water (Barilaro 2012); (v) climate, spring water composition and (vi) the source of CO2.
Different travertine facies have been determined at different geographic settings and depositional environments (Chafetz and Folk 1984; Guo and Riding 1998; Minissale et al. 2002; Özkul et al. 2002; Pentecost 2005; Rainey and Jones 2005; Gandin and Capezzuoli 2008; Kele et al. 2011; Barilaro 2012; Koşun 2012). To find which factors were influential may be achieved by comparing recent spring deposits at differing geological settings.
This study describes and elucidates the depositional, mineralogical, and geochemical characteristics of four Quaternary travertine mounds and two recent travertine sites around Kavakköy (Konya, central Turkey) with the view of establishing factors that controlled their development. The data obtained from this study will be used in evaluating similar deposits throughout the World.
Geological setting
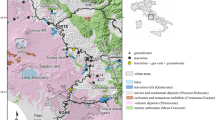
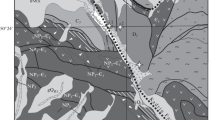
The study area is located at southwest of Kavakköy, 15 km to north of Seydişehir (Konya, central Turkey; Fig. 1). The Early–Middle Cambrian Çaltepe Formation is the oldest unit in the study area and comprises weakly metamorphosed recrystallized limestone and dolomite. The Seydişehir Formation, which rests conformably on the Çaltepe Formation, is Late Cambrian-Early Ordovician in age and is represented by alternated metasandstone, shale and phyllite. The Triassic-Lower Cretaceous carbonates come unconformable on these units (Özgül 1976). The Miocene–Pliocene volcanic rocks are andesitic–basaltic in character and cover a large area at the eastern site of the study area (Fig. 1; İnnocenti et al. 1975). The Neogene Taşlıca Formation lies unconformably on the above-mentioned units and comprises highly fossiliferous, fractured thin-bedded limestone and weakly consolidated sandstone, mudstone and marl. Quaternary travertines occur as mounds, which are roughly aligned in a row along the Seydişehir Fault Zone. Recent travertine deposition is taking place around the output of an artificial artesian well (230 m deep) and a spring located 2 km further. The temperature and the pH of the well and spring water are 39 °C; 6.51 and 19 °C; 6.41, respectively.

Modified after Özgül (1997)
Simplified geological map of the study area.
The Bozkır and Hadim nappes formed in relation to the closing of the Neo-Tethys ocean as well as the Seydişehir Fault Zone (SFZ) developed during the neo-tectonic period are the main tectonic features in the study area (Özgül 1976, 1997; Koçyiğit 1984). The Seydişehir Fault Zone extending NW–SE in the study area can be followed for 7–8 km and it cut across travertine mounds around Kavakköy (Fig. 1).
Methods
The sedimentological characteristics and lateral–vertical relations of travertine and tufa (?) facies were determined during field studies by recognizing the characteristics introduced by Guo and Riding (1998). Systematic samples were collected from all travertine facies in quarries (Fig. 2a) opened on travertine mounds for mineralogical, petrographical, geochronologic and geochemical evaluation. The temperature and pH of two water outlets in the study area were measured by means of Hanna Combo pH&EC instruments and recent tufa deposits were also sampled (Fig. 2b).
Of 104 samples, 23 samples were subjected to elemental analysis, 56 samples for stable isotope analysis, 3 samples for U-Th dating and 83 samples for XRD analysis. Scanning electron microscope investigations were performed on gold coated samples using a “Zeiss evo Is 10” microscope. Mineralogical compositions of the randomly oriented powdered travertine samples were determined by X-ray powder diffraction (XRD) technique using a Bruker D-8 Advance diffractometer with CuKα radiation at 40 kV and 40 mA. The SEM and XRD analyses were performed at the ILTEK center of Selcuk University, Konya, Turkey. Stable carbon and oxygen isotope measurements of the travertines and four water samples were performed at the Institute for Geological and Geochemical Research Institute, Hungarian Academy of Sciences, Budapest, Hungary. Carbon and oxygen isotope analyses of bulk travertine samples were carried out using both the conventional phosphoric acid method (H3PO4 digestion method at 25 °C) of McCrea (1950) and the continuous flow technique of Spötl and Vennemann (2003). δ 18O analysis of waters was conducted using the CO2–water equilibration method of Epstein and Mayeda (1953). All samples were measured at least in duplicate and the mean values are given in the standard δ notation in parts per thousand (‰) relative to Vienna Pee Dee Belemnite (VPDB; δ13C and δ18O) and Vienna Standard Mean Ocean Water (VSMOW). 50–100 mg of gently crushed, ultrasonically treated and dried three samples, which were chosen among compact, light colored and primarily deposited samples, were subjected to U-Th dating analysis (Shen et al. 2012) at the High-precision Mass Spectrometry and Environment Change Laboratory (HISPEC), Department of Geosciences, National Taiwan University, Taipei, Taiwan.
Facies of the Kavakköy Travertine
Travertine facies and their depositional environments have been defined by many scientists from different parts of the world and in different geological settings (Chafetz and Folk 1984; Guo and Riding 1998; Minissale et al. 2002; Özkul et al. 2002, 2013, 2014; Pentecost 2005; Rainey and Jones 2005; Gandin and Capezzuoli 2008; Kele et al. 2011; Koşun 2012; Orhan and Kalan 2015). Six different facies based on Guo and Riding (1998) classification were determined in the Kavakköy Travertine.
Crystalline crust facies
This facies is made up of a few millimeters to centimeter thick layers of coarse radial calcite crystals which were oriented with their long axis perpendicular to the growing surface. It is white and compact and has a few tens of meters of lateral continuity. Its thickness is in the range of 80–115 cm. Vertically elongated voids were observed in some levels (Fig. 3a). Microscopic analysis reveals that the crystalline crust facies is formed by finely laminated dendritic architectures exhibiting, in general, well-developed fan-shaped morphologies (Fig. 3b, c).
Interpretation: Crystalline crust facies develops on a low to high angle, mainly with dips of 10° ± 4°, non-terraced smooth slopes and reflects that deposition took place in an agitated and rapidly flowing water (Guo and Riding 1998). Thick layers of crystalline crust represent fast deposition in relatively fast flowing water and thin layers represent slow precipitation in a relatively slow flow (Folk et al. 1985; Guo and Riding 1992, 1998; Özkul et al. 2002, 2014; Jones and Renaut 2010).
Raft facies
Paper-thin raft facies is characterized by white to cream colored, thin, delicate, brittle and flat crystalline layers. Raft deposits are often associated with the coated bubble facies (Fig. 4a). Recent raft formations were observed on the surface of pool water in the study area (Fig. 4b).
Interpretation: Paper-thin rafts, also known as “hot water ice” (Allen and Day 1935) and “calcite ice” (Bargar 1978), are developed on the surface of stagnant water bodies where surface degassing of CO2 causes an increase in saturation re CaCO3 leading to calcite or aragonite precipitation (Baker and Fiosticks 1951; Folk et al. 1985; Chafetz et al. 1991; Guo and Riding 1998; Özkul et al. 2002). They generally occur as a few individual sheets but locally they are found as dense concentration by accumulations on the floor of small stagnant pools and as sheets of still water (Guo and Riding 1998; Barilaro 2012).
Coated bubble facies
The coated bubble facies consists of sub-spherical to circular or elongated micritic/microsparitic coatings around porous structures with a diameter ranging from 2 mm to 2 cm (Fig. 5a). Some of gas hollows are filled with sparitic calcite (Fig. 5b). This facies is generally observed beneath the raft facies.
Interpretation: Coated bubbles mainly form in places, such as near pool surfaces below paper-thin rafts, or among crystals or vegetation in pools and porous sediments, where bubbles are trapped (Guo and Riding 1998; Özkul et al. 2002; Barilaro 2012). The source of these bubbles is mainly from microbial activity in underlying sediments but in some places the degassing of the water near its outlet produces bubbles as it was seen in the study area.
Pisoid facies
This facies comprises white or pale yellow pisoids ranging in shape from spherical to irregularly rounded and varies in size from a few millimeters to 2 cm (Fig. 6a). Recent pisoids are present in small pool of micro terrace (Fig. 6b). Generally, they have a sparitic core and concentrically laminated micrite/sparit interlaminates (Fig. 6c). They are either loose or consolidated with micrite (Fig. 6d) and show very little abrasion.
Interpretation: Pisoids in a travertine may form as (1) concentrically laminated grains (Folk and Chafetz 1983); (2) dendroids (Folk and Chafetz 1983); and (3) stromatolitic mammillate (Guo and Riding 1998). The concentrically laminated pisoids present in the study area have been interpreted as being formed in splashing and turbulent water and have been regarded as inorganic precipitates (Folk and Chafetz 1983; Guo and Riding 1998; Barilaro 2012).
Lithoclast facies
Lithoclast travertine facies is interbedded with other light colored facies (Fig. 7a) and comprises light colored clasts of travertine, mainly from crystalline crust facies (Fig. 7b), mixed with a dark orange to brown colored micritic matrix. It has a thickness ranging from 20 cm to 1 m.
Interpretation: Lithoclast travertine facies develops either as a result of penecontemporaneous erosion of slope travertines and deposition of fragments from terrace rims or collapsed crystalline travertine on the lower slopes and depressions, where it mixes with in situ precipitates, or as a result of the collapse of low cliffs, as in waterfalls (Chafetz and Folk 1984; Casanova 1986; Pentecost 1990; Guo and Riding 1994, 1998).
Shrub facies
This facies is characterized by small shrub-like growth. Shrubs are a few millimeters to a few centimeters in size, white to gray in color and, show a dendritic morphology with vertically aligned calcite crystals (Fig. 8a). It occurs typically as several layers, 1–3 cm thick, which are separated by thin micritic laminae (Fig. 8b).
Interpretation: Three types of shrubs were defined by Chafetz and Guidry (1999). Bacterial shrubs have highly irregular forms, while crystal shrubs and ray crystal crusts display regular geometric patterns. They interpreted that the bacterial and crystal shrubs are bacterially mediated, while ray crystal crusts are formed by a combination of bacterial and abiotic processes. Shrub travertine facies in the study area has been interpreted as crystal shrub and as being deposited in depressions where the water energy is low.
Depositional systems of facies
Three depositional systems for travertine have been introduced by Guo and Riding (1998) for Rapolano Terme travertine in Italy. These depositional systems include (i) the slope depositional system; (ii) the depression depositional system; and (iii) the Reed mound depositional system.
The sedimentological, morphological and mineralogical characteristics of facies in the Kavakköy Travertine point to the fact that they were developed in slope and depressional depositional systems (Fig. 9). Also, the absence of unconformities and paleosol supports this interpretation.

Adapted from Özkul et al. (2002)
Depositional model for Kavakköy Travertine (Tkk: crystalline crust facies, Tp: pisoid facies; Ts: raft facies, Tgk: coated bubble facies, Tç: shrub facies, Tlk: lithoclast facies).
Travertine geochemistry
Major and trace elements
All facies are almost composed of pure calcite (in XRD analysis, Fig. 10) and contain very little detrital material. The major oxide, trace and rare earth element contents of recent travertine around two water outlets and four Quaternary travertine mounds in the study area are given in Tables 1, 2 and 3. The CaO content of Quaternary travertines ranges between 44.92 and 55.86%, while the content of recent travertines around hot water outlet changes between 52.6 and 54.63% and those around cold water changes between 54.43 and 54.61%. MgO content of the older travertines changes between 0.17 and 0.98% while the MgO of the recent travertines ranges from 0.30 to 0.50% mol.
There are positive correlations between Al2O3, Fe2O3, SiO2 and total REE contents, whereas there are negative correlations between REE and CaO contents (Fig. 11a–e). Even though trace element contents of different facies are slightly distinctive, there are significant differences in trace elements between recent and older travertines (Table 2).
The results of analyses show no significant differences in the rare earth element content and isotopic values of different facies. But on the diagrams drawn using CaO versus rare earth element content (Sr, Ba, As, Zr, Y, Zn, Rb), two separate clusterings were realized. These groupings are interpreted as being most probably related to the temperatures of travertine formation water (Fig. 12a–g).
Stable isotopes
The Quaternary travertine samples in the study area have δ18O values ranging from − 14.6 and − 6.8‰ (VPDB) and recent travertines between − 13.5 and − 8.7‰ (VPDB); similarly, older travertine samples have δ13C values between + 0.2 and + 8‰ (VPDB) and recent travertine between + 5.4 and + 8 ‰ (VPDB) (Table 4). Based on the δ13C and δ18O composition (Pentecost 2005; Teboul et al. 2016), the studied travertines are mainly classified as hypogenic travertine. High δ13C values imply that the parent water was charged with CO2 from a deep origin, which is either associated with decarbonation of carbonate resulted from thermal, magmatic and metamorphic processes or with magmatic CO2 from active volcanism (Kele et al. 2011; Teboul et al. 2016).
Discussion
The sedimentological and mineralogical characteristics of six different facies in the Kavakköy Travertine, which were developed around a water discharge area related to the Seydişehir Fault Zone point that the Kavakköy Travertine was deposited on a slope and depression depositional setting. Similar interpretation was proposed for Rapolano Terme travertine in Italy (Guo and Riding 1998) and for Pamukkale travertine in Denizli, Turkey (Özkul et al. 2002, 2013).
Of the six facies determined from the Kavakköy Travertine, Crystalline crust facies was formed through rapid precipitation at the terrace pool rims and on the slopes where the water flows fast. Paper-thin raft facies and coated bubbles facies were developed on the calm water bodies in terrace pools and depressions. The pisoidic facies was deposited in agitated terrace pools and large depressions with biotic and abiotic processes. Lithoclast travertine facies formed as a result of deposition of fragments broken from terrace rims or collapsed crystalline travertine facies in terrace pools or depressions. The shrub travertine facies is commonly deposited on subhorizontal surfaces on terrace pools, depressions and slope flats (Fig. 9).
Generally, travertine has more positive δ13C (VPDB) values, in the range of − 1 to 10‰ (Pentecost 2005; Koşun 2012; Özkul et al. 2014), than tufa. Having different isotopic values is acceptable because, in a travertine depositional environment, several differing facies may develop under differing conditions (Özkul et al. 2002). The differences in travertine isotope geochemistry are controlled by physical and chemical processes such as CO2 degassing, cooling water, evaporation, temperature, saturation, water flow, biological activities and photosynthesis, the origin of CO2, type of bedrock and karstic reservoir, tectonism, local climate, distance to the hydrothermal vents (Minissale et al. 2002; Pentecost 2005; Kele et al. 2011; Barilaro 2012; Özkul et al. 2014; Teboul et al. 2016). Negative δ18O values are resulted from high saturation and high temperature. Similarly, light isotopes (δ16O and δ12C) in the water may be removed from the system by biological activities and photosynthesis, leading to δ18O and δ13C enrichment in the water and the resultant carbonates (Barilaro 2012; Kele et al. 2011).
On the δ18O (VPDB) versus δ13C (VPDB) diagram, the recent samples from the hot water artesian site (39 °C) and those from the relatively cool spring site (19 °C) clustered into separate fields and, samples from crystalline crust facies are clustered around the field of hot water samples whereas other facies are scattered between these two fields (Fig. 13). The stable isotope values of the travertine at the Kavakköy region fall in the positive field on the diagram introduced by Gandin and Capezzuoli (2008) (Fig. 14), which points that the Kavakköy Travertine, especially the crystalline crust facies, was deposited by relatively hot water.
Combined plot of δ18O (VSMOW/VPDB) and δ13 (VPDB) values derived from carbonates presently forming in terrestrial (travertine, calcareous tufa, speleothems) and marine (skeletal-ooid sediments and pelagic muds) environments and from lithified marine limestones (Gandin and Capezzuoli 2008)
The significant differences in the isotopic values of the recent travertines from two different sites having a water temperature of 19 °C and the other 39 °C are related to water circulation path and source of CO2 (Table 4, Fig. 13). The travertine precipitated by water with a temperature of 19 °C has comparatively short and shallow fluid flow paths and low (normal) heat flow and has CO2 isotopic signatures, indicating a comparatively large quantity of CO2 contribution from decarbonation of limestone. In contrast, the travertines formed by relatively hot water (39 °C) which were conductively heated during percolation through the thick Paleozoic–Cenozoic sequence have incorporated a comparatively large quantity of CO2 derived from mantle sources. The Quaternary travertine deposits are scattered as mounds along the Seydişehir Fault Zone and are considered as being formed in relation with this fault zone. Therefore, the differences in stable isotope values in the Quaternary travertine are also interpreted to be the result of different water circulation paths.
The composition of isotopic carbon in travertine changes depending on the main source of CO2 (Minissale, 2004). Panichi and Tongiorgi (1976) proposed an equation (\(\delta^{13} {\textrm{C}}_{({\text{CO}}_2 )} = 1.2\delta^{13} {\textrm{C}}_{\text{ (trav}.)}- 10.5\)) for recalculating the original δ13C values of CO2 from the measured δ13C values in fossil travertines on the basis of data obtained from of Italian thermal springs and associated travertines. The studied travertine samples have high δ13C, between + 0.2 and + 8‰ (VPDB), and the calculated δ13C values, using the Panichi and Tongiorgi’s (1976) equation, range from − 10.3 to − 0.7‰ (VPDB). Both measured and calculated δ13C values imply that the parent water of the Kavakköy Travertine was charged with CO2 mainly from a deep mantle source (Figs. 15, 16; Minissale et al. 2002) with a little contribution from decarbonation of carbonate bedrocks (i.e., Paleozoic marbles and Miocene lacustrine carbonate).

Modified after Minissale et al. (2002)
A simplified model for sources of CO2 in two recent travertine deposition sites in the study area (Location 1: hot water artesian well; Location 2: relatively cold spring).
δ13C of CO2 vs. δ13C in CaCO3 from actively depositing travertine areas (black squares) and δ13C values of CO2 ‘recalculated’ from δ13C values (gray circles) of travertines according to the formula shown at the top of the diagram (Minissale et al. 2002)
Conclusions
-
1.
In the study area, six different facies were determined from four Quaternary travertine mounds which are roughly aligned along the Seydişehir Fault Zone. These are crystalline crust travertine, paper-thin raft travertine, coated bubble travertine, pisoid, lithoclast travertine, shrub travertine facies.
-
2.
The sedimentological, morphologic and geochemical characteristics of travertine facies point that the depositions took place in slope and depressional depositional systems.
-
3.
The δ18O (VPDB) values indicate that the deposition of the facies in the Kavakköy Travertine is closely related to the water temperature. The crystalline crust facies was deposited by hot water, while shrub facies were deposited by relatively warm water.
-
4.
High δ13C and the calculated δ13C values of the Kavakköy Travertine has been explicated as that the majority of CO2 was derived from a mantle source and that CO2 contribution from metamorphism of limestone during thermometamorphic decarbonation of the Paleozoic and Mesozoic carbonate basement rocks was low.
References
Allen ET, Day AL (1935) Hot springs of the Yellowstone National Park. Carnegie Inst. Washington Pub. 466
Altunel E (1996) Pamukkale Travertenlerinin morfolojik özellikleri, yaşları ve neotektonik önemleri. MTA Dergisi 118:47–64
Baker G, Fiosticks AC (1951) Pisoliths, ooliths and calcareous growths in limestone caves at Port Campbell Victoria, Australia. J Sediment Petrol 21:85–104
Bargar KE (1978) Geology and thermal history of Mammoth hot springs Yellowstone National Park Wyoming, U.S. Geol Surv Bull 1444:1–55
Barilaro F (2012) The character and spatial distribution of Holocene and Pleistocene hydrothermal travertines Albegna Valley Southern Tuscany, Central Italy. Ph. D Thesis, Unıversita Degli Studi Milano, p 176
Brogi A, Capezzuoli E (2009) Travertine deposition and faulting: the fault related travertine fissure ridge at Terme S Giovanni, Rapolano Terme, Italy. Int J Earth Sci 98:931–947
Casanova J (1986) East African Rift stromatolites, In: Frostick RW (ed) Sedimentation in the African Rifts, Geological Society of London Special Publication 25. London, pp 201–210
Chafetz HS, Folk RL (1984) Travertines: depositional morphology and the bacterially constructed constituents. J Sediment Petrol 54:289–316
Chafetz HS, Guidry SA (1999) Bacterial shrubs, crystal shrubs and ray-crystal shrubs: bacterial vs. abiotic precipitation. Sediment Geol 126:57–74
Chafetz HS, Lawrence JR (1994) Stable isotopic variability within modern travertines. Geogr Phys Quat 48(3):257–273
Chafetz HS, Rush PF, Utech NM (1991) Microenvironmental controls on mineralogy and habitat of CaCO3 precipitates: an example from an active travertine system. Sedimentology 38:107–126
Dandurand JL, Gout R, Hoefs J, Menschel G, Schott J, Usdowski E (1982) Kinetically controlled variations of major components and carbon and oxygen isotopes in a calcite-precipitating spring. Chem Geol 36:299–315
Epstein S, Mayeda T (1953) Variation of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:89–103
Folk RL, Chafetz HS (1983) Pisoliths (pisoids) in quaternary travertines of Tivoli, İtaly. In: Peryt TM (ed) Coated grains. Springer, Berlin, pp 474–487
Folk RL, Chafetz HS, Tiezzi PA (1985) Bizarre forms of depositional and diagenetic calcite in hot spring travertines, Central Italy: In: Schneiderman N, Harris PM (eds), Carbonate cements, Special Publication, Society Economical Paleontologist Mineralogist 36. Tulsa, pp 249–369
Ford TD, Pedley HM (1996) A review of tufa and travertine deposits of the world. Earth Sci Rev 41:117–175
Fouke BW, Farmer JD, Des Marais DJ, Pratt L, Sturchio NC, Burns PC, Discipulo MK (2000) Depositional facies and aqueous—solid geochemistry of travertine—depositing hot springs, Angel Terrace Mammoth Hot Springs Yellowstone National Park, U.S.A. J Sediment Res 70(3):565–585
Gandin A, Capezzuoli E (2008) Travertine versus calcareous tufa: distinctive petrologic features and stable isotopes signatures. II Quternario. Ital J Quat Sci 21(1B):125–136
Guo L, Riding R (1992) Aragonite laminae in hot water travertine crusts, Rapolano Terme Italy. Sedimentology 39:1067–1079
Guo L, Riding R (1994) Origin and diagenesis of quaternary travertine shrub fabrics, Rapolano Terme, central Italy. Sedimentology 41:499–520
Guo L, Riding R (1998) Hot-spring travertine facies and sequences: late Pleistocene Rapolano Terme, Italy. Sedimentology 45:163–180
Hancock PL, Chalmers RML, Altunel E, Çakır Z (1999) Travitonics: using travertines in active fault studies. J Struct Geol 21:903–916
Herman JS, Lorah MM (1987) CO2 outgassing and calcite precipitation in Falling Spring Creek Virginia, U.S.A. Chem Geol 62:251–262
Innocenti F, Mazzuoli R, Pasquare G, Radicati di Brozola F, Villari L (1975) The Neogene calcalkaline volcanism of Central Anatolia, Geochronological data on Kayseri-Niğde Area. Geol Mag 112(4):349–360
Jacobson RL, Usdowski E (1975) Geochemical controls on a calcite precipitating spring. Contrib Miner Petrol 51:65–74
Jones B, Renaut RW (2010) Calcareous spring deposits in continental settings. In: Alonso Zarza AM, Taner LH (eds) Carbonates in continental settings: facies, environments, and processes: developments in sedimentology, vol 61. Elsevier, Oxford, pp 177–224
Kele S, Özkul M, Fórizs I, Gökgöz A, Baykara MO, Alçiçek MC, Németh T (2011) Stable isotope geochemical study of Pamukkale travertines: new evidences of low-temperature non-equilibrium calcite-water fractionation. Sediment Geol 238:191–212
Koçyiğit A (1984) Güneybatı Türkiye ve yakın dolayında levha içi yeni tektonik gelişim. Türkiye Jeoloji Kurumu Bülteni 27(1):1–16
Koşun E (2012) Facies characteristic and depositional environments of Quaternary tufa deposits, Antalya, SW Turkey. Carbonates Evaporites 27(3):269–289
Lorah MM, Herman JS (1988) The chemical evolution of a travertine-depositing stream: geochemical processes and mass transfer reaction. Water Resour Res 24:1541–1552
McCrea JM (1950) On the isotopic chemistry of carbonates and a paleotemperature scale. J Chem Phys 18:849–857
Minissale A (2004) Origin, transport and discharge of CO2 in central Italy. Earth Sci Rev 66:89–141
Minissale A, Kerrick DM, Magro G, Murrell MT, Paladini M, Rihs S, Vaselli O (2002) Geochemistry of quaternary travertines in the region north of Rome (Italy): structural, hydrologic and paleoclimatic implications. Earth Planet Sci Lett 203(2):709–728
Orhan H, Kalan F (2015) Sedimentological characteristics of Quaternary Aydıncık tufa (Mersin- Türkiye). Carbonates Evaporites 30(4):451–459
Özgül N (1976) Torosların bazı temel jeolojik özellikleri. Türkiye Jeoloji Kurumu Bülteni 19(1):65–78
Özgül N (1997) Bozkır-Hadim-Taşkent (Orta Toroslarʹın Kuzey Kesimi) dolaylarında yer alan Tektono – Stratigrafık Birliklerin Stratigrafisi. MTA Dergisi 119:117–174
Özkul M, Varol B, Alçiçek MC (2002) Denizli travertenlerinin petrografik özellikleri ve depolanma ortamları. MTA Dergisi 125:13–29
Özkul M, Kele S, Gökgöz A, Shen CC, Jones B, Baykara MO, Fόrizs I, Nemeth T, Chang YW, Alçiçek MC (2013) Comparison of the quaternary travertine sites in the Denizli Extensional Basin based on their depositional and geochemical data. Sediment Geol 294:179–204
Özkul M, Gökgöz A, Kele S, Baykara OM, Shen C, Chang Y, Kaya A, Hançer M, Aratman C, Akın T, Örüş Z (2014) Sedimentological and geochemical characteristics of fluvial travertine: a case from the eastern Mediterranean region. Sedimentology 61:291–318
Panichi C, Tongiorgi E (1976) Carbon isotopic composition of CO2 from springs, fumaroles, mofettes and travertines of central and southern Italy: a preliminary prospection method of geothermal areas. In: Proceedings of the 2nd UN symposium on the development and use of geothermal resources, San Francisco CA, 20–29 May 1975, pp 815–825
Pedley HM (1990) Classification and environmental models of cool freshwater tufas. Sediment Geol 68:143–154
Pentecost A (1990) The formation of travertine shrubs: mammoth hot springs, Wyoming. Geol Mag 127:159–168
Pentecost A (2005) Travertine. Springer, Berlin, p 445
Rainey DK, Jones B (2005) Radiating calcite dendrites- precursors for coated grain formation in the Fairmont Hot Springs Travertine, Canada. In: Özkul M, Yağız S, and Jones B (eds) Proceedings of 1st International Symposium on Travertine September 21–25 2005, Denizli, Turkey, pp 25–33
Shen CC, Wu CC, Cheng H, Edwards RL, Hsieh YT, Gallet S, Chang CC, Li TY, Lam DD, Kano A, Hori M, Spötl C (2012) High-precision and high-resolution carbonate 230Th dating by MC-ICP-MS with SEM protocols. Geochim Cosmochim Acta 99:71–86
Spötl C, Vennemann TW (2003) Continuous-flow isotope ratio mass spectrometric analysis of carbonate minerals. Rapid Commun Mass Spectr 17:1004–1006
Teboul PA, Durlet C, Gaucher EC, Virgone A, Girard JP, Curie J, Lopez B, Camoin GF (2016) Origins of elements building travertine and tufa: new perspectives provided by isotopic and geochemical tracers. Sediment Geol 334:97–114
Zhang DD, Zhang Y, Zhu A, Cheng X (2001) Physical mechanism of river waterfall tufa (travertine) formation. J Sediment Res 71:205–216
Acknowledgements
We are grateful to the Selcuk University Research Fund for the financial support (Project No. 12101018). Special thanks go to Prof. Dr. Ada Haynes from Tennessee Tech. University, USA for her contribution in improving the language of this manuscript. The critical comments on an earlier version of this manuscript by anonymous reviewers are greatly appreciated. This article is produced from Master Thesis of Seda Karaisaoğlu supervised by Hükmü Orhan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaisaoğlu, S., Orhan, H. Sedimentology and geochemistry of the Kavakköy Travertine (Konya, central Turkey). Carbonates Evaporites 33, 783–800 (2018). https://doi.org/10.1007/s13146-018-0436-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-018-0436-z