Abstract
The role of brown adipocytes and adipocytes of a new beige type in the energy metabolism of a healthy person and in the pathogenesis of obesity has extensively been discussed in recent years. The interest to these cells has been stimulated owing to the application of new noninvasive methods for studying the metabolic activity of tissues. Using these methods, the presence of thermogenically active adipocytes in adults and their reactivity to cold stimuli have been proved. These data, together with the results of animal experiments support the idea of thermogenic fat being a direct regulator of the energy balance of man. However, for several reasons there are some objections to this viewpoint. The main objection is that the total activity of the human thermogenic adipocytes is about 100 kJ/day, i.e., it is negligible. In addition, the burn of excessive nutrients is biologically inappropriate for an organism. Therefore, the idea that obesity is caused by the decreased activity of thermogenic adipocytes is erroneous. The statement that the causes of obesity are associated with the increased efficiency of energy-dependent processes seems more reasonable. The consequence is a reduction in energy expenditure to perform a unit of biological work. This results in excess of nutrients deposited in the form of fat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown adipose tissue in small mammals increases the production of heat in response to two different stimuli, cold [28] and overfeeding [48]. Based on these observations, a concept was formulated that the thermogenesis in brown fat could significantly change the energy balance of the organism [27]. Although the presence of brown adipose tissue in humans was confirmed by more than a century ago [24], this concept was applied to humans with caution [7, 40]. However, in recent years the situation has changed. New methods such as computed positron emission tomography using 18F-fluorodeoxyglucose (18F-FDG PET/CT) [15, 57, 60] and functional magnetic resonance imaging prove that thermogenically active brown fat is present in humans throughout life [12]. Thus, to date, the hypothesis has been formulated that the lack of thermogenic activity of brown adipose tissue may cause spontaneous human obesity [8, 10, 33, 51]. Our little review is devoted to the discussion of this hypothesis.
The types of thermogenic adipocytes and their anatomical localization
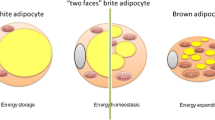
In recent years, the knowledge concerning the adipose tissue has quickly evolved. At present, there is no doubt that in addition to typical or canonical white and brown adipocytes, the adipose tissue includes the third type of cells [33, 36, 47]. These cells diffusely scattered in white fat depots as brown adipocytes have mitochondria with uncoupling protein 1. These cells are mostly referred to as beige [33, 36] or "brite" ones (brite—an acronym for "brown-in-white") [47], therefore they are called bright/beige adipocytes ((Brite/beige)Ad) in the review. (Brite/beige)Ad arise from the precursors, which differ from the lineage of brown adipocytes and/or as a result of reversible transdifferentiation from white adipocytes [14, 23, 47]. Despite the different origins of brown and (Brite/beige)Ad, the differentiation process is similar in many respects. The transcription factors C/EBP-β and PR domain, containing 16 (PRDM16) play a crucial role in the differentiation process [20, 33]. Hereinafter, the common name "thermogenic adipocytes" (TherAd) is used for brown and beige adipocytes when the information on the origin of these cells is not important for the discussion of their functions.
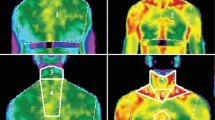
The comparison of gene expression patterns indicates the presence of both types of TherAd with a significant predominance of (Brite/beige)Ad cells in the fat depots of adult humans [16, 32, 37]. TherAd in adult humans are located in the cervical, supraclavicular, mediastinal, perirenal areas, i.e., in the so-called protective collar in the upper body, and along the major blood vessels [26, 57, 60]. Owing to such an anatomical location, the generated heat can protect the thorax from cooling and warm the blood moving to vital organs including the brain, kidneys, etc. [7, 50].
Amount and functional activity of non-stimulated thermogenic fat
The earlier data on the content of UCP1 and its mRNA which were obtained on postmortem material showed that obesity in humans could be associated with the decrease in the amount and functional activity of brown fat [44, 52].
The technique of 18F-fluorodeoxyglucose positron emission tomography provides wide opportunities to assess the amount and activity of thermogenic adipocytes in the investigations in vivo despite the indications of the relatively low spatial resolution of PET images, not very accurate measurements of the total volume of the thermogenic depot, and the difficulty in comparing the values of the metabolic activity of the tissues obtained in different studies [56].
In most studies, thermogenic fat, mainly in the cervical and supraclavicular zones, was detected in 6 % of patients who underwent tomographic examination for medical reasons and healthy people at room temperature without the use of any stimulation [13, 15, 45]. It is stated that thermogenic fat with a dense network of capillaries and sympathetic nerve fibers was detected in 33 % of patients undergoing surgery for thyroid diseases and subsequent treatment with thyroxine replacement therapy [65]. An inverse relationship between the weight/activity of thermogenic zones and body mass index (BMI, kg · m−2) was observed in the cohorts of patients with the thermogenic fat identified [13, 15, 45]. This means that patients with morbid obesity (BMI > 35) have a thermogenic area with much lower weight and activity compared with those who had BMI lower than 25. The detection frequency of thermogenic fat increased during the cold season [2, 13, 45].
Of course, a question arises about a relatively rare occurrence of TherAd in the studied cohorts. It can not be ruled out that this result is due to the low resolution of the method. In any cases, the obtained results are partially consistent with the hypothesis on the role of TherAd in fat depot stabilization in humans.
The response of thermogenic adipocytes to the intravital stimulation
In accordance with the hypothesis on the involvement of brown fat in the stabilization of fat depot weight, the nutritional factor is of interest as a stimulus which triggers the adaptive thermogenesis in TherAd. In fact, this stimulation was used very rarely in humans, as opposed to mild cold stimulation. Apparently, such a strategy is chosen by analogy with the experiments in which the cold exposure and the "excess" of nutrients caused qualitatively the same enhancement of thermogenesis in brown fat of animals. However, these two ways of stimulation of TherAd are not equivalent, because they have completely different biological contents. This can be seen even from the fact that a moderate food restriction increased the amount of TherAd [19, 41]. Mild cold exposure until shivering clearly increased the frequency of PET scans with metabolically active thermogenic fat. Thermogenic fat after stimulation was detected in 84 % of patients on the average [1, 60, 64]. Moreover, the cold raised the blood flow [12], the content of uncoupling protein 1 and its mRNA [60], i.e., the functional activity of thermogenic fat. It is also important that the activity of thermogenic fat after the cold and ephedrine stimulation was inversely proportional to BMI [11, 57] and weakly expressed in obese patients [58]. Obviously, this is the evidence for the rapid activation of thermogenesis in TherAd, so these cells can probably play an essential role in the regulation of energy balance of humans. However, one could not exclude that the reduced cold sensitivity of thermogenic adipocytes in obese people was a consequence rather than the cause of metabolic disorders. In addition, reducing the thermogenic activity of adipocytes of such patients may result from the improved thermal insulating properties of the body [10].
Therefore, the study in which the energy metabolism was stimulated by high-calorie food was of crucial importance for understanding the role of TherAd [61]. The study was conducted on young men with BMI of 22.4. The metabolic activity of the supraclavicular fat and other organs was detected using [18F]FDG PET/CT. [18F]FDG was injected at the peak of postprandial energy expenditure after consuming a high-calorie, carbohydrate-rich meal. Postprandial [18F]FDG uptake in TherAd increased, although to a lesser extent than after the cold exposure. Simultaneously, postprandial [18F]FDG uptake by muscles also increased and their contribution into the increase of energy metabolism of the whole organism would be dominant if their weight is taken into account. While [18F]FDG uptake into supraclavicular fat closely correlated with the cold-induced thermogenesis, no direct relation was found between postprandial [18F]FDG uptake into the fat depot and diet-induced thermogenesis.
The result of this study, showing a minor role of thermogenic fat in human energy metabolism, may seem unexpected only at first sight. In our opinion, the exaggeration of the role of thermogenic fat in human energy balance may be due to different reasons. One of them may be the unreasonable straightforward transfer of the experimental results obtained from small rodents to humans. In addition, one can not exclude the psychology of a researcher, when the interpretation of the experimental data is largely determined by the currently accepted "brown-thermogenic" paradigm. Finally, the overestimation may arise from the methodological error when the indirect estimates and their relative changes are used instead of the absolute values of thermogenesis. For example, the relevance of the UCP1 mRNA levels for estimating the degree of recruitment and thermogenic capacity of adipocytes is doubtful. The observations that cold acclimation barely increases the UCP1 mRNA levels in canonical brown fat while leading to a 200-fold increase in the UCP1 mRNA levels in brite/beige adipose tissue may overestimate the physiological significance of these depots [43]. In fact, the absolute values of UCP1 mRNA are initially very low and remain lower in (Brite/Beige)Ad than in the canonical brown adipocytes, even after such a significant recruitment. The direct measurements of oxygen consumption by mitochondria of cold-adapted mice show that the additional thermogenesis of (Brite/Beige)Ad located in the inguinal fat is not more than one third of this value of canonical brown fat [53]. Thus, the adequate assessment of the thermogenic contribution of (Brite/Beige)Ad is only possible using the direct bioenergetic indices.
In order to verify the adequacy of [18F]FDG PET/CT for the estimates of thermogenic activity the daily energy expenditure and the tissue energy metabolism were investigated in 25 healthy men and women and the obtained results were compared with the PET scans [42]. The daily energy expenditure was determined by indirect calorimetry. The tissue energy metabolism was determined from the regional blood oxygen saturation measured by near-infrared spectroscopy. The energy metabolism was stimulated by mild cold exposure. The results confirmed the dynamics of brown fat thermogenic responses which had previously been detected by PET in normal and obese individuals. However, the absolute contribution of the cervical and supraclavicular brown fat thermogenesis to the total energy exchange even in the cold did not exceed 62–104 kJ/day. Therefore, according to the authors, despite the correlations between the daily energy expenditure and thermogenic activity of brown fat, the latter represents a minor source of heat in the human organism. Apparently, the low thermogenic capacity of fat raises doubts concerning a direct metabolic link between morbid obesity and human thermogenic adipocytes.
Energy burn or saving?
In terms of bioenergetics, there are two explanations of the positive energy balance of an animal organism. One envisages the existence of a special mechanism which transforms the energy of excessive nutrients, if any, into heat. Its inability to function or insufficiently high activity results in spontaneous obesity. This is the essence of the hypothesis on the connection between obesity and TherAd. There are two difficulties in accepting this hypothesis. Firstly, the direct evidence that this mechanism includes thermogenic adipocytes is still absent. To support the "brown-thermogenic" hypothesis, one actually has a small amount of correlations between the linear scan size of brown fat and BMI. Secondly, it is impossible to provide a rational explanation for the necessity of such a mechanism for animals in nature [9, 32]. On the contrary, the appearance of adipose tissue was an important evolutionary acquisition, which made the survival of individuals and their offspring possible in adverse environmental conditions. Furthermore, in the natural environment, overeating and obesity is impossible for many reasons even in the abundance of food. Among these reasons is high energy consumption for thermoregulation and for physical activity both of predators and their prey during hunting [35] and on the other hand, a forced reduction in the duration of animal feeding in the environment with predators [38]. Moreover, the absorption of nutrients in the gut is impaired due to intestinal parasitic infections widespread in wildlife [62], and the maintenance of immunity requires additional energy [25]. For many centuries of human civilization the imbalance between the consumption of food energy and the expenses for heavy exhausting labor and for fighting infections impeded the obesity for a vast majority of people [22]. It is worth noting again that the "burn" of nutrients in brown fat if it occurs in thermoneutral conditions, is not consistent with the biological logic. It is therefore necessary to take into consideration the viewpoint that the concept of diet-induced thermogenesis is due to the erroneous interpretation of the experimental data [34].
The second explanation is diametrically opposed to the first one. It consists in the fact that due to certain circumstances, one or more energy-dependent processes begin to operate with high energy efficiency. This implies a reduction in energy consumption per unit of work. Consequently, if the feeding behavior is not properly regulated, the excess of nutrients is deposited in the adipose tissue. One does not know these circumstances for sure, but the basic energy-dependent processes are known: mitochondrial ATP synthesis, the active membrane transport of ions against their electrochemical gradient, anabolic biosynthesis, and locomotion. Saving energy resources corresponds to the biological basis of the functioning of the animal organism. Thus, it is not appropriate to confuse the biological expediency of the triglyceride accumulation in fat depots with the health impact of this process.
For current and future research a more common approach is required to solve the problem of energy balance. In this context, thermogenic adipocytes with uncoupling protein represent a specialized mechanism realizing the general principle of the energy balance regulation. The principle is that living organisms are capable to change the energy efficiency of energy-dependent processes at the molecular-genetic level. This is performed in two ways: on the one hand, the organism adapts the energy efficiency according to its current demands and needs; on the other hand, this is realized on the phylogenetic level. For the first time this principle was formulated and proved for skeletal muscles in the seventies of the last century [29, 30].
From all our knowledge about thermogenic adipocytes and uncoupling protein 1, it follows that this mechanism is physiologically significant for a small group of animals and for a quite narrow range of situations. Brown fat is involved in maintaining the temperature homeostasis of newborn and adult animals of small size, especially during hibernation and arousal [7, 28]. In all these cases, the UCP1 thermogenesis in brown fat is dominant relative to other mechanisms of thermogenesis only during hibernation. In all other cases, one should take into account the heat generated by muscles, since even newborns have significant mobility under the real conditions as opposed to the model experiments. The energy efficiency of contractile act, as previously noted, is reduced in the cold-adapted animals [29, 30]. The reduced effectiveness of muscle contraction is seemingly due to the increase of the mitochondrial uncoupling. But UCP1 in the skeletal muscle is absent, and UCP3 does not reproduce any thermogenic effects of UCP1 [5]. The channel of the basal H+ conductivity, such as adenine nucleotide translocase, is not regulated [17, 31]. The adaptation to the cold does not increase the non-shivering thermogenesis of the skeletal muscle [21], which is also not consistent with the idea of muscle mitochondrial uncoupling.
The skeletal muscle is the main source of additional heat in the cold adaptation of another phylogenetic group of animals, i.e., birds which do not have brown fat. Their muscle mitochondria become more thermogenic while remaining in the coupling state as before the cold adaptation [54]. This can have only one implication: the increased synthesis of ATP to compensate its high rate of hydrolysis for some energy-consuming processes of the muscle fiber. Indeed, ATP is intensively used to recycle Ca2+ in the sarcoplasmic reticulum, i.e., for the futile cycle [18]. Recent findings show that Ca2+ recycling is also an important thermogenic mechanism in skeletal muscle of mammals [4, 39, 49]. In this regard, particular attention is paid to the features of free energy of ATP hydrolysis. It is generally known that its change in any cellular process, including muscle contraction, depends on the activity of the all reactants: ATP, ADP, Pi, H+, and Mg2+ [3]. Therefore, free energy of ATP hydrolysis in cells may vary widely from 35 to 60 kJ/mol. It should be borne in mind that ATP has a monopoly for all types of biological work, and the turnover of this substance in humans is higher than 100 mol/day [46]. Consequently, the heat of the reaction and the load on mitochondria are considerably varied with the increase or decrease of the output of free energy from the hydrolysis of ATP.
It is known that oxygen consumption and proton leak in poikilotherms are four to five times lower than those of homeothermic animals with an equal body weight under similar assay conditions. Energy saving, i.e., the high energy efficiency of poikilotherms is due to different amounts of molecules of adenine nucleotide translocase in the inner mitochondrial membrane [6, 31].
All these examples are given with the aim to demonstrate various ways of the adaptive change in the energy effectiveness of biological processes. In our opinion, this is especially actual with regard to the problem of obesity because thermogenic adipocytes with UCP1 still have uncertain prospects as participants in such processes. The skeletal muscles with their high energy capacity are also worthy candidates for localizing energy conservation sites. However, the search for the relationship between the state of mitochondria in muscles and obesity has not led to any definite result, except for the observation of their dysfunction in obese patients [55]. The problem of the primacy of changes in muscle mitochondria in relation to the development of obesity remains unsolved as well as the same can be mentioned in connection with brown fat. It is therefore necessary to monitor all the energy-dependent processes to find a site of metabolism, where the energy saving occurs.
Conclusions
The last two decades have dramatically changed our understanding of the physiology of adipose tissue. Currently, it presents a very dynamic and plastic structure with auto-, para-, and endocrine properties [59, 63] and, without exaggerating, can be called an adipose organ [14]. The new evidence indicates that white adipocytes are not only a passive depot of triglycerides, and brown adipocytes are not just a heat source. In view of all this, (Brite/Beige)Ad reflect the complicated structural and functional organization of the fat organ. Therefore, the insufficient conclusiveness of the facts and arguments in favor of the hypothesis of the connection between thermogenic adipocytes and obesity in humans, in our opinion, leads to two conclusions. Firstly, it is necessary to carry out additional studies involving direct energy estimates of thermogenesis of (Brite/beige)Ad and brown adipocytes, as well as using animal models with the adipose organ similar to the human adipose tissue. Secondly, the correlation between the functional activity of thermogenic adipocytes and obesity can be realized through other functions of the cells, where the energy component is not critical. But in any case, it is necessary to find out which energy-dependent process exactly results in nutrient saving.
References
Admiraal WM, Holleman F, Bahler L, Soeters MR, Hoekstra JB, Verberne HJ (2013) Combining 123I-metaiodobenzylguanidine SPECT/CT and 18F-FDG PET/CT for the assessment of brown adipose tissue activity in humans during cold exposure. J Nucl Med 54:208–212
Au-Yong IT, Thorn N, Canatra R, Perkins AC, Symonds ME (2009) Brown adipose tissue and seasonal variation in humans. Diabetes 58:2583–2587
Bagshaw CR (1982) Muscle contraction. New York, London
Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, Carpentier AC, Haman F (2015) Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol 593:701–714
Brand MD, Esteves TC (2005) Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2:85–93
Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EI (2005) The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J 392:353–362
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Cannon B, Nedergaard J (2010) Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes 34:S7–S16
Cannon B, Nedergaard J (2011) Non-shivering thermogenesis and its adequate measurement in metabolic studies. J Clin Invest 214:242–253
Cannon B, Nedergaard J (2012) Yes, even human brown fat is on fire! J Clin Invest 122:486–489
Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MN, Kingwell BA (2013) Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 56:147–155
Chen Y-CI, Cypess AM, Chen Y-C, Palmer M, Kolodny G, Kahn CN, Kwong KK (2013) Measurement of human brown adipose tissue volume and activity using anatomic MRI and functional MRI. J Nucl Med 54:1584–1587
Cheng WY, Zhu ZH, Ouyang M (2009) Patterns and characteristics of brown adipose tissue uptake of 18F-FDG positron emission tomograph/computed tomography imaging. Acta Acad Med Sin 31:370–373
Cinti S (2012) The adipose organ at a glance. Dis Model Mech 5:588–594
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
Cypess AM, White AP, Vernochet C, Schulz TI, Xue R, Sass CA et al (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19:635–639
Divakaruni AS, Brand MD (2011) The regulation and physiology of mitochondrial proton leak. Physiology 26:192–205
Dumonteil E, Barré H, Meissner G (1995) Expression of sarcoplasmic reticulum Ca2+ transport proteins in cold-acclimated ducklings. Am J Physiol 269:C955–C960
Elsukova EI, Mizonova OV, Medvedev LN, Taidonov SV (2012) Effect of calorie-restricted diet on brown adipose tissue in mice. Bull Exp Biol Med 152:286–288
Emont MP, Yu H, Wu J (2015) Transcriptional control and hormonal response of thermogenic fat. J Endocrinol 225:R35–R47
Foster DO (1986) Quantitative role of brown adipose tissue in thermogenesis. In: Trayhurn P, Nicholls DG (eds) Brown adipose tissue, Arnold, Baltimore, pp 31–51
Genne-Bacon E (2014) Thinking evolutionarily about obesity. Yale J Biol Med 87:99–11
Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S (2014) White, brown, and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol 170:R159–R171
Hatai S (1902) On the presence in human embryos of an interscapular gland corresponding to the so-called hibernating gland of lower mammals. Anat Anz 21:369–373
Hawlena H, Krasnov BR, Abramsky Z, Khokhlova IS, Saltz D, Kam M, Tamir A, Degen AA (2006) Flea infestation and energy requirements of rodent hosts: are there general rules? Funct Ecol 20:1028–1036
Heaton JM (1972) The distribution of brown adipose tissue in the human. 113:35–39
Himms-Hagen J (1984) Brown adipose tissue thermogenesis, energy balance, and obesity. Can J Biochem Cell Biol 62:610–617
Horwitz BA (1989) Biochemical mechanisms and control of cold-induced cellular thermogenesis in placental mammals. In: Wang LH (ed) Comparative and Environmental Physiology 4. Springer, Berlin, pp 84–116
Ivanov KP (1980) The muscle heat production after adaptation to cold. Med Biol 58:76–81
Ivanov KP, Pchelenko LD (1978) Increase of the heat production by muscle contraction after adaptation to the cold. Dokl Akad Nauk SSSR 240:227–230
Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD (2010) Mitochondrial proton and electron leaks. Essays Biochem 47:53–67
Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A et al (2013) A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17:798–805
Kajimura S, Saito M (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Ann Rew Physiol 76:13.1–13.25
Kozak LP (2010) Brown fat and myth of diet-induced thermogenesis. Cell Metab 11:263–267
Laundre JW (2014) Ecology, how large predators manage the cost of hunting. Science 346:33–34
Lee P, Werner CD, Kebebew E, Celi FS (2014) Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes 38:170–176
Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander E, Slawik M et al (2013) Evidence for two types of brown adipose tissue in humans. Nat Med 19:631–634
Liesenjohann T, Eccard JA (2008) Foraging under uniform risk from different types of predators. BMC Ecol 8:19
Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, Periasamy M (2015) Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J Biol Chem 290:10840–10849
Medvedev LN, Elsukova EI (2002) Brown fat tissue in humans. Usp Fiziol Nauk 33:17–29
Mizonova OV, Elsukova EI, Medvedev LN (2013) Energy metabolism and biochemical features of adipose tissue in ICR mice after long-term calorie-restricted diet. Bull Exp Biol Med 155:745–747
Muzik O, Mangner TJ, Leonard VR, Kumar A, Janisse A, Granneman JG (2013) 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54:523–531
Nedergaard J, Cannon B (2013) UCP1 mRNA does not produce heat. Biochim Biophys Acta 1831:943–949
Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W (1997) Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res 38:2125–2133
Perkins AC, Mshelia DS, Symonds ME, Sathekge M (2013) Prevalence and pattern of brown adipose tissue distribution of 18F-FDG in patients undergoing PET-CT in a subtropical climatic zone. Nucl Med Commun 34:168–174
Rich P (2003) Chemiosmotic coupling: the cost of living. Nature 421:583
Rosenwald M, Wolfrum C (2014) The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 3:4–9
Rothwell NJ, Stock MJ (1979) A role of brown adipose tissue in diet-induced thermogenesis. Nature 281:31–36
Rowland LA, Bal NC, Kozak LP, Periasamy M (2015) Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem 290:12282–12289
Sacks H, Symonds ME (2013) Anatomical locations of human brown adipose tissue. Functional relevance and implications in obesity and type 2 diabetes. Diabetes 62:1783–1790
Saito M (2013) Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J 37:22–29
Santos GC, Araujo MR, Silveira TC, Soares FA (1992) Аccumulation of brown adipose tissue and nutritional status. A prospective study of 366 consecutive autopsies. Arch Pathol Lab Med 116:1152–1154
Shabalina IG, Petrovic N, De Jong JM, Kalinovich AV, Cannon B, Nedergaard J (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5:1196–1203
Teulier L, Rouanet J-L, Letexier D, Romestaing C, Belouze M, Rey B, Duchamp C, Roussel D (2010) Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J Exp Biol 213:2476–2482
Thrush AB, Dent R, McPherson R, Harper M-E (2013) Implications of mitochondrial uncoupling in skeletal muscle in the development and treatment of obesity. FEBS J 280:5015–5029
Van der Lans AA, Wierts R, Vosselman MJ, Schrauven P, Brans B, van Marken Lichtenbelt W (2014) Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol 307:R103–R113
Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GI, Bouvy ND et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Vijgen GH, Bouvy ND, Teule GI, Brans B, Schrauwen P, Van Marken Lichtenbelt W (2011) Brown adipose tissue in morbidly obese subjects. РLoS ONE. doi:10.1371/journal.pone.0017247
Villarroya J, Cereijo R, Villarroya F (2013) An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 305:E567–E572
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Vosselman MJ, Brans B, Van der Lans AA, Wierts R, Van Baak MA, Mottaghy FM et al (2013) Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr 98:57–64
Wang T, Hildebrandt M, Thrasher S, Appleton JA, Ahima RS, Wu GD (2007) Divergent metabolic adaptations to intestinal parasitic nematode infection in mice susceptible or resistant to obesity. Gastroenterology 133:1979–1988
Wang GX, Zhao XY, Lin JD (2015) The brown fat secretom: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 26:231–237
Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada T, Kawai Y, Saito M (2011) Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity 19:13–16
Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S (2009) The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23:3113–3120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medvedev, L.N., Elsukova, E.I. Can thermogenic adipocytes protect from obesity?. J Physiol Biochem 71, 847–853 (2015). https://doi.org/10.1007/s13105-015-0443-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0443-7