Abstract
Hepatocellular carcinoma is one of the leading causes of death in cancer and yet no drug has proven to be a successful candidate for its treatment in advanced stages. Ursolic acid stearoyl glucoside (UASG) is a newly discovered triterpene in Lantana camara and there lies a possibility that it possess anti-hepatocellular carcinoma property. In the present study, we induced hepatocellular carcinoma in Wistar rats by diethylnitrosamine (DENA) and treated it with ursolic acid stearoyl glucoside. The ability to treat hepatocellular carcinoma was measured by comparing biochemical serum markers such as serum alanine aminotransferase, serum aspartate aminotransferase, serum alkaline phosphatase, and the specific marker for hepatocellular carcinoma, alpha fetoprotein. The histological studies of the livers were also performed. The results have shown significant elevated levels of these parameters as compared to normal control and the drug receiving groups have shown significant reduction in these marker levels. Histopathological studies also indicated the reduced liver damage in drug-treated groups. It was noted that a significant and dose-dependent reversal of DENA-diminished activity of antioxidant enzymes like superoxide dismutase, catalase, glutathione peroxidase, glutathione transferase, and the reduced DENA-elevated level of lipid peroxidation (LPO) with a marked change. UASG significantly suppressed free radical formation by scavenging the hydroxyl radicals. It also modulates the levels of LPO and markedly increases the endogenous antioxidant enzymes level in DENA-induced hepatocellular carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lantana camara L. has been used for a long time by the local populations for treating various ailments including tumors. The presence of ursolic acid derivative that is stearoyl glucoside of it, and the use by local populations created a possibility that this triterpene derivative might be able to cure cancer as ursolic acid already has been proven as apoptosis-inducing and angiogenesis inhibitor compound. Now, this possibility steered authors’ interest for evaluating ursolic acid stearoyl derivative as anticancer agent in chemically induced hepatocellular carcinoma [1].
Epidemiology and risk factors
From a long time, cancer has been the dreadful of all the diseases of which hepatocellular carcinoma (HCC) is sixth most common neoplasm and the third most frequent cause of cancer death. More than 700,000 cases of this malignant disease were diagnosed in 2008, with age- and age-adjusted worldwide incidence of 16 cases per 100,000 [14]. The highest incidence is in southeastern and eastern Asia with a rate of 18.3–35.5 per 100,000 populations and lowest in Central America with a rate of 2.1 per 100,000 populations [38, 52]. In most cases, hepatocellular carcinoma develops within an established background of chronic liver disease (70–90 % of all patients) [44]. Most cases of hepatocellular carcinoma (80 %) arise in eastern Asia and sub-Saharan Africa, where the dominant risk factor is chronic infection with hepatitis B virus, together with exposure to aflatoxin B1, whereas in North America, Europe, and Japan, infection with hepatitis C virus is the main risk factor, together with chronic alcohol use [10]. Diabetes is an independent risk factor [11], and alcohol and tobacco are among other synergistic risk factors whereas coffee reduces it [4, 32].
Molecular pathogenesis
Hepatocarcinogenesis is a complex multistep process in which many signaling cascades are altered, leading to a heterogeneous molecular profile [13, 50]. The main mutations include the tumor suppressor gene p53 (present in about 25–40 % of cancers, depending on tumor stage) and the gene for β-catenin, CTNNB1 (about 25 %, predominantly in HCV-related hepatocellular carcinoma). Other mutations are less frequent [13].
Treatments and possible pathways
Potentially efficacious therapies for HCC and ultrasonography for early detection have primed the establishment of surveillance plans in the population at risk, namely patients with cirrhosis [5]. This intervention has resulted in a steady increase in the numbers identified early. Early detection with therapies centered on the patients and stage allows longer survival. The conventional therapies at early stages are surgical resection, liver transplantation, and ablation by radiofrequency or ethanol injection. With these options, survival at 5 years ranges between 50 and 70 %. Promising signaling pathways which can be used as therapeutic targets are MAPK pathways, β-catenin pathways, Akt pathway, and IGF signaling. Other important targets are apoptosis and angiogenesis [9, 28, 33]. At present, the only systemic targeted therapy which is used in advanced HCC for improving survival is Sorafenib. It is a recently developed multitarget drug. It inhibits the kinase activity of wild type B-Raf and mutant Raf, VEGF receptors, PDGFR, c-kit, FLT3, and RET. This molecule is also antiproliferative and anti-angiogenic [39].
Ursolic acid
3β-Hydroxy-urs-12-en-28-oic acid is a pentacyclic triterpenoid, a member of the cyclosqualenoid family, found in many kinds of medicinal plants, and present in human diet [1, 29]. Ursolic acid has been shown to inhibit DNA replication [26], tyrosine kinase activity [20], and the expression of lipoxygenase, cyclooxygenase-2, inducible nitric oxide synthase, and matrix metalloproteinase-9 [34, 45, 47, 48]. Ursolic acid also activates caspases [17] and induces Ca+2 releases [3]. All these molecular effects can give account of the reported pleiotropic biological effects of ursolic acid, including antibacterial, hepatoprotective, immunomodulatory, antiproliferative, antitumoral, anti-inflammatory, and anti-angiogenic activities [21, 30, 46, 47]. In cancer, angiogenesis is described as one of its hallmarks, and it is required for both tumor progression and dispersal of metastatic cells [16]. Ursolic acid has been proved as an anti-angiogenic compound which inhibits key steps of angiogenesis in vitro [6] and promotes apoptosis [30, 36, 51]. Recently, a new derivative of ursolic acid, ursolic acid stearoyl glucoside (urs-12-en-3β-ol-28-oic acid 3β-d-glucopyranosyl-4′-octadecanoate) (Fig. 1), is discovered in L. camara L. [23]. Some derivatives of ursolic acid have shown in vitro and in vivo anticancer activity [43].
Material and methods
Animals
Wistar albino rats (150–200 g) were obtained from Animal house facility at Siddhartha Institute of Pharmacy, Dehradun and were acclimatized for 2 weeks with light and dark cycle of 12 h and temperature was maintained at 25 ± 1 °C and humidity 55 ± 5 %. The animals were given standard pellet diet obtained from Lipton Rat Feed Ltd. Pune and water was provided ad libitum. The experimental protocol was approved by Institutional Animal Ethics Committee (SIP/IAEC/05/2011).
Chemicals
All chemicals used were of analytical grade. Diethylnitrosamine (Sigma-Aldrich, St. Louis, MO) was used for hepatocellular carcinoma induction. Test compound (UASG) was provided by Siddhartha Institute of Pharmacy, Dehradun as gift sample.
Experimental protocol
Induction of hepatocellular carcinoma
Animals were divided into five groups, each having six animals. Diethylnitrosamine (200 mg/kg) (DENA) was administered in phosphate buffer solution intraperitoneally to induce hepatocellular carcinoma, as single dose, after animals were fasted overnight [35]. Induction of hepatocellular carcinoma was confirmed after 7 days of administration of DENA by measuring serum alpha fetoprotein (AFP) in different group animals. Animals were grouped as follows: group I was kept normal control and administered saline, group II was given DENA only. Groups III, IV, and V rats were treated with UASG (20, 40, and 80 mg/kg, p.o.) once daily for 16 weeks, respectively.
Estimation of anti-hepatocellular carcinoma activity
Blood was collected from all groups directly from retro-orbital plexus after anesthetized by a mixture of chloroform–ether (2:3) at the end of protocol period and animals were sacrificed followed by liver specimen collection. Serum was separated after coagulating blood for 30 min and centrifuged at 1,500 rpm for 20 min; serum was then separated and was used for estimation of biochemical parameters.
Biochemical estimation
At the end of experimental period of 16 weeks, animals were subjected to ether anesthesia, blood was collected from retro-orbital plexus, and serum was separated by centrifugation. Anti-hepatocellular carcinoma activity of the drug was determined by measuring serum levels of enzymes such as serum aspartate transferase (AST), serum alanine transferase (ALT), serum alkaline phosphatase (ALP), and serum AFP.
Animals were sacrificed by cervical decapitation, and the liver was excised, washed in ice-cold saline, and blotted to dryness. A 1 % homogenate of the liver tissue was prepared in phosphate buffer (0.1 M; pH 7.4), centrifuged, and the clear supernatant used for further biochemical assays. The unbroken cells and debris were removed by centrifugation at 10,000 rpm for 15 min at 4 °C using a REMI cooling centrifuge and the supernatant was used for the estimation of lipid peroxidation (LPO), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione transferase (GST) [40].
Histology
Autopsy specimens were taken from the livers of rats in all groups directly after sacrificing. Specimens were immediately fixed in 10 % formalin (pH 7.4) for at least 24 h. Tissue specimens were dehydrated in graded series of ethanol, cleared in xylene, embedded in paraplast wax, and sectioned at 5 μM thicknesses. Tissue sections were mounted on positively charged coated slides. Tissue sections were deparaffinized and stained with hematoxylin and eosin for general histological structure.
Statistical analysis
All the data are expressed as mean ± S.E.M (n = 6). Significant differences between the mean values were statistically analyzed using one-way analysis of variance. The p values less than 0.001 were considered significant.
Results
Biochemical parameters
After the end of protocol period of 16 weeks, animals were sacrificed and blood was collected for estimation of serum levels of enzymatic parameters of serum aspartate transferase, serum alanine transferase, serum alkaline phosphatase, and serum alpha fetoprotein.
The level of serum aspartate transferase in group II which was given DENA only is 149.5 ± 2.837 which is significantly higher than the group I which was kept as normal control (22.17 ± 2.167) (p < 0.001). The groups receiving UASG 20 mg/kg (group III), 40 mg/kg (group IV), and 80 mg/kg (group V) have AST levels 73.67 ± 1.626, 27.67 ± 1.116, and 25.67 ± 2.376, respectively, which is significantly below (p < 0.001) than the level of that of group II (Table 1).
Serum alanine transferase in group II which was given DENA only is 76.33 ± 2.996 and this level is significantly higher than the group I which was kept as normal control (35.67 ± 1.542) (p < 0.001). The groups receiving UASG 20 mg/kg (group III), 40 mg/kg (group IV), and 80 mg/kg (group V) have ALT levels 58.67 ± 2.929, 40.00 ± 1.317, and 38.83 ± 2.136, respectively, which is significantly below than the levels of that of group II (p < 0.001) (Table 1).
Enzyme alkaline phosphatase level in group II which was given DENA only is 129.3 ± 3.180 which is significantly higher (p < 0.001) than the group I which was kept as normal control (72.17 ± 1.701). The groups receiving UASG 20 mg/kg (group III), 40 mg/kg (group IV), and 80 mg/kg (group V) have ALP levels 103.8 ± 1.470, 79.17 ± 2.600, and 73.83 ± 2.762, respectively, which is significantly below than the levels of that of group II (p < 0.001) (Table 1).
The level of serum alpha fetoprotein in group II which was given DENA only is 71.37 ± 9.546 which is significantly higher than the group I (p < 0.001)which was kept as normal control (1.183 ± 0.3321). The groups receiving UASG 20 mg/kg (group III), 40 mg/kg (group IV), and 80 mg/kg (group V) have AST levels 27.63 ± 5.768, 2.817 ± 0.4854, and 2.067 ± 0.7315, respectively, which is significantly below than the levels of that of group II (p < 0.001) (Table 1).
As shown in Table 2, DENA increased the level of LPO, which was significantly (p < 0.01, p < 0.001) reduced by UASG (20, 40, 80 mg/kg). The levels of different antioxidant enzymes SOD, CAT, GPx, and GST were reduced in serum of DENA-treated rats, which were significantly (p < 0.01) restored by UASG (20, 40, 80 mg/kg).
Histology
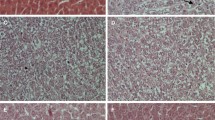
The histological sections of livers from animals of normal control group I (Fig. 2a) show the normal organization of hepatic lobules consisting of one to two cell thickness of hepatic cords radiating from a central vein towards the lobular periphery. In contrast to it, the sections of animal livers from group II (Fig. 2b) of cancer control group show disorganized hepatic parenchyma with trabeculae of polyhedral cells bordering wide sinusoids which represent cellular necrosis and hepatocellular carcinoma development by diethylnitrosamine treatment. Liver specimen histology from group III (Fig. 2c) showed minor disorganization and sinusoidal congestions, whereas liver from rat of groups IV and V (Fig. 2d, e) displayed very less damage of hepatocellular organization and low index of necrosis damage.
Discussion
The compound under has been recently discovered in L. camara L. and has never been tested for anticancer properties. The ursolic acid, of which the test compound is derivative, has shown anti-angiogenic and apoptosis-inducing properties. There are assured evidences that indicate the importance of angiogenesis for the progression of tumors. Angiogenesis also permits the shedding of metastatic tumors from the primary site; therefore, inhibition of angiogenesis may lead to control of tumor growth and metastasis and inhibitors of angiogenesis could be inhibitors of tumor growth. Ursolic acid induces apoptosis via inhibition of NF-κB-induced bcl-2-mediated antiapoptotic pathway leading to activation of P53-induced and caspase 3-mediated proapoptotic pathways [31].
UASG is a pleiotropic agent and shows potent antidiabetic [23], anxiolytic [25], and antiepileptic [24] activity. Toxicity study indicates that it is safe up to 500 mg/kg dose in rats [22]. Hence, it is a very useful compound, to further exploration. Further, clinical trial study, dose designing, formulation, etc. required to its complete exploration.
DENA is frequently used to induce hepatocarcinoma in animal models [2] possibly by causing oxidative stress and cellular injury with enhanced formation of detrimental free radicals [49]. DENA has been shown to be metabolized to its active ethyl radical, which can interact with DNA causing mutation and subsequent oncogenesis [7]. It is present in tobacco smoke, water, cheese, cured, and flamed meats [41].
The results in Table 1 showed that the serum parameters have raised significantly in group II which was given DENA as compared to parameter levels in group I which was kept normal, indicating induction of hepatocellular carcinoma. Groups III, IV, and V have shown significant reduction in the serum parameters after they have been administered ursolic acid stearoyl glucoside.
Among the evaluated parameters, serum alpha fetoprotein level which is considered the gold standard marker for hepatocellular carcinoma has shown consistent results along with other serum parameters as it declines in groups receiving ursolic acid stearoyl glucoside [51]. It is similar in size, structure, and amino acid composition to serum albumin, but it is detectable only in minute amounts in the serum of normal adults. Elevated serum concentrations of this protein can be achieved in the adult by exposure to hepatocarcinogenic agents. It has the high specificity for hepatocarcinoma. Its serum concentration confirms hepatocarcinoma and for the diagnosis of tumor response to therapy. In concurrent work, the levels of AFP decline after giving UASG, the altered levels of AFP indicated that UASG in dose-dependent manner have anticancer activity.
These results are consistent with the fact that ursolic acid prevents angiogenesis and the fact that angiogenesis prevention provides a novel therapeutic approach to hepatocellular carcinoma [15, 37].
Lower level of total protein showing the reduction in biosynthesis of protein due to destructions and dissociation of polyribosome’s on endoplasmic reticulum [8] caused by toxicity of DENA. UASG restricted the protein synthesis by protecting the polyribosome. These biochemical findings were further substantiated by histopathological studies.
LPO is one major mechanisms of corpuscle damage caused by free radicals [12]. Administration of DENA has been reported to generate lipid peroxidation products like MDA and 4-hydroxynonenal that may interact with various molecules leading to oxidative stress and carcinogenesis [19]. Administration of DENA increases the level of LPO, which shows carcinoma. This change of process may further lead to uncompromised production of free radicals overcome the corpuscle antioxidant protection [27]. Animals co-treated with UASG exhibited markedly lower level of LPO in serum, as compared to DENA-administered animals. This shows the anti-lipid peroxidation activity of UASG which may be due to its ability to scavenge free radicals.
It is reported that SOD converts superoxide anion into H2O2 and O2, whereas CAT reduces H2O2 to H2O, resulting in the detoxification of free radicals. GPx reduces reactive peroxides to alcohol and water. GST generally is viewed as a phase 2 enzymes, primarily involved in the detoxification of electrophilic compounds. Many reported studies mentioned that GST plays an important role in protecting cells against oxidant-mediated injury by catalyzing the decomposition of lipid hydroperoxides generated from oxidative damage of cellular lipid molecules [18, 42].
Reduction in SOD, CAT, GPx, and GST are observed in hepatic carcinoma cells. The compounds that can destroy excessive free radicals are suggested to inhibit the process of carcinogenic activity in the body. Such studies give a basis to our results that those activities of the enzyme antioxidants are returned to near normal in UASG-pretreated rats and therefore counter the initiation of carcinogenesis by DENA.
On histopathological examination of the liver cell, UASG treatment revealed that it had antitumor activity, evidenced from the absence of cellular necrosis and inflammatory infiltrates in the liver section of rats pretreated with the highest dose tested. UASG at concentrations 40 and 80 mg/kg is capable to revert the histological changes caused by the development of hepatocellular carcinoma.
From our study, it is concluded that the evaluation of UASG in dose-dependent manner prevented the carcinogenesis in the liver because it significantly prevent the markedly increase in the levels of serum marker enzymes and also suppressed the free radical processes by scavenging hydroxyl radicals. It also modulates the levels of LPO and markedly increases the endogenous antioxidant enzymes level in DENA-induced hepatocellular carcinogenesis. Hence, the treatment of hepatocarcinogenesis with UASG could be a novel approach in the field of chemotherapy. The present study will help and assist designing proper medication for patients suffering from hepatic cancer.
References
Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A (2011) Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol 133:945–972
Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, Fatani AG (2009) Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol 15:1373–1380
Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, Kim KW (1997) Intracellular Ca+2 release mediates ursolic acid-induced apoptosis in human leukaemic HL-60 cell. Int J Cancer 73:725–728
Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C (2007) Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology 46(2):430–435
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1226
Cardenas C, Quesada AR, Medina MA (2004) Effects of ursolic acid on different steps of the angiogenesis process. Biochem Biophys Res Commun 320:402–408
Chakraborty T, Chatterjee A, Rana A, Dhachinamoorthi D, Kumar PA, Chatterjee M (2007) Carcinogen-induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: chemopreventive role of vanadium on this process. Biochim Biophys Acta 1772:48–59
Clawson GA (1989) Mechanism of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res 8:104–112
Coleman WB (2003) Mechanisms of human hepatocarcinogenesis. Curr Mol Med 3(6):573–588
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127
El-Serag HB, Tran T, Everhart JE (2004) Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 126:460–468
Esterbauer H, Chesseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxy-nonenal. Methods Enzymol 186:407–421
Farazi PA, DePinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6:674–687
Ferlay J, Shin HR, Bray F, Forman D, Mathers C (2010) GLOBOCAN 2008, cancer incidence and mortality worldwide: no. 10. Lyon, France: International Agency for Research on Cancer. Int J Cancer 127:2893–2917
Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009) Angiogenesis in liver disease. J Hepatol 50:604–620
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Harmand PO, Duval R, Liagre B, Jayat-Vignoles C, Beneytout JL, Delage C, Simon A (2003) Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int J Oncol 23:105–112
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Hietanen E, Ahotupa M, Bartsch H (1987) Lipid peroxidation and chemically induced cancer in rats fed lipid rich diet. In: Lapis K, Kcharst S (eds) Carcinogenesis and tumor progression, vol. 4. Akademiaikiado, Budapest, pp 9–16
Hollosy F, Meszaros G, Bokonyi G, Idei M, Seprodi A, Szende B, Keri G (2000) Cytostatic, cytotoxic and protein tyrosine kinase inhibitory activity of ursolic acid in A431 human tumour cells. Anticancer Res 20:4653–4670
Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH (1994) Inhibition of skin tumourigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res 54:701–708
Kazmi I, Gaurav G, Afzal M, Anwar F (2012) Anticonvulsant and depressant-like activity of ursolic acid stearoyl glucoside isolated from Lantana camara L. (Verbanaceae). Asian Pac J Trop Dis 2(1):S453–S456
Kazmi I, Rahman M, Afzal M, Gupta G, Saleem S, Afzal O, Shaharyar MA, Nautiyal U, Ahmed S, Anwar F (2012) Anti-diabetic potential of ursolic acid stearoyl glucoside: a new triterpenic gycosidic ester from Lantana camara. Fitoterapia 83:142–146
Kazmi I, Afzal M, Gupta G, Anwar F (2012) Antiepileptic potential of ursolic acid stearoyl glucoside by GABA receptor stimulation. CNS Neurosci Ther 18(9):799–800
Kazmi I, Gupta G, Afzal M, Rahman M, Anwar F (2012) Pharmacological evaluation of anxiolytic activity of ursolic acid stearoyl glucoside isolated from Lantana camara. CNS Neurosci Ther 18(8):707–708
Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Chung HY, Kim ND, Choi YH, Lee SH, Kim KW (2000) Apoptotic activity of ursolic activity may correlate with the inhibition of initiation of DNA replication. Int J Cancer 87:629–636
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Ann Rev Pharmacol Toxicol 44:239–267
Li W, Tan D, Zhang Z, Liang JJ, Brown RE (2008) Activation of Akt-mTOR signaling in angiogenesis in hepatocellular carcinoma. Oncol Rep 20(4):713–719
Liu J (1995) Pharmacology of oleanoic acid and ursolic acid. J Ethnopharmacol 49:57–68
Manez S, Recio MC, Giner RM, Rios JL (1997) Effect of selected triterpenoids on chronic dermal inflammation. Eur J Pharmacol 334:103–105
Manu KA, Kuttan G (2008) Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-κB mediated activation of bcl-2 in B16F-10 melanoma cells. Int Immunopharmacol 8:974–981
Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS (2005) Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 42:218–224
Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM (2009) Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol 25(3):86–94
Najid A, Simon A, Cook J, Chable-Rabinovitch H, Delage C, Chulia AJ, Rigaud M (1992) Characterisation of ursolic acid as a lipoxygenase and cyclo-oxygenase inhibitor using macrophages, platelets and differentiated HL60 leukaemic cells. FEBS Lett 299:213–217
Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM (2008) Experimental models of hepatocellular carcinoma. J Hepatol 48:858–879
Ovesna Z, Kozics K, Slamenova D (2006) Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat Res 600:131–137
Pang R, Poon RTP (2006) Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett 242:151–167
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Peng JW, Cheng AL, Hsu C, Chen CM (2010) Clinical development and future direction for the treatment of hepatocellular carcinoma. J Exp Clin Med 2(3):93–103
Plaa GL, Hewitt WR (1989) Detection and evaluation of chemically induced liver injury. In: Wallace Hyes A (ed) Principle and methods of toxicology, 2nd edn. Raven, New York, p 399
Rajeshkumar NV, Kuttan R (2000) Inhibition of N-nitrosodiethylamine-induced hepatocarcinogenesis by Picroliv. J Exp Clin Cancer Res 19:459–465
Robak J, Glyglewsi RJ (1988) Flavonoids are scavengers of superoxide anions. Biochem Pharmacol 37:837–841
Shao JW, Dai YC, Xue JP, Wang JC, Lin FP, Guo YH (2011) In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur J Med Chem 46:2652–2661
Sherman M (2010) Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 30:3–16
Shishodia S, Majumdar S, Banerjee S, Aggarwal BB (2003) Ursolic acid inhibits nuclear factor-KB activation induced by carcinogenic agents through suppression of. I KBa kinase and p65 phosphorylation: correlation with down-regulation of cyclo-oxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res 62:4375–4383
Sohn KH, Lee HY, Chung HY, Young HS, Yi SY, Kim KW (1995) Anti-angiogenic activity of triterpene acids. Cancer Lett 94:213–218
Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ (2000) Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res 60:2399–2404
Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB (1998) Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res 58:717–723
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM (2007) Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis 27:55–76
Wright LM, Kreikemeier JT, Fimmel CJ (2007) A concise review of serum markers for hepatocellular cancer. Cancer Detect Prev 31:35–44
Yan SL, Huang CY, Wu ST, Yin MC (2010) Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol Vitr 24:842–848
Acknowledgments
Authors are thankful to the Department of Pharmacology, Siddhartha Institute of Pharmacy, Dehradun for the all possible help it has provided for conducting the research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kazmi, I., Narooka, A.R., Afzal, M. et al. Anticancer effect of ursolic acid stearoyl glucoside in chemically induced hepatocellular carcinoma. J Physiol Biochem 69, 687–695 (2013). https://doi.org/10.1007/s13105-013-0245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0245-8