Abstract
Even though intense exercise has traditionally been associated with a statistically significant accumulation of blood-borne biomarkers of free radical-mediated lipid peroxidation, it remains to be determined if the oxidative stress response is biologically significant. To examine biological significance, we calculated the critical difference of selected biomarkers of oxidants–antioxidants in the peripheral circulation of ten male subjects aged 24 ± 3 years. Venous blood was drawn in the resting supine position every hour over an 8-h period (Study 1). As proof-of-concept, supine venous blood was also obtained at rest and following maximal cycling exercise in a separate group of 13 males, mean age 22 ± 3 years (Study 2). The critical difference of electron paramagnetic resonance spin-trapped alkoxyl free radicals, lipid hydroperoxides, malondialdehyde, ascorbic acid, retinol, lycopene, α-tocopherol, β-carotene and α-carotene was calculated as 121%, 28%, 50%, 9%, 29%, 106%, 13%, 28% and 107%, respectively (Study 1). Maximal exercise was associated with a statistically significant (P < 0.05 vs. rest) reduction in α-tocopherol and retinol, and a corresponding rise in alkoxyl free radicals and lipid hydroperoxides (Study 2). However, these changes were all within the critical difference percentage value. In conclusion, these findings highlight the importance of distinguishing biological from statistical significance when assessing the physiological and clinical impact of exercise-induced oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence suggesting that free radicals increase susceptibility to certain disease processes has stimulated much research in the area of oxidative stress [20]. Likewise, exercise biochemists have primarily utilised markers of lipid peroxidation to determine whether or not exercise causes damage to cell membranes. For example, Ashton et al. [3] and more recently Fogarty et al. [16] have demonstrated a significant rise in malondialdehyde and lipid hydroperoxides following exhaustive aerobic exercise, and this increase in cell membrane oxidation was attributed to a greater production of free radicals and subsequent attack on membrane phospholipids. Furthermore, Davison et al. [13] has shown that type 1 diabetic patients have a greater basal and exercise-induced oxidative stress in comparison to healthy control subjects. These studies commonly aim to describe the metabolic and biochemical status of healthy and disease groups, and to examine the effect of an intervention such as aerobic exercise.
The metabolic status of an individual is represented by measurements obtained at a specific time of day; however, some parameters have circadian rhythms that may be daily, monthly or seasonal [17]. With this in mind, studies often pay little attention to how such measurements fluctuate from person to person, with most individuals having inherent fluctuations, which can be described as random variation close to a homeostatic set point [17]. Critical difference according to Fraser and Fogarty [17] is the change that must occur before a true biological difference can be claimed. For example, whilst the response to exercise may change the concentration of a metabolite mathematically (P < 0.05), the magnitude of that change may be biologically insignificant. Therefore, a clear distinction between statistical and biological significance must be determined before drawing conclusions as to whether any meaningful physiological or biochemical changes have occurred.
The concept and importance of critical difference is well recognised in clinical biochemistry but is often neglected in the field of exercise biochemistry. Reference data exist for a wide range of clinical and haematological analytes; however, to our knowledge, there is no reference data for any oxidative stress metabolite associated with the lipid peroxidation biochemical pathway and for lipid-derived free radical species. Thus, the primary aim of this investigation and of Study 1 was to determine the critical difference of selected oxidative stress and aqueous and lipid antioxidant indices. Proof of concept (Study 2) was determined by comparing the critical difference values with oxidative stress data obtained before and following exhaustive exercise, and as such we hypothesise that (a) exercise significantly increases oxidative stress and (b) the mathematical change following exercise exceeds the critical difference value for each oxidative stress metabolite.
Methods
Subject characteristics
Following local medical research ethics committee approval, ten (n = 10) apparently healthy male volunteers were recruited from the student population of a local university (age, 24 ± 3 years; stature, 178 ± 0.07 cm; body mass, 80 ± 14 kg; body mass index, 25 ± 3 kg/m2 and body fat, 14 ± 6%). Ten subjects were chosen as it has previously been stated that valid estimates of the components of variation can be obtained from a relatively small group of subjects over a reasonably short period of time [18].
Experimental design
Subjects were instructed to abstain from dietary antioxidant intervention for 6 weeks prior to the experimental phase, and also instructed to refrain from exercise, caffeine and alcohol for 24 h and to maintain their usual dietary pattern.
Experimental protocol
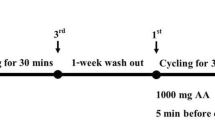
Subjects entered the laboratory at 8.00 am after a 12-h overnight fast, and body mass and stature were measured according to standard methods. An intravenous 18-gauge cannula (Venflon IV cannula, Becton-Dickinson, Sweden) connected to a three-way sterile stopcock (Connecta plus 3, Ohmeda, Sweden) was inserted into a prominent forearm antecubital vein. The cannula line was flushed with 3 ml physiological saline (Norton Steri-Amp, 0.9% NaCl, Steripak Ltd., Chesire, UK) every 30 min to keep the line patent (Fig. 1). Whilst in the supine position, blood was drawn from each individual by the same phlebotomist (this practice helped minimise pre-analytical variance) once every hour for an 8-h period (i.e. 9.00 am to 5.00 pm). Immediately after blood sampling, subjects were allowed to slowly stand up and walk for 15 min if desired but confined to the laboratory. Subjects remained in a supine position for 45 min prior to each blood collection. Arterialised capillary blood was taken at the same time as venous collection for packed cell volume and haemoglobin determination. Subjects abstained from food throughout the day to control for hormone fluctuations; however, controlled water intake (2 ml/kg body weight) was permitted after each blood sample.
Exercise protocol (study 2)
Thirteen (n = 13) apparently healthy male volunteers (age, 22 ± 3 years; stature, 179 ± 7 cm; body mass, 81 ± 9 kg; body mass index, 25 ± 2 kg/m2 and maximal oxygen uptake (VO2max), 41 ± 13 ml kg−1 min−1) were also recruited to perform a VO2max test. Subject number was determined by a previous exercise-induced oxidative stress study performed by our group [13]. The exercise test was designed to be progressive and incremental in order to elicit VO2max. A cadence of 60 rpm was maintained while workload was increased by 0.5 kg every 3 min until volitional fatigue. Validation of VO2max was obtained if the respiratory exchange ratio was >1.15 at the termination of test, had a plateau in the oxygen uptake/exercise intensity relationship (<2 ml kg−1 min−1) and had a heart rate value within 10 bpm of age predicated maximum (220 bpm-age). Oxygen uptake (VO2) was monitored continuously during exercise using an on-line automated gas analysis system (Medgraphics, CPX/D, Manchester, UK). Heart rate was also continuously recorded using a short angle telemetry system (Polar Sports Tester, Finland). Subjects were instructed to refrain from exercise and alcohol for 24 h before the test and were instructed to maintain their usual dietary pattern.
Metabolic measurements
To minimise analytical variance, all samples from each individual were assayed in the same batch, using the same batch of reagents, standards, and quality control materials, and the same biochemists performed all analysis. Following blood collection in EDTA and serum separation vacutainers, samples were centrifuged at 3,000 rpm for 10 min at 4°C, and serum/plasma aliquots were stored at −80°C. Blood for free radical determination was assayed on the same experimental day, while the remaining aliquots were assayed within 8 weeks of collection. Venous blood samples were drawn in a supine position before and following exhaustive exercise and corrected for a possible exercise-induced haemoconcentration.
Electron paramagnetic resonance spectroscopy
Electron paramagnetic resonance (EPR) spectroscopy in conjunction with ex vivo spin trapping using α-phenyl-tert-butylnitrone (PBN) was used to investigate the formation of free radicals as previously described [11]. It was considered not necessary to correct the EPR–PBN spectral lines for molecular decay. Intra-assay coefficient of variation (CV) at 1,795 arbitrary units (AU) was 5%. A stable synthetic radical diphenylpicrylhydrazil was measured ten consecutive times to determine analytical precision for the PBN-adduct.
Lipid peroxidation
Lipid peroxidation was estimated by measuring malondialdehyde and lipid hydroperoxide concentration using the respective assays of Young and Trimble [27] and Wolff [26]. Intra- and inter-assay CV for malondialdehyde at 0.56 μmol L−1 was 6.2% and 9.1%, respectively, and for lipid hydroperoxide at 0.57 μmol L−1 was 4.6% and 6%, respectively.
Aqueous and lipid antioxidants
The plasma concentrations of α-tocopherol, retinol, lycopene, α- and β-carotene were determined using the simultaneous HPLC assay of Thurnham et al. [24] and Catignani and Bieri [9]. Intra- and inter-assay CV were both <5%. The fluorometric assay of Vuilleumier and Keck [25] was used to determine plasma ascorbic acid concentration using metaphosphoric acid as a stabiliser. The inter-assay CV at 51.11 μmol L−1 was 0.72%.
Packed cell volume and haemoglobin
Packed cell volume (PCV) and haemoglobin concentration was measured on whole blood to correct for a possible change in exercise-induced plasma volume using the equations of Dill and Costill [15]. PCV (%) was measured using the standard microcapillary reader technique and corrected by 1.5% for plasma trapped within erythrocytes [10]. Haemoglobin (grammes per deciliter) was measured using a β-haemoglobin photometer (Hemocue Ltd., Angelholm, Sweden).
Statistical analysis
Data were analysed using parametric statistics following mathematical confirmation of a normal distribution by repeated Shapiro–Wilk test. Between-hour differences were analysed using a one-way ANOVA with a posteriori Tukey Honestly Significant Difference test. Pre- and post-exercise data were analysed using a paired samples t test. Alpha was established at P < 0.05 (95% confidence interval), and all values are reported as a mean ± standard deviation.
Analytical variation
Analytical variation (CVA) was calculated using the following equation:
Biological variation (CVB)
Assay data from each subject, collected at periodic times (eight specimens) as previously described (Study 1), was used to calculate within subject biological coefficient of variation (CVw) using the following equation:
Critical difference
The critical difference was assessed using the equation of Fraser and Fogarty [17]:
where:
- k :
-
constant determined by the probability level (2.77 at P < 0.05)
- CVA :
-
coefficient of analytical variation
- CVw :
-
coefficient of within subject variation
Results
There were no differences observed for any metabolite within or over the course of an 8-h period (P > 0.05).
Critical difference (study 1)
Table 1 summarises the critical difference and its residuals (CVA and CVB) for all oxidative stress indices measured. It is observed that the highest critical difference was obtained for the PBN-adduct concentration (121%), whilst the critical difference for malondialdehyde and lipid hydroperoxides was comparatively lower. For the antioxidants, ascorbic acid had the lowest critical difference percentage value (9%), while α-carotene had the highest (107%).
Exercise (study 2)
Exercise resulted in a statistically significant (P < 0.05 vs. rest) reduction in α-tocopherol and retinol and a corresponding rise in PBN alkoxyl adducts and lipid hydroperoxides. However, these changes were all within the critical difference percentage values as shown in Table 2.
Typical EPR spectra at rest and post-exercise in serum are illustrated in Fig. 2a, b, exhibiting the characteristic triplet of doublets from nitroxide PBN spin trapping. The hyperfine coupling constants were a N 13.6 G and aβH = 1.8 G, as confirmed by computer simulation (Fig. 2c) and are consistent with the trapping of a secondary oxygen-centred lipid-derived free radical species [11].
Typical EPR spectra of PBN-adducts extracted ex vivo from human blood in one volunteer at rest (a) and following maximal exercise (b). Computer simulation (c) identified a single species with nuclear hyperfine splittings of a N = 13.6 G and aβH = 1.8 G. All spectra were filtered and scaled identically
Discussion
This work highlights the importance of consulting with published critical difference values when assessing the physiological impact associated with exercise-induced oxidative stress.
Little is currently known regarding the critical difference of lipid peroxidation per se, with only a few studies examining the circadian variation of malondialdehyde in humans [7, 8] and one using animals [21]. To date, no attention has been given towards the critical difference of free radical species. This study presents novel data which describes a critical difference of 121%, 50% and 28% for the free radical components, PBN-adduct, malondialdehyde and lipid hydroperoxides, respectively.
We suggest that the EPR free radicals detected within the current investigation (Study 1 and 2) evolve during the metal-catalyzed reductive decomposition of lipid hydroperoxides formed subsequent to free radical-mediated damage to membrane phospholipids and are secondary alkoxyl free radical species [11]. The availability and concentration of fatty acid molecules in vivo is paramount to the detection of ex vivo PBN-trapped free radicals. Although the components of lipids were not measured in the present study, it may be postulated that as the day progressed, the predominant energy source was from an increased utilisation of fatty acid molecules derived from adipose tissue sites [23] and therefore an increase in circulating fats would provide more substrate within the systemic circulation for oxidation.
The present study describes a biological variation of 17.5% for malondialdehyde, somewhat lower than the PBN-adduct variation and slightly higher than the biological variation for lipid hydroperoxides. Since malondialdehyde is formed primarily by alkoxyl radical attack within the lipid peroxidation cascade [2], it is suggested that the detection of the PBN-adduct in the present study contributed towards malondialdehyde formation. This is supported by the low biological variation of the main lipid soluble antioxidants, α-tocopherol and β-carotene, thus, the lack of potent antioxidant variation (decreased scavenging capacity) in the venous circulation would inevitably allow the alkoxyl radicals to progress further within the cascade, and in so doing produce malondialdehyde. However, some degree of radical scavenging and protection is offered, as apparent by the much larger biological variation of the lipid soluble antioxidants, lycopene and α-carotene.
Gallagher et al. [19] report similar values for retinol (11%) and β-carotene (16%), whilst contrasting data are reported for the main lipid and aqueous antioxidants, α-tocopherol (17%) and ascorbic acid (15%), respectively. Since the analytical methods of antioxidant determination were the same in our study as those of Gallagher et al. [18], the only other explanation is perhaps a greater variance in subject antioxidant consumption, as there was no mention of excluding individuals supplementing with antioxidants prior to the experimental phase. In agreement with the present data, Maes et al. [22] display virtually identical α-carotene (38%) and retinol (9%) values in 20 apparently healthy Belgian men. In comparison, a larger value for ascorbic acid (18%) is shown, which may reflect a difference in the sensitivity of the various analytical methods employed.
This study used EPR spectroscopy to measure free radicals directly and observes that a single bout of high-intensity aerobic exercise statistically and mathematically enhances lipid-derived free radical and lipid hydroperoxide production, thus demonstrating the presence of oxidative stress. Published literature supports the postulation that exercise causes oxidative stress (see Table 3), and there is a clear statistical change in lipid hydroperoxides and free radicals when measured from baseline to immediately following exhaustive exercise.
Ashton et al. [2] postulates that the alkoxyl radical is generated from the lipid peroxidation of cellular membranes by primary free radical attack resulting in increased levels of plasma lipid hydroperoxides post-exercise. Direct free radical detection, in addition to a rise in lipid hydroperoxide concentration in the present study, suggesting that the damage inflicted to cellular membranes during exercise may well be primary free radical mediated through mitochondrial leakage and/or extracellular leukocyte activation [14]. Furthermore, since there is evidence of membrane peroxidation and damage following exercise, perhaps the integrity of the muscle cell membrane was compromised, although the extent of sarcolemmal permeability was not assessed in the current work.
Although the magnitude of change expressed as delta from pre- to post-exercise in our study agrees with that of other published literature, the mathematical differences are all found to be within the observed critical difference values for both lipid hydroperoxide and alkoxyl free radicals, thus causing an interpretive dilemma for the exercise biochemist. This ‘dilemma’ highlights the importance of observing statistical differences as a function of exercise, whilst the data would seem not to be physiologically or even clinically relevant. It is widely regarded that physiological stress brought about by exercise damages important biomolecules such as lipid, protein and DNA, and Halliwell [20] suggests that damage to these indices may be detrimental to normal cell and bodily function, and in some cases may be related to the onset of disease. Thus, in this instance, although mathematical significance may be achieved, if the data are not physiologically different, one must treat the data with caution in terms of interpretation as to the overall importance and relevancy of conclusions that may be drawn.
On the other hand, it may be important to observe a change in free radical production in the absence of any real physiological difference. While it was once thought that all free radical generation was harmful to the human system, it is now known that controlled amounts of free radicals and reactive oxygen species play important physiological roles, such as coordinating the body’s responses to injury and infection, and that some genes promoting free radical production and modulating antioxidant levels are beneficial for human survival and reproduction [20]. With regard to exercise, there has been a plethora of investigations documenting the importance of exercise training and free radical involvement in complex redox biochemical reactions for cell adaptation. Therefore, it is important in this type of scenario, where exercise-induced free radicals are used as cell signalling molecules for muscle adaptation, that investigators make sound judgements in terms of the importance and use of the critical difference concept.
Conclusion
This work demonstrates that exhaustive exercise can induce oxidative stress, and the magnitude of change in biochemical markers from rest to exercise, although mathematically significant, may not be biologically relevant. Critical difference is an important concept in clinical and exercise biochemistry, and we emphasise that investigators who measure oxidative stress take into consideration critical difference values in order to determine whether or not exercise contributes towards a meaningful physiological/clinical change. Moreover, the critical difference in the present work was calculated using biochemical data from apparently healthy male individuals. Future work should concentrate on determining the critical difference specifically for pathology.
References
Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL (2000) Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sport Exerc 32:1576–1581
Ashton T, Rowlands CC, Jones E, Young IS, Jackson SK, Davies B, Peters JR (1998) Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol 77:498–502
Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC (1999) Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol 87:2032–2036
Bailey DM, Davies B, Young IS (2001) Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin Sci 101:465–475
Bailey DM, Lawrenson L, McEneny J, Young IS, James PE, Jackson SK, Henry RR, Mathieu-Costello O, McCord JM, Richardson RS (2007) Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic Res 41:182–190
Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS (2010) Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol 109:449–456
Bridges AB, Fisher TC, Scott N, McLaren M, Belch JJF (1992) Circadian variation of white blood cell function and free radical in normal volunteers. Free Rad Res Comm 16:89–97
Bridges AB, Scott NA, McNeill GP, Pringle TH, Belch JJF (1992) Circadian variation of white blood cell aggregation and free radical indices in men with ischaemic heart disease. Eur Heart J 13:1632–1636
Catignani GL, Bieri JG (1983) Simulataneous determination of retinol and α-tocopherol in serum or plasma by liquid chromatography. Clin Chem 29:708–712
Dacie JV, Lewis SM (1968) Practical haematology. Churchill, London
Davison GW, Ashton T, Davies B, Bailey DM (2008) In vitro electron paramagnetic resonance characterisation of free radicals: relevance to exercise-induced lipid peroxidation and implications of ascorbate prophylaxis. Free Radic Res 42:379–386
Davison GW, Ashton T, George L, Young IS, McEneny J, Davies B, Jackson SK, Peters JR, Bailey DM (2008) Molecular detection of exercise-induced free radicals following ascorbate prophylaxis in type I diabetes mellitus: a randomised controlled study. Diabetologia 5:2049–2059
Davison GW, George L, Jackson SK, Young IS, Davies B, Bailey DM, Peters JR, Ashton T (2002) Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free Radic Biol Med 33:1543–1551
Davison GW, Morgan RM, Hiscock N, Garcia JM, Grace F, Boisseau N, Davies B, Castell L, McEneny J, Young IS, Hullin D, Ashton T, Bailey DM (2006) Manipulation of systemic oxygen flux by acute exercise and normobaric hypoxia: implications for reactive oxygen species generation. Clin Sci 110:133–141
Dill DB, Costill DL (1974) Calculation of the percentage changes in volumes of blood, plasma and red cells in dehydration. J Appl Physiol 37:247–248
Fogarty MC, Hughes CM, Burke G, Brown J, Trinick TR, Duly E, Bailey DM, Davison GW (2011) Exercise-induced lipid peroxidation: implications for deoxyribonucleic acid (DNA) damage and systemic free radical production. Environ Mol Mut 52:35–42
Fraser CG, Fogarty Y (1989) Interpreting laboratory results. BMJ 298:1659–1660
Fraser CG, Harris EK (1989) Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 27:409–437
Gallagher SK, Johnson LK, Milne DB (1992) Short- and long-term variability of selected indices related to nutritional status. II. Vitamins, lipids, and protein indices. Clin Chem 38:1449–1453
Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46:531–542
Kolosova NC, Mel’nikov VN, Shorin IP, Khasnulin VI (1983) Role of photoperiodicity and circadian rhythm of glucocorticoids in synchronising the fluctuations in free radical oxidation of lipids in rats. Biull Eksp Biol Med 96:99–101
Maes M, Weeckk S, Wauters A, Neels H, Scharpe S, Verkerk R, Demedts P, Desnyder R (1996) Biological variation in serum vitamin E concentrations: relation to serum lipids. Clin Chem 42:1824–1831
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Thurnham DI, Smith E, Flora PS (1988) Concurrent liquid-chromatographic assay of retinol, α-tocopherol, β-carotene, α-carotene, lycopene, and β-cryptoxanthin in plasma with tocopherol acetate as internal standard. Clin Chem 34:377–381
Vuilleumier JP, Keck E (1989) Fluorometric assay of vitamin C in biological materials using a centrifugal analyser with fluorescence attachment. J Micronutr Anal 5:25–34
Wolff SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hypoperoxides. Methods Enzymol 233:183–189
Young IS, Trimble ER (1991) Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Ann Clin Biochem 28:504–508
Acknowledgements
The authors extend their sincere gratitude and appreciation to Professor Alan Nevill (University of Wolverhampton, UK) for the helpful discussions regarding statistics used within the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davison, G.W., Ashton, T., McEneny, J. et al. Critical difference applied to exercise-induced oxidative stress: the dilemma of distinguishing biological from statistical change. J Physiol Biochem 68, 377–384 (2012). https://doi.org/10.1007/s13105-012-0149-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0149-z