Abstract
Computed tomography perfusion (CTP) is used as a tool to select ischemic stroke patients for endovascular treatment (EVT) and is currently investigated in the setting of extensive stroke with low Alberta Stroke Program Early CT scores (ASPECTS). The purpose of this study was to perform a comprehensive quantitative analysis of cerebral blood flow within the ischemic lesion compared to threshold-derived core lesion volumes. We hypothesized that the degree of cerebral blood volume (CBV) reduction within the ischemic lesion is predictive of irreversible tissue injury and functional outcome in patients with low ASPECTS. Ischemic stroke patients with an ASPECTS ≤ 5 who received multimodal CT on admission and underwent thrombectomy were analyzed. The ischemic lesion on CTP was identified, and CTP-derived parameters were measured as absolute means within the lesion and relative to the physiological perfusion measured in a contralateral region of interest. The degree of irreversible tissue injury was assessed using quantitative net water uptake (NWU). Functional endpoint was good outcome defined as modified Rankin Scale (mRS) scores 0–3 at day 90. One hundred eleven patients were included. The median core lesion volume was 71 ml (IQR: 25–107), and the median quantitative NWU was 9.5% (IQR: 6–13). Relative CBV (rCBV) reduction and ASPECTS at baseline were independently associated with NWU in multivariable linear regression analysis (ß: 12.4, 95%CI: 6.0–18.9, p < 0.0001) and (ß: − 0.78, 95% CI: − 1.53 to − 0.02; p = 0.045), respectively. Furthermore, rCBV was significantly associated with good outcome in patients with core volumes > 50 ml (OR: 0.16, 95% CI: 0.05–0.49, p = 0.001). Our study shows that rCBV reduction serves as an early surrogate for increase of NWU as a marker of irreversible tissue injury and lesion progression. Thus, the analysis of rCBV reduction within ischemic lesions may add another dimension to acute stroke triage in addition to core volumes or ASPECTS as indicators of the infarct extent and viability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefit of endovascular treatment (EVT) for acute ischemic stroke with extensive signs of infarction (i.e., low ASPECTS) is yet uncertain and currently investigated in several randomized clinical trials [1]. Accordingly, patient selection and treatment decision-making is a matter of current debate, as there is still no reliable threshold of infarct extent to guide EVT based on ischemic core volume, ASPECTS, or other neuroimaging tools. In the ongoing SELECT-2 trial (NCT03876457), CT perfusion (CTP) is utilized to guide EVT in patients with low ASPECTS (3–5), or high core volumes (≥ 50 ml). Even though CTP can quantify cerebral blood flow (CBF) and volume (CBV) within an area of hypoperfused brain tissue, in daily clinical practice, it is mainly confined to estimate total volumes of irreversible tissue injury (i.e., ischemic core) in relation to the volume of metabolically viable brain tissue that may be salvageable with rapid CBF restoration (i.e., penumbral tissue). This concept has been increasingly criticized for two reasons: (1) CTP-derived core lesion volumes may overestimate the true volume of irreversibly injured tissue (i.e., ghost core) and consequently lead to an exclusion of endovascular treatment in patients potentially benefitting from acute reperfusion therapy-options; and (2) the utilization of binary thresholds to assess viability of tissue on CTP (i.e., voxel infarcted yes/no) represents an oversimplified concept that does not encompass the complexity of pathophysiological processes, especially in transition zones of infarcted to normal tissue [2, 3].
Quantitative lesion net water uptake (NWU) is a CT imaging tool that quantifies the degree of edematous water uptake, which is the hallmark of ischemic brain undergoing infarction [4]. Recently, it has been observed that NWU is highly specific to capture irreversible tissue injury in anterior circulation large vessel occlusion (LVO) stroke [5]. Notwithstanding the latter, the quantification of NWU still requires manual post-processing and is therefore not yet available as an ad hoc imaging biomarker in acute stroke triage. In contrast, CTP is widely used in the assessment of stroke patients, and the relationship of quantitative brain perfusion and NWU could help to understand the early dynamics of ischemic hypoperfusion, reduction of CBF and CBV, and the formation of vasogenic edema as indicator of irreversible infarction.
The purpose of this study was to perform a comprehensive analysis of the relationship of CBV and CBF within the ischemic lesion and quantitative NWU as indicator of irreversible tissue injury in extensive baseline stroke. We hypothesized that quantitative CBV and CBF analysis within the ischemic lesion in low ASPECTS stroke is (1) directly associated with the degree of NWU and (2) predicts functional outcome more accurately compared to CTP-derived ischemic core volumes.
Methods
Patients
For this study, we retrospectively analyzed all consecutively treated patients with an acute ischemic stroke patients due to a LVO between June 2015 and May 2020 at a high-volume stroke center. Retrospective and fully anonymized data were analyzed after ethical review board approval, and the local ethics committee waived informed consent after review (WF 04/13). The data that support the findings of this study are available from the corresponding author upon reasonable request. The study was conducted in accordance with the ethical guidelines of the local ethics committee and in accordance with the Declaration of Helsinki.
All patients were retrospectively screened based on the following a priori–defined inclusion criteria: (1) acute anterior circulation stroke (2) due to a LVO of the MCA (3) with an ASPECTS of 0–5; (4) multimodal CT imaging protocol at admission including CT angiography (CTA) and CT perfusion (CTP); (5) known time window from symptom onset to admission imaging; (6) EVT procedure performed; and (7) absence of intracranial hemorrhage with significant mass effect (parenchymal hemorrhage (PH) type 2) according to Fiorelli et al. [6] and preexisting thromboembolic or hemodynamic infarctions in admission non-enhanced CT (NECT) or preexisting significant carotid stenosis. There were individual reasons for attempting MT in case of low ASPECTS: early presentation after symptom onset, significant perfusion mismatch despite large early ischemic core with tissue-at-risk in eloquent regions beyond areas contributing to the low ASPECTS, younger patient age, or specific request for therapy by family members. All treatment decisions were made by a board-certified neurointerventionalist in consensus with a board-certified attending stroke neurologist.
ASPECTS was rated on admission-NECT by a board-certified neuroradiologist and verified by an attending neuroradiologist. Discrepancies were settled in a joint discussion. A binary clinical good outcome was defined based on modified Rankin Scale (mRS) after 90 days with a score of 0 to 3 as previously reported in low ASPECTS patients [7]. The modified Thrombolysis in Cerebral Infarction (mTICI) scale was used to assess the degree of recanalization defining a result of mTICI 2b-3 as successful.
Image Acquisitions
All patients received multimodal stroke imaging at admission with NECT, CTA, and CTP performed in equal order on 256 dual slice scanners (Philips iCT 256): NECT, 120 kV, 280–340 mA, 5.0-mm slice reconstruction, 1-mm increment; CTA, 100–120 kV, 260–300 mAs, 5.0-mm slice reconstruction, 1-mm increment, 80-mL highly iodinated contrast medium, and 50 mL NaCl flush at 4 mL/s; and CTP, 80 kV, 200–250 mA, 5-mm slice reconstruction (max. 10 mm), slice sampling rate 1.50 s (min. 1.33 s), scan time 45 s (max. 60 s), biphasic injection with 30 ml (max. 40 ml) of highly iodinated contrast medium with 350 mg iodine/ml (max. 400 mg/ml) injected with 6 ml/s, followed by a 30-ml sodium chloride chaser bolus.
Image Analysis
Anonymized data was processed at an imaging core lab. All analyses were conducted by an experienced neuroradiologist (> 10 years of experience). Subsequently, all cases were screened in a consensus reading with a second experienced neuroradiologist. Infarct core and penumbra have been assessed using CT perfusion (CTP) with whole brain coverage. The hypoperfusion volume has been defined as the volume of tissue with a prolonged Tmax of at least 6 s (Tmax > 6 s), and infarct core has been defined by the volume of tissue with a relative cerebral blood flow (rCBF) of 30% or less of the maximum (rCBF ≤ 30%) as established gold standard [8, 9]. For quantitative analysis of the perfusion parameters, the total ischemic area was first visually identified using MTT maps. Within this ischemic area of bolus delay, the core lesion for quantitative analysis has been defined as lesion of high infarct probability by CBV parameter maps, and a region of interest (ROI) was placed within the CBV lesion using IntelliSpace Portal analysis software (Philips Healthcare, Best, Netherlands). The mean CBV in ml × 100 g−1 of the lesion was quantified. The corresponding CBV-ROI was then placed automatically in the CBF/MTT and TTP parameter maps and used to measure CBF in in ml × 100 g−1/ min, MTT in s, and TTP in s, respectively. Simultaneously, every ROI was automatically mirrored to the contralateral hemisphere to measure the physiological cerebral perfusion of every parameter in a corresponding ROI. Relative differences were calculated. In cases without certain CBV lesion, a CBF lesion was used to define the ROI for quantitative measurement of the perfusion parameters. This ROI was then placed equally on all parameter maps. Subsequently, NWU was quantified as described elsewhere [10, 11]. As previously reported by Minnerup et al., the ROI representing the core lesion was interpolated in the NECT map within the early hypoattenuated lesion, and densitometric measurements were performed (Dischemic) [10]. A mirrored ROI was placed within normal tissue of the contralateral hemisphere (Dnormal). ROIs in NECT were segmented with semiautomatic edge detection and sampled between 20 and 80 HU using commercially available software (Analyze 11.0, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) [4].
Collateral status was assessed using an established 5-point scoring system [12]. Intracranial CTA maximum intensity projections (MIPs) were used for grading: 0 = absent collaterals in > 50% of an MCA-M2 branch (superior or inferior division) territory; 1 = diminished collaterals in > 50% of an MCA-M2 branch territory; 3 = collaterals equal to the contralateral hemisphere; and 4 = increased collaterals [12]. Good collaterals were defined as CS 2–4 and poor collaterals as CS 0–1 according to Kim et al. and modified by Broocks et al. [13, 14].
Statistical Analysis
Variable distribution was described by medians and interquartile ranges (IQRs). Absolute and relative frequencies are given for categorical data. In order to compare independent samples of metric or categorical outcome, Mann–Whitney U test or χ2 test was used, respectively (Table 1). Primary outcome was quantitative NWU and dichotomized NWU (malignant versus non-malignant NWU). Malignant NWU was defined as baseline quantitative NWU > 12.7%, which has previously been described as accurate predictor of malignant infarction [11]. Secondary endpoint was favorable functional outcome defined as mRS 0–3 at day 90. We tested the correlation of absolute and relative cerebral perfusion parameters as well as the correlation of the covariables (ASPECTS, hypoperfusion volume, ischemic core volume, time from onset to imaging, and NIHSS) with NWU. Subsequently, the medians of the aforementioned independent variables were tested separately for patients with malignant NWU versus non-malignant NWU using Mann–Whitney U tests. Multivariable linear regression was performed with NWU as dependent parameter and relative CBV reduction, ASPECTS, age, sex, hypoperfusion, and core volume as independent parameters. Moreover, receiver-operating characteristic (ROC) curve analyses were performed to assess the diagnostic accuracy of the independent variables with malignant NWU as binary outcome. Multivariable logistic regression analysis was used to assess the independent association of the baseline variables with malignant NWU. Regarding functional outcome, logistic regression analyses were performed to assess the association of the independent variables with favorable outcome. To compare the diagnostic accuracy to classify outcome of NWU and rCBV reduction versus core and penumbra volume, the AUC of two models were compared (model 1, NWU + rCBV; model 2 = core volume and hypoperfusion volume). Additionally, the association of the baseline variables and outcome were analyzed only including patients with an rCBF-derived core volume of > 50 ml (as defined in SELECT-2 [15]). Finally, a sub-analysis was performed to assess the impact of the collateral status.
A statistically significant difference was accepted at a p value of less than 0.05. Analyses were performed using Stata 17.0 (StataMP, StataCorp, TX, USA).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Patients
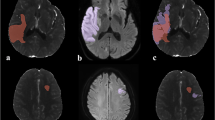
A total of 111 patients were analyzed. The median age was 77 years (IQR: 66–84) and 53 (48%) patients were female. The median NIHSS of this low ASPECTS cohort was 17 (IQR: 14–20). The median time from symptom onset to imaging was 3.5 h (IQR: 2.3–5.5). 14 patients (13%) had an ASPECTS of 0–2 (ASPECTS 0: 2, ASPECTS 1: 7, ASPECTS 2: 5), while 13 patients (12%) had an ASPECTS of 3, 27 (24%) patients an ASPECTS of 4, and 57 (51%) an ASPECTS of 5. The median core volume based on rCBF ≤ 30% was 71 ml (IQR: 25–107) and the hypoperfusion volume based on Tmax > 6 s was 91 ml (IQR: 50–145). Sixty-three patients (57%) had a core volume of ≥ 50 mL. The mean CBV of the ischemic lesion was 1.08 (SD ± 0.64; range, 0.013–3.51) ml × 100 g−1 and the mean relative CBV (rCBV) reduction was 0.70 (SD ± 0.18; range, 0.04–0.99). The median NWU was 9.5% (IQR: 5.9–13). Sixty-eight patients (61%) underwent successful EVT (mTICI 2b-3). Out of 111 patients, pre-stroke mRS was known in 37 cases. The pre-stroke mRS was known and documented in 37 cases (33.3%). Eight patients showed a pre-stroke dependency with an mRS of 3. Nineteen patients had a known pre-stroke mRS of 0, 8 patients with mRS 1, and 2 patients with mRS 2, respectively. Favorable outcome at day 90 (mRS 0–3) was observed in 20 patients (18%); 37 patients (33%) died. The rate of patients with functional independence defined as mRS 0–2 was 12 (11%). PH type 2 was observed in 10 patients (9.0%). Patient characteristics are displayed in Table 1. An example is illustrated in Fig. 1.
Comparing patients with favorable outcome at day 90 (mRS 0–3) to patients with an mRS 4–6, we observed that patients with favorable outcomes were younger (65 versus 78 years, p = 0.007), showed a lower median NIHSS on admission (14 versus 19, p < 0.001), had a higher ASPECTS (5 versus 4, p < 0.001), and evidenced better intracranial collaterals (Table 1). The median NWU was lower in patients with favorable outcome (5.9% versus 10.9%, p < 0.001). Regarding the CTP-derived parameters, rCBV reduction (0.65 versus 0.77, p < 0.001) and ischemic core lesion volume (rCBF < 30%; 35 ml versus 72 ml, p = 0.04) were different when comparing both patient groups.
Quantitative NWU as Indicator of Irreversible Tissue Injury
Based on pairwise correlation analysis of the perfusion derived parameters, hypoperfusion volume (− 0.24, p = 0.01), core volume (0.23, p = 0.02), CBV of the ischemic lesion (− 0.24, p = 0.01), and relative CBV reduction (0.45, p < 0.001) correlated significantly with quantitative NWU. Absolute CBF, TTP, MTT, and relative CBF, MTT, or TTP changes did not correlate with NWU. Among further baseline parameters, ASPECTS correlated with NWU (− 0.28, p = 0.003). Subsequently, multivariable linear regression was performed with NWU as dependent parameter and relative CBV reduction, ASPECTS, age, sex, hypoperfusion, and core volume as independent parameters. Absolute CBV was excluded due to collinearity. The independent predictors of NWU were relative CBV reduction (ß = 12.4, 95% CI: 6.0–18.9, p < 0.0001) and ASPECTS (ß = − 0.78, 95% CI: − 1.53 to − 0.02, p = 0.045) (Fig. 2, Table 2).
Association with Early “Malignant NWU”
Twenty-nine patients (26%) showed “malignant NWU” at baseline (defined as NWU > 12.7% [11]). Relative CBV reduction was significantly higher in patients with malignant NWU (0.83 versus 0.71, p < 0.01). Further baseline parameters showed no statistical difference when comparing patients with malignant NWU versus non-malignant NWU. Using the median rCBV reduction (0.74) to distinguish two groups (high versus low rCBV reduction), the proportion of patients with malignant NWU was 72% for patients with high rCBV reduction compared to 42% for patients with low rCBV reduction (p < 0.01).
In univariable ROC curve analysis, rCBV (AUC: 0.70, 95% CI: 0.6–0.78, p < 0.01) showed the highest values for discriminating between malignant and non-malignant NWU, with > 0.78 as optimal cut-off yielding a sensitivity of 69% and specificity of 76%. Further baseline parameters showed an AUC between 0.51 and 0.63 (Supplemental Table I).
Prediction of Favorable Outcome
Comparing patients with favorable and poor outcome, the median rCBV reduction was significantly higher in patients with poor outcome (0.77 versus 0.65, p < 0.01). After dichotomization, the proportion of patients with favorable outcome was 30% for patients with high rCBV reduction compared to 55% in patients with low rCBV reduction. Only 4% of the patients with high rCBV reduction achieved functional independence at day 90, compared to 18% in patients with low rCBV reduction (p = 0.01). In univariable logistic regression analysis with favorable outcome as dependent parameter, rCBV reduction (OR per 10% rCBV reduction: 0.77, 95% CI: 0.60–0.99, p = 0.047) and ASPECTS (OR: 2.37, 95% CI: 1.12–4.99, p = 0.02) were significant predictors. Ischemic core volume was by trend associated with outcome (OR: 0.99, 95% CI: 0.98–1.00, p = 0.06). The association of rCBV reduction and functional outcome was significantly modified by baseline core volumes (Fig. 3). In patients with core volumes of > 50 ml, a rCBV reduction of 10% was associated with an OR of 0.16 (95% CI: 0.05–0.49, p = 0.001), while there was no significant association between rCBV reduction and functional outcome in patients with a core volume < 50 ml (OR: 7.48, p = 0.34). The interaction term of rCBV reduction and core was significant (p = 0.007) (Fig. 4). To compare the diagnostic accuracy to classify outcome of NWU and rCBV reduction versus core and penumbra volume, the AUC of two models were compared (model 1, NWU + rCBV; model 2 = core volume and hypoperfusion volume). The dependent variable was favorable outcome at day 90 defined as described using mRS scores 0–3. The AUC of the rCBV/NWU model was 0.83, and the AUC for the mismatch model was 0.68.
Prediction of functional outcome by rCBV reduction and core volume. Impact of rCBV reduction (x axis) on the probability for favorable outcome at day 90 separately for patients with core volume < 50 ml and > 50 ml. There was no significant association between rCBV reduction and outcome in patients with a smaller core volume (blue graph)
Sub-analysis Impact of Intracranial Collaterals
Thirty patients (27%) had intermediate-good collaterals, and 81 patients (73%) had poor collaterals. The median rCBV reduction was 65% (IQR: 47–76) if better collaterals were present, which was significantly lower than the rCBV reduction in patients with poor collaterals (77%, IQR: 68–85, p < 0.001). A better collateral score was inversely associated with a lower rCBV reduction (8%, 95% CI: 5–12, p < 0.0001, for every 1-point increase) and lower baseline NWU (− 1.6%, 95% CI: − 2.8 to − 0.4, p = 0.01, for every 1-point increase).
Discussion
Our study assessing the relationship of quantitative NWU as marker of irreversible tissue injury and cerebral perfusion parameters in extensive baseline stroke revealed several findings: (1) rCBV reduction was the only CTP-derived parameter that was significantly associated with quantitative NWU together with ASPECTS; (2) rCBV reduction showed the highest diagnostic accuracy as a classifier to discriminate between malignant and non-malignant NWU as indicator of aggravated ischemic lesion progression; (3) rCBV reduction was significantly associated with favorable outcome at day 90 in patients with a large ischemic core, while core volume itself was no significant predictor of outcome, emphasizing the value of rCBV reduction as a marker of “ischemia depth” in large cores; (4) better collaterals (occurring in 27% of this low ASPECTS cohort) were associated with significantly lower rCBV reduction highlighting the association of favorable collateral profiles and comparably lower ischemic edema development (i.e., NWU).
Currently, several ongoing trials evaluate the effect of thrombectomy in patients with low ASPECTS [1]. Yet, it remains uncertain how to select these patients for treatment, which is reflected by the different study protocols of the present trials. In daily clinical practice, CTP is mainly used to estimate ischemic core volume (i.e., volume that is thought to represent irreversible tissue injury), compared to the total volume of hypoperfused brain tissue [16,17,18]. Accordingly, the presence of ischemic penumbra (metabolically viable brain tissue that may be salvageable with rapid CBF restoration) is the fundamental rationale for acute reperfusion therapy [19]. Still, the dynamics of early lesion progression and particularly the assessment of irreversible tissue injury remains ambiguous also considering different inter-individual progression rates (i.e., slow versus fast progressors) [20]. Previously, thresholds of the perfusion derived parameters were determined that optimally estimate the aforementioned volumes, for instance, a relative MTT of 145% to define the penumbra or absolute cerebral blood volume below 2 ml × 100 g−1 as ischemic core [21], while currently, rCBF < 30% and Tmax > 6 s are used as gold standard, respectively. In SELECT-2, CTP is utilized to guide EVT in patients with low ASPECTS (3–5), or high core volume (rCBF/apparent diffusion coefficient ≥ 50 ml). A threshold for voxel classification into “core” and “penumbra” may seem pathophysiologically appealing suggesting a switch-like cut-off that indicates whether the affected brain tissue evolves into infarction [2]. While some studies implied that these CTP thresholds may predict final infarct volume, others suggest that threshold-derived maps substantially overestimate ischemic changes, which may lead to an exclusion of patients for EVT, despite a potential clinical benefit [3, 22, 23]. If, and to what extent, CTP leads to an overestimation of the true volume of irreversible tissue injury in clinical routine is yet not well understood. Accordingly, a previous analysis of the HERMES group showed that despite its prognostic value, core volumes were not helpful in the prediction of a treatment benefit of EVT [19].
In contrast, the data of the present study supports a more granular delineation of early lesion progression dynamics suggesting a multi-stage chronological approach [24]. After onset of an arterial occlusion, the affected brain tissue undergoes oxygen deprivation, which leads to a compensatory CBF increase. After failure of these compensatory mechanisms and CBF decline, the metabolically viable tissue (i.e., penumbra) shows transformation to an irreversible stage over time. Subsequently, water homeostasis disrupts even before breakdown of the blood–brain barrier resulting in net uptake of water and cations into the ischemic tissue. Hence, the assessment of quantitative CTP could help to reflect lesion progression particularly in patients with large lesion extent. These lesion characteristics within a defined ischemic core may show significant variation which might be a hint of its viability. Concretely, two patients with a similar ischemic lesion size but differences in rCBV reduction may have a different level of NWU and, therefore, irreversible tissue injuries. This pathophysiological evolution of a multi-stage, chronological process from hypoperfusion to unviable tissue, was supported by the results of our study showing that the rCBV reduction in patients with low ASPECTS and core volume > 50 ml ranged from 42 to 99% (median 79%, IQR: 71–88%) and were significantly associated with quantitative NWU, as indicator of irreversible tissue injury, and functional outcome. This finding is in-line with the results of previous studies observing an association of rCBV reduction and diffusion restriction, which is often considered as gold standard for defining early ischemic core volumes, in stroke patients within 9 h of onset [25]. In contrast, CBF, MTT, or TTP changes did not correlate with NWU, which emphasizes that these parameters are less suitable to define true irreversible tissue injury.
Illustrated by the results of this study, further specification of lesion properties could have high clinical relevance in extensive baseline stroke, particularly regarding the discrimination of reversible versus irreversible tissue injury, treatment decision-making, and long-term outcome. Moreover, early prediction of malignant infarction characterized by aggravated edema formation might be of high clinical interest. Recently, it has been observed that the overestimation of CTP-derived core volumes was significantly associated with quantitative NWU, particularly in patients with large core volumes [24]. However, the assessment of quantitative NWU still requires post-processing and is therefore not available as imaging tool in acute stroke triage. Therefore, the significant interrelation of rCBV with quantitative NWU might help to estimate the degree of irreversible tissue injury. In 34 patients of this cohort (30%), rCBV reduction was > 80%, which showed a specificity of 83% to classify early malignant edema in ROC curve analysis. Correspondingly, the mean quantitative NWU of these patients was 13.6% (compared to 9.8% of the total cohort). This is higher than the reported thresholds that predict malignant infarction (i.e., 12.7% [11]), or futile recanalization (i.e., 10% [26]). The comparably low sensitivity (69% when using the optimal cut-off to classify early malignant edema) may be caused by partially increased CBV as a result of autoregulation processes following arterial vessel occlusion, which results in a drop of the cerebral perfusion pressure. Subsequently, intracranial resistance vessels dilate to maintain blood flow, which results in a CBV increase. Therefore, severe ischemia may temporarily be associated with normal to elevated CBV values which is often visually evident in an absence of a lesion in the CBV parameter map. Nonetheless, patients with low ASPECTS are characterized per definition by significant hypoattenuation in the brain tissue, which directly reflects edematous water uptake and is therefore most likely accompanied by significant CBV reduction. Still, the existence of good intracranial collaterals in low ASPECTS stroke should be considered (which might be linked to higher CBV values). Previously, it was reported that up to 1/3 of low ASPECTS stroke patients might show good collaterals, which has recently been linked to lower edematous water uptake and better functional outcome [27, 28]. In this study, 27% of the patients evidenced intermediate-good collaterals, which is similar to previous reports analyzing the impact of collaterals in patients with low ASPECTS [14, 27], and these patients showed a significantly lower rCBV reduction (65% versus 77%) and lower quantitative NWU (6% versus 11%).
The current patient cohort represents patients with a time window of < 6 h (median 3.5 h). Recently, Almallouhi et al. investigated the effect of recanalization in patients with extended time and lesion window and observed a potential treatment benefit with regard to 90-day outcomes [29]. Still, no randomized data exists whether thrombectomy may be beneficial in patients with extended time and lesion window. Several randomized trials are currently running to investigate the effect of thrombectomy in this patient group applying different inclusion criteria including patients up to 24 h after onset and with different lower ASPECTS thresholds. The reduction of rCBV might be an interesting tool for this patient subgroup as it may further classify lesion progression.
This study has all limitations that come along with a retrospective study design including a relatively small number of patients, partially due to rigorous inclusion criteria. The monocentric analysis may contain selection bias and needs to be validated externally. The intention was to obtain a homogenous patient cohort (i.e., only including patients with CT-based ASPECTS 0–5). Further studies are needed to translate our findings in the clinical context including a more granular approach in functional endpoints, for instance, by using the NIHSS at day 90 and respective of the definitive demarcation in follow-up imaging. Although quantitative NWU is thought to reflect irreversible tissue injury, rare cases of edema reversibility have recently been discussed [30]. This phenomenon was associated with higher degree of reperfusion and faster times from imaging to reperfusion; however, its occurrence in patients with extensive infarction is unknown and requires further investigation. Patients with parenchymal hemorrhage type 2 were excluded and therefore not analyzed. The reduction of rCBV could also be used to predict secondary hemorrhage which constitutes the second major injury volume following stroke besides edema [31]. The present study may directly serve future efforts to implement quantifiable image parameters to be used for automated algorithms in acute stroke triage or clinical studies [32].
Conclusion
In this study focusing on low ASPECTS patients, the reduction of rCBV was an indicator of quantitative NWU and, thus, irreversible tissue injury and lesion progression. The additional analysis of rCBV reduction as marker of the ischemia depth could be used as a complementary tool, especially together with NECT-ASPECTS and collateral profiles, to assess lesion characteristics in extensive baseline stroke (i.e., low ASPECTS or core volumes > 50 ml) and to guide treatment decision-making. Future research is needed to investigate how rCBV reduction mediates treatment effects in low ASPECTS stroke.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bendszus M, Fiehler J, Thomalla G. New interventional stroke trials. Clin Neuroradiol. 2019;29:1.
Flottmann F, Broocks G, Faizy TD, Ernst M, Forkert ND, Grosser M, et al. Ct-perfusion stroke imaging: a threshold free probabilistic approach to predict infarct volume compared to traditional ischemic thresholds. Sci Rep. 2017;7:6679.
Goyal M, Ospel JM, Menon B, Almekhlafi M, Jayaraman M, Fiehler J, et al. Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke. 2020;51:3147–55.
Broocks G, Flottmann F, Ernst M, Faizy TD, Minnerup J, Siemonsen S, et al. Computed tomography-based imaging of voxel-wise lesion water uptake in ischemic brain: relationship between density and direct volumetry. Invest Radiol. 2018;53:207–13.
Broocks G, McDonough R, Meyer L, Bechstein M, Kniep H, Schon G, et al. Reversible ischemic lesion hypodensity in acute stroke ct following endovascular reperfusion. Neurology. 2021;97:e1075–84.
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the european cooperative acute stroke study i (ecass i) cohort. Stroke. 1999;30:2280–4.
Meyer L, Bechstein M, Bester M, Hanning U, Brekenfeld C, Flottmann F, et al. Thrombectomy in extensive stroke may not be beneficial and is associated with increased risk for hemorrhage. Stroke. 2021;52:3109–17.
Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal ct perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–40.
Lee TY, Murphy BD, Aviv RI, Fox AJ, Black SE, Sahlas DJ, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37:2201; author reply 2203
Minnerup J, Broocks G, Kalkoffen J, Langner S, Knauth M, Psychogios MN, et al. Computed tomography-based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: a multicenter observational study. Ann Neurol. 2016;80:924–34.
Broocks G, Flottmann F, Scheibel A, Aigner A, Faizy TD, Hanning U, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke. 2018;49:1906–12.
Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant cta collateral profile is highly specific for large admission dwi infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–6.
Kim JT, Liebeskind DS, Jahan R, Menon BK, Goyal M, Nogueira RG, et al. Impact of hyperglycemia according to the collateral status on outcomes in mechanical thrombectomy. Stroke. 2018;49:2706–14.
Broocks G, Kniep H, Schramm P, Hanning U, Flottmann F, Faizy T, et al. Patients with low alberta stroke program early ct score (aspects) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg. 2020;12:747–52.
Sarraj A, Hassan AE, Abraham M, Ribo M, Blackburn S, Chen M, et al. Express: a randomized controlled trial to optimize patientas selection for endovascular treatment in acute ischemic stroke (select2): Study protocol. Int J Stroke. 2021:17474930211035032
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21.
Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–803.
Campbell BCV, Majoie C, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55.
Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: Clinical and research implications. Stroke. 2017;48:2621–7.
Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-ct assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–85.
Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in swift prime. Ann Neurol. 2016;79:76–89.
Boned S, Padroni M, Rubiera M, Tomasello A, Coscojuela P, Romero N, et al. Admission ct perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9:66–9.
McDonough R, Elsayed S, Meyer L, Ewers T, Bechstein M, Kniep H, et al. Low baseline ischemic water uptake is directly related to overestimation of ct perfusion-derived ischemic core volume. Preprint at Scientific Reports. 2022
Knash M, Tsang A, Hameed B, Saini M, Jeerakathil T, Beaulieu C, et al. Low cerebral blood volume is predictive of diffusion restriction only in hyperacute stroke. Stroke. 2010;41:2795–800.
Nawabi J, Flottmann F, Kemmling A, Kniep H, Leischner H, Sporns P, et al. Elevated early lesion water uptake in acute stroke predicts poor outcome despite successful recanalization - when “tissue clock” and “time clock” are desynchronized. Int J Stroke. 2021;16:863–72.
Tan BY, Wan-Yee K, Paliwal P, Gopinathan A, Nadarajah M, Ting E, et al. Good intracranial collaterals trump poor aspects (alberta stroke program early ct score) for intravenous thrombolysis in anterior circulation acute ischemic stroke. Stroke. 2016;47:2292–8.
Broocks G, Kemmling A, Meyer L, Nawabi J, Schon G, Fiehler J, et al. Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke. 2019;50:3424–30.
Almallouhi E, Al Kasab S, Hubbard Z, Bass EC, Porto G, Alawieh A, et al. Outcomes of mechanical thrombectomy for patients with stroke presenting with low alberta stroke program early computed tomography score in the early and extended window. JAMA Netw Open. 2021;4:e2137708.
Broocks G, McDonough R, Meyer L, Bechstein M, Dipl Ing HK, Schon G, et al. Reversible ischemic lesion hypodensity in acute stroke ct following endovascular reperfusion. Neurology. 2021
Nawabi J, Kniep H, Schon G, Flottmann F, Leischner H, Kabiri R, et al. Hemorrhage after endovascular recanalization in acute stroke: lesion extent, collaterals and degree of ischemic water uptake mediate tissue vulnerability. Front Neurol. 2019;10:569.
Nowinski WL, Gupta V, Qian G, He J, Poh LE, Ambrosius W, et al. Automatic detection, localization, and volume estimation of ischemic infarcts in noncontrast computed tomographic scans: method and preliminary results. Invest Radiol. 2013;48:661–70.
Author information
Authors and Affiliations
Contributions
WH, LM, AK, JF, GB have contributed in conception and design of the study. GB, CR, WH, WH, LM, MW, RM, SE, MB, and GB have contributed in acquisition and analysis of data.
WH, LM, GS, HK, AK, JF, UH, and GB have contributed in drafting a significant portion of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was conducted in accordance with the ethical guidelines of the local ethics committee (Ethikkommission der Ärztekammer Hamburg) and in accordance with the Declaration of Helsinki. Additionally, approval from local ethics committees of the participating hospitals was obtained. Only anonymized data were analyzed. The requirement of informed consent was waived by ethics committees.
Conflict of Interest
JF: research support—German Ministry of Science and Education (BMBF), German Ministry of Economy and Innovation (BMWi), German Research Foundation (DFG), European Union (EU), Hamburgische Investitions- und Förderbank (IFB), Medtronic, Microvention, Route92, Stryker. Consultant for: Acandis, Bayer, Boehringer Ingelheim, Cerenovus, Evasc Neurovascular, MD Clinicals, Medtronic, Microvention, Penumbra, Phenox, Stryker, Transverse Medical. Stock holder: Tegus Medical.
Competing Interests
All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work is dedicated to the memory of Wolfgang Haupt, MD.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haupt, W., Meyer, L., Wagner, M. et al. Assessment of Irreversible Tissue Injury in Extensive Ischemic Stroke—Potential of Quantitative Cerebral Perfusion. Transl. Stroke Res. 14, 562–571 (2023). https://doi.org/10.1007/s12975-022-01058-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01058-9