Abstract
The poor prognosis of subarachnoid hemorrhage (SAH) might be associated with sympathetic nerve activation (catecholamine surge) initiated by hypothalamic injury. As renal denervation (RD) has been shown to exert protective effects on cardiovascular dysfunction by suppressing increased central sympathetic nerve activation, we examined whether RD improved the experimental SAH prognosis in this study. Two hundred thirty-eight male Sprague-Dawley rats were divided into sham-operated and SAH-operated groups, and then each rat was further separated into Sham-operated and RD-operated groups. Bilateral RD was performed approximately 45 min after SAH induction. We examined the effect of RD on early brain injury (EBI) and delayed cerebral ischemia (DCI) as a primary endpoint, and also explored the effect on cerebral vasospasm (CVS) as a secondary endpoint. Although RD did not exert significant effects on primary endpoint, RD significantly prevented CVS and reduced SAH-induced increases in the number of phosphorylated extracellular signal-regulated kinase (ERK)-positive endothelial cells, cyclooxygenase-2 expression, and macrophage infiltration in major cerebral arteries. Moreover, RD significantly decreased the areas displaying dopamine β-hydroxylase and glial fibrillary acidic protein immunopositivity in the paraventricular nucleus of the hypothalamus and serum angiotensin II levels, all of which were increased by SAH. Although RD decreased systolic blood pressure, significant changes in cerebral blood flow were not observed compared with SAH + Sham group. Based on the findings, RD improved CVS by reducing endothelial cell damage and the effects were associated with the stabilization of central sympathetic nerve activation in a SAH model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is one of the most serious diseases in patients with stroke, and the outcome has not yet improved. The main factors determining the SAH prognosis are early brain injury (EBI) and delayed cerebral ischemia (DCI) [1]. EBI develops within the first 72 h after a cerebral aneurysm rupture, and the pathogenesis is initiated by increased intracranial pressure and a subsequent decrease in cerebral blood flow (CBF), which is characterized by a disturbance in the microcirculation, microthrombosis, disrupted blood-brain barrier (BBB), cerebral edema, glutamate excitotoxicity, and cellular apoptosis [1, 2]. On the other hand, DCI commonly occurs from 4 to 14 days after SAH. As patients with SAH presenting with DCI do not always show anatomical narrowing of the major cerebral arteries (cerebral vasospasm: CVS), the pathogenesis may be multifactorial and includes microcirculatory constriction, cortical spreading depression, inflammation, apoptosis, increased levels of vasoconstrictor peptides, and disrupted BBB [1, 3]. Those two share many of the same pathogenic factors and contribute the functional poor outcome in the patients with SAH [2, 4].
The high mortality and morbidity of SAH are sometimes caused by non-neurological complications such as arrhythmia, cardiomyopathy, and neurogenic pulmonary edema, all of which are associated with sympathetic nerve activation (catecholamine surge) [5]. The activation of this nerve is initiated by hypothalamic injury in a hyperacute phase after SAH [6]. On the other hand, researchers have studied the potential correlation between sympathetic nerve activation and the pathogenesis of SAH brain for many years. Although some reports revealed positive correlations between these factors [7, 8], others suggested a negative correlation [9, 10]. Therefore, the role of sympathetic nerve activation on SAH brain currently remains to be defined.
Renal denervation (RD) has been introduced to exert a blood pressure (BP)-lowering effect and decrease sympathetic outflow in patients with severe hypertension [11, 12]. Although recent studies have reported conflicting results regarding whether RD exerts a positive effect on BP in the hypertensive patients [13], the potential mechanism of protection against cardiovascular injuries is still being investigated. The proposed mechanism underlying the antihypertensive effect of RD is described below; RD inhibits activated renal nerves that contain afferent sensory nerves and efferent sympathetic nerves. The afferent sensory nerves have complicated connections to the center of sympathetic nervous system, including the rostral ventrolateral medulla (RVLM) in the medulla and paraventricular nucleus (PVN) in the hypothalamus. Then, the efferent sympathetic nerves modulate renal sympathetic stimulation and inhibit the upregulation of the renin-angiotensin system [14,15,16,17].
Recently, we have explored the protective effects of RD on experimental stroke. First, pretreatment with RD slowed stroke onset in hypertensive rats, which was associated with reductions in oxidative stress and glial activation in the PVN and cerebral cortex [18]. Next, we revealed that posttreatment with RD improved ischemic neurological deficit and cerebral infarction in hypertensive rats with transient middle cerebral artery occlusion by suppressing the elevated BP after ischemic induction [19]. Therefore, we postulated that the protective effects of RD on stroke might be promising, and RD represents a good candidate to improve the SAH prognosis by stabilizing sympathetic nerve activity.
The aim of this study was to clarify whether the stabilization of sympathetic nerve activation by RD improved the SAH prognosis. Therefore, we evaluated the effect of posttreatment with RD on EBI and DCI as primary endpoints, and our secondary objective was to explore the effect of RD on CVS in rats with SAH.
Materials and Methods
Experimental Animals and Study Groups
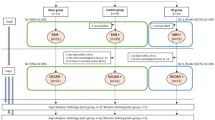
All experiments were approved by the Institutional Animal Care and Use Committee of Kumamoto University. Two hundred thirty-eight male Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) weighting 259 to 397 g (from 9 to 10 weeks-old) were housed in a room with constant environmental conditions (temperature 22° ± 2 °C; humidity 55 ± 5%; 12/12 h light/dark cycle) and provided assessment to food and water ad libitum through this study. Rats were randomly divided into the following two groups: (1) sham-operated group (S group) and (2) SAH-operated group (SAH group). The rats in both groups were further randomly separated into the following four groups: (1) S group with RD (S + RD group), (2) S group with sham-operation (S + Sham group), (3) SAH group with RD (SAH + RD group), and (4) SAH with sham-operation (SAH + Sham group). We planned to use at least 10 animals for each arm to assess primary and secondary outcomes but did not analyze additional animals if no changes were observed in 5–7 animals per group. We planned to collect those data from 5 to 7 animals per group to further evaluate the molecular changes.
Experiment 1 was performed to assess EBI during 120 min after SAH induction. We measured neurological functions, temporal changes in CBF, the amount of subarachnoid blood, and Akt activation in the ipsilateral hemisphere, all of which are involved in the pathogenesis of EBI [1, 20,21,22] . Experiment 2 was performed to investigate DCI for 48 h after SAH induction [23]. We measured neurological functions, CBF, the amount of subarachnoid blood, and brain edema [1]. Experiment 3 was performed to examine CVS at 24 h after SAH induction. We measured neurological functions, CBF, arterial vessel narrowing, and evaluated the mechanism underlying the effect of RD. Detailed protocols for the each experiment are shown in the Supplemental Figs. 1–3.
Surgery
Anesthesia was maintained with 2% isoflurane, and the rats were intubated and mechanically ventilated (tidal volume 7.5 ml/kg and 70 breaths/min, MK-V100, Muromachi Kikai Co., Ltd., Tokyo, Japan). SAH was induced via endovascular perforation methods using a hollow polytetrafluoroethylene tube (SUBL-120; Braintree Scientific, Braintree, MA, USA) and a tungsten rod (diameter, 0.004 in.; A-M Systems, Sequim, WA, USA), as previously described [24, 25]. The polytetrafluoroethylene tube was inserted up to 16 mm from the left internal-external carotid arterial bifurcation, and the tungsten rod was then advanced an additional 2 mm to produce SAH.
Bilateral RD was performed as previously described [18, 19]. The procedure was started at 15 min and performed approximately 45 min after SAH induction. Renal arteries of the rats were exposed, and all visible renal nerves along the arteries were cut and painted with 10% phenol in absolute ethanol to remove both afferent and efferent renal nerves. The dorsal skin of rats in the S + Sham and SAH + Sham groups was cut, and the animals received the same operation as the RD group, except for incision and removal of renal nerves with phenol. After the surgery, the operative region was disinfected with iodine and a subcutaneous injection of meloxicam (1 mg/kg) was administered for appropriate analgesia. At the time of euthanasia in each experiment, the animals were decapitated after they did not move in response to painful stimuli under deep anesthesia with an overdose of isoflurane.
Neurological Findings
Two neurological scoring tests were performed in all groups throughout the study. First, a 22-point scoring system (modified Garcia neurological score) consisting of an 18-point composite score (Garcia neurological score), and a 4-point beam walking score was used [22, 26]. In addition, the rotarod test was performed to evaluate activity and determine the consciousness status of each animal (MK-630B, Muromachi Kikai Co., Ltd., Tokyo, Japan) [26]. Briefly, animals were placed on the rotating spindle and walked at a constant race of 4 rotations per min (RPM) for 1 min. Then, the rats were subjected to the trial on an accelerating spindle (4–40 RPM) for 5 min, and the latency to fall off the cylinder was recorded. The mean latency values for three trials of the rotarod test were recorded for each animal.
Measurement of BP, HR, and CBF
Systolic BP (SBP) and heart rate (HR) were measured before (0–3 days) and after (23 or 47 h) SAH induction via the tail artery using a tail cuff (BP-98A; Softron Co., Tokyo, Japan) [26]. CBF was measured in the ipsilateral somatosensory cortex using a laser speckle flowmetry (Omega Zone; Omegawave, Tokyo, Japan) [19, 27] and expressed % of the value in the S + Sham group.
Amount of Subarachnoid Blood and Brain Edema
The severity of SAH was scored using the SAH grading score, as previously described [28]. Rats with SAH grading score < 7 at euthanasia were excluded from the study [22]. The brain water content (BWC) was measured to evaluate brain edema using a previously described method, with slight modifications [22]. Briefly, the brains were removed and both hemispheres of each brain were quickly separated and weighted (wet weight). Each hemisphere was incubated in an oven at 105 °C for 72 h and weighted again (dry weight). The following formula was used to calculate the percentage of BWC: [(wet weight-dry weight) / wet weight] × 100%.
Visualization of Major Cerebral Arteries with Latex Perfusion
The animals underwent black-ink angiography as previously described [24]. Briefly, a mixture of white latex compound (Chicago Latex Products Inc., Cristal Lake, IL, USA) and black ink was perfused through the left ventricle at a rate of 20 ml/min (the perfusion pressure was approximately 110 mmHg) [29] and then the brains were immersed in a 4% paraformaldehyde solution. After 24 h, major cerebral arteries were photographed and their diameters were measured at a distance of 1 mm from the top of internal carotid artery or basilar artery (BA) using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Brain and Serum Samples
Blood samples were collected from the animals via the abdominal artery, and the rats were subsequently perfused with phosphate-buffered saline under deep anesthesia. Then, animals were decapitated and their brains were quickly removed and divided at the bregma. The ipsilateral hemisphere on the rostral side was used for western blot analysis at 120 min after SAH induction in experiment 1. The caudal side of each cerebrum was immediately frozen in Tissue-tek OCT embedding medium (Sakura Finetek, Tokyo, Japan). Eight-micrometer slices were cut at approximately 1.72–1.92 mm from the bregma and at a 1 mm proximal portion from the top of BA for the histological evaluation in experiment 3. In addition, major cerebral arteries were isolated, collected, and crushed using Malti-Beads Shocker system (YASUI. CO., LTD., Osaka, Japan) at 24 h after SAH induction and the samples were used for the western blot analysis in experiment 3.
Immunohistochemistry
We immunostained the brain sections from each rat with anti-phosphorylated extracellular signal-regulated kinase (pERK; 1:400, Cell Signaling Technology, Danvers, MA, USA), anti-cluster of differentiation 68 (ED-1; 1:400, Cell Signaling Technology), anti-dopamine β-hydroxylase (DBH;1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-glial fibrillary acidic protein (GFAP, Dako Envision FLEX-GFA, Dako, Glostrup, Denmark) antibodies as previously described [22]. The numbers of pERK-positive endothelial and smooth muscle cells were counted separately and ED-1-positive cells around the BA were counted at × 200 magnification. DBH and GFAP staining were quantified in the paraventricular nucleus (PVN) of the hypothalamus, including the dorsal cap, ventral part, lateral magnocellular part, and medial parvicellular part, in both hemispheres at a × 200 magnification, and the mean values are presented as the relative density of the values in the S + Sham group.
Western Blot Analysis
Protein concentrations were determined using a DC protein assay (Bio-Rad, Hercules, CA, USA). Western blot analyses were performed according to the methods described in our previous studies, with slight modifications [18, 22, 30]. The following primary antibodies were used: anti-pAkt (1: 2000, Cell Signaling Technology), anti-Akt (1: 2000, Cell Signaling Technology), anti-pERK (1:1000, Cell Signaling Technology), anti-ERK (1:1000, Cell Signaling Technology), anti-phosphorylated p38 (1:1000, Cell Signaling Technology), anti-p38 (1:1000, Cell Signaling Technology), anti-phosphorylated c-Jun N-terminal kinase (JNK; 1:2000, Cell Signaling Technology), anti-JNK (1:2000, Cell Signaling Technology), anti-cyclooxygenase-2 (COX-2; 1:500, Origene Technologies, Inc. Rockville, MD, USA), anti-angiotensin II (AT II) receptor type 1 (AT1R; 1:200, Santa Cruz Biotechnology), and anti-α-tubulin (1:5000, Calbiochem, La Jolla, CA, USA) antibodies. Images were analyzed semiquantitatively using the ImageJ software (National Institutes of Health, Bethesda, MD), and changes in the Akt, mitogen-activated protein kinase (MAPK), COX-2, and AT1R levels were described as relative densities of the values in S + Sham group.
Enzyme-Linked Immunosorbent Assay
Serum levels of the AT II protein were analyzed using a commercially available Enzyme-Linked Immunosorbent Assay (ELISA) kit (Sigma-Aldrich, St. Louis, MO, USA).
Statistical Analysis
We performed all measurements in a blinded manner. Rats were numbered, and measurements were performed by an examiner without knowledge of the group to which the rats belonged. All data are presented as means ± SEM, and statistical analyses were evaluated using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA) and Statcel (OMS publication, Saitama, Japan) software. The mortality rate was assessed using Fisher’s exact test. Parametric evaluations were performed using a one-way ANOVA with the Tukey-Kramer test in four groups (CBF at 75 min and 150 min, and pAkt/Akt in experiment 1, SBP, CBF, and BWC in experiment 2) and with Fisher’s LSD test in three groups (SBP, HR, rotarod test, cerebral arterial diameters without left PCA, immunohistochemical analyses, western blot analyses except for COX-2, and ELISA). Non-parametric analyses were performed in other evaluations of the difference among three and four groups using a Kruskal-Wallis test followed by the Steel-Dwass test. A non-paired t test (BWC) or Mann-Whitney U test (SAH grade and CBF at preoperation and at 45 min after the operation in experiment 1) was performed to compare the data between two groups. P < 0.05 was considered significant.
Results
Experiment 1: The Effect of RD on EBI
No animals in the S group died. The morality rate of SAH rats was 37.9% (11 of 29 rats) within 15 min after SAH induction (Supplemental Fig. 1b). A significant difference in the mortality rate was not observed between the SAH + Sham (0%) and SAH + RD (30%) groups. RD did not improve the changes of modified Garcia neurological score (8.2 ± 1.4 vs. 9.0 ± 1.6), rotarod (6.7 ± 4.8 vs. 9.8 ± 5.6 s), CBF at 60 min (59.9 ± 4.7 vs. 47.6 ± 1.1%), 75 min (64.9 ± 7.0 vs. 49.3 ± 1.6%), 90 min (69.5 ± 8.2 vs. 47.9 ± 1.8%), 150 min (57.6 ± 8.0 vs. 59.8 ± 5.5%), SAH grading score (14.7 ± 0.7 vs. 13.4 ± 0.6), and Akt phosphorylation (1.6 ± 0.1 vs. 1.5 ± 0.1) compared to SAH + Sham, although SAH resulted in a significant deterioration of neurological functions, reduced CBF, and increased Akt phosphorylation (n = 6 - 7, Fig. 1a–e).
The effect of RD on EBI. Differences in the modified Garcia neurological score (a), latency to fall in the accelerating rotarod test (b), temporal changes in CBF (c), SAH grading score (d), and pAkt levels in the ipsilateral hemisphere (e) among the S + Sham, S + RD, SAH + Sham, and SAH + RD groups. Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include CBF, cerebral blood flow; EBI, early brain injury; RD, renal denervation; S, sham; SAH, subarachnoid hemorrhage
Experiment 2: The Effect of RD on DCI
No animals in the S group died. The mortality rate of SAH rats was 42.4% (14 of 33 rats) within 15 min after SAH induction (Supplemental Fig. 2b). A significant difference in the mortality rate was not observed between the SAH + Sham (22.2%) and SAH + RD (40%) groups. RD decreased SBP both S (from 117.0 ± 4.6 to 101.6 ± 2.2 mmHg vs. from 120.7 ± 2.3 to 130.8 ± 5.0 mmHg) and SAH groups (from 110.0 ± 3.0 to 102.7 ± 3.6 mmHg vs. from 115.6 ± 2.6 to 134.3 ± 3.9 mmHg) (n = 6, Fig. 2a), while HR were similar among the groups (data not shown). RD did not improve the changes of modified Garcia neurological score (17.8 ± 0.8 vs. 16.8 vs. 2.2), rotarod (31.8 ± 7.7 vs. 29.2 ± 9.2 s), CBF (108.5 ± 4.3 vs. 95.4 ± 4.4%), SAH grading score (14.3 ± 0.4 vs. 11.2 ± 1.3), and BWC (79.1 ± 0.1 vs. 79.4 ± 0.1%) compared to SAH + Sham, although SAH resulted in a significant deterioration of modified Garcia neurological score and increased BWC (n = 6, Fig. 2b–f).
The effect of RD on DCI. Differences in systolic BP (a), modified Garcia neurological score (b), latency to fall in the accelerating rotarod test (c), value of CBF (d), SAH grading score (e), and brain water content in the ipsilateral hemisphere (f) among the S + Sham, S + RD, SAH + Sham, and SAH + RD groups. Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include CBF, cerebral blood flow; DCI, delayed cerebral ischemia; RD, renal denervation; S, sham; SAH, subarachnoid hemorrhage; SBP, systolic blood pressure
Because the results from the S + RD group were similar to the S + Sham group in experiments, rats in the S + RD group were excluded from further analysis in experiment 3.
Experiment 3: The Effect of RD on CVS
Mortality, SBP, HR, Neurological Functions, CBF, and Brain Edema
No animals in the S + Sham group died. The mortality rate of SAH rats was 41.7% (53 of 127 rats) within 15 min after SAH induction (Supplemental Fig. 3b). A significant difference in the mortality rate was not observed between the SAH + Sham (13.2%) and SAH + RD (13.9%) groups. Although SAH did not modulate the changes in SBP (n = 6) and HR (n = 6) at 24 h after SAH, RD significantly lowered the SBP (from 128.5 ± 3.4 to 112.7 ± 7.0 mmHg vs. from 125 ± 3.6 to 129.7 ± 5.0 mmHg) and increased HR (from 352.8 ± 15.2 to 431.3 ± 10.1 bpm vs. from 346.7 ± 6.4 to 368.5 ± 14.7 bpm) compared with the SAH + Sham group (Supplemental Fig. 4a and b). SAH significantly decreased neurological functions (n = 23), and RD did not result in a significant improvement (Supplemental Fig. 4c and d). On the other hand, a significant difference was not observed in resting CBF (n = 11) at 24 h after SAH induction among the three groups (Sham, 100.0 ± 17.8; SAH + Sham, 101.4 ± 8.5; SAH + RD, 102.1 ± 4.7) (Supplemental Fig.4e). Significant differences in the SAH grading and BWC in ipsilateral cortex at 24 h after SAH induction were not observed between the SAH + Sham and SAH + RD groups (n = 5, data not shown).
Measurement of Major Cerebral Arterial Diameters
Although SAH significantly decreased the diameters of all major cerebral arteries, RD significantly attenuated vasospasm in the left middle cerebral artery (0.25 ± 0.02 vs. 0.20 ± 0.01 mm), right posterior cerebral artery (0.16 ± 0.01 vs. 0.12 ± 0.01 mm), and BA (0.28 ± 0.01 vs. 0.23 ± 0.01 mm) compared with the SAH + Sham group (n = 10, Fig. 3).
Diameters of each major cerebral artery, including the bilateral ACA, MCA, PCA, and BA, among the Sham, SAH + Sham, and SAH + RD groups. The panels in the upper left corner show representative angiograms. Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include ACA, anterior cerebral artery; BA, basilar artery; Lt, left; MCA, middle cerebral artery; PCA, posterior cerebral artery; RD, renal denervation; Rt, right; SAH, subarachnoid hemorrhage
Expression of Phosphorylated MAPKs, COX-2, and ED-1 in Cells in the Major Cerebral Arteries
We evaluated MAPKs to determine whether RD improved vascular cytotoxic responses accompanied by CVS, because these kinases were the main kinases modulated in an experimental SAH study [31]. Immunohistochemical evaluations (n = 6) revealed significant increases in the numbers of pERK-positive smooth muscle (Fig. 4a) and endothelial cells (Fig. 4b) after SAH, whereas RD significantly decreased the number of pERK-positive endothelial cells compared with the SAH + Sham group (23.0 ± 2.0 vs. 32.8 ± 1.7 cells). The western blot analysis (n = 5) revealed a significant decrease in pERK levels in the SAH + RD group compared with the SAH + Sham group (1.0 ± 0.03 vs. 1.2 ± 0.04, Fig. 5a). On the other hand, no differences in the levels of phosphorylated p38 and JNK were observed between the SAH + Sham and SAH + RD groups (data not shown).
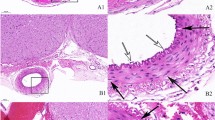
Immunohistochemical staining for pERK in the SMCs (a) and ETCs (b) in the BA of the Sham, SAH + Sham, and SAH + RD groups. Upper panels show representative pictures of pERK-positive cells in SMCs (arrowhead) and ETCs (arrow). Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include BA, basilar artery; ETC, endothelial cell; pERK, phosphorylated extracellular signal-regulated kinase; RD, renal denervation; SAH, subarachnoid hemorrhage; SMC, smooth muscle cell
Expression of pERK (a) and COX-2 (b) in major cerebral arteries from Sham, SAH + Sham, and SAH + RD groups. Upper panels show representative western blot bands for pERK (a) and COX-2 (b). Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include COX-2, cyclooxygenase-2, pERK, phosphorylated extracellular signal-regulated kinase; RD, renal denervation; SAH, subarachnoid hemorrhage
As shown in the Figs. 5b and 6, the expression of COX-2 in major cerebral arteries and number of ED-1-positive cells around the BA were increased by SAH, whereas RD significantly reduced both COX-2 levels (1.2 ± 0.2 vs. 7.0 ± 4.7, Fig. 5b) and ED-1-positive cells (2.3 ± 1.2 vs. 7.2 ± 1.9, Fig. 6) compared with the SAH + Sham group.
Immunochemical staining for ED-1 in cells around the BA in the Sham, SAH + Sham, and SAH + RD groups. Upper panels show representative pictures of ED-1-positive cells (arrow). Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include BA, basilar artery; RD, renal denervation; SAH, subarachnoid hemorrhage
Sympathetic Nerve Activation and Number of Reactive Astrocytes in the PVN of the Hypothalamus
We focused on the PVN of the hypothalamus, which is involved in the nuclei implicated in sympathetic regulation, to explore how RD reduced CVS and cerebrovascular injuries. SAH increased DBH and GFAP immunoreactivity, whereas RD significantly reduced the levels of DBH (1.6 ± 0.2 vs. 1.1 ± 0.1) and GFAP (1.1 ± 0.04 vs. 1.0 ± 0.02) compared with the SAH + Sham group (n = 7, Fig. 7a, b).
Immunohistochemical staining showing DBH- (a) and GFAP-positive (b) areas in the PVN of the hypothalamus from the Sham, SAH + Sham, and SAH + RD groups. Rectangles in the left panel identify the area in the PVN at which the immunoreactivities were evaluated. Upper panels show representative pictures of DBH- (a) and GFAP-positive (b) areas. Values are means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include DBH, dopamine β-hydroxylase; GFAP, glial fibrillary acidic protein; Lt, left; PVN, paraventricular nucleus; RD, renal denervation; Rt, right; SAH, subarachnoid hemorrhage
Changes in the Expression of AT1R on the Basilar Artery and the Serum AT II Level
We measured serum AT II levels to confirm that the reduction in central sympathetic nerve activation in the PVN reflected peripheral sympathetic nerve activity because AT II is strictly associated with sympathetic activation [32]. Although SAH increased serum AT II levels, RD significantly reduced the levels compared with the SAH + Sham group (63.7 ± 3.6 vs. 84.6 ± 6.4 pg/ml) (n = 5, Fig. 8a). On the other hand, significant differences in AT1R expression in major cerebral arteries were not observed among the three groups (n = 5, Fig. 8b).
Comparison of serum AT II concentration (a) and AT1R expression (b) in the major cerebral arteries among the Sham, SAH + Sham, and SAH + RD groups. Upper panels show representative western blot bands for AT1R (b). Values are presented as means ± SEM. Asterisk indicates P < 0.05. Abbreviations used include AT II, angiotensin II; AT1R, angiotensin II receptor type 1; RD, renal denervation; SAH, subarachnoid hemorrhage
Discussion
In our previous study, RD rescued acute ischemic stroke in hypertensive rats, but did not exert a protective effect on normotensive animals [19]. The significant result was postulated to arise from the difference in sympathetic nerve activity in addition to the hypertensive background [33, 34]. Moreover, RD slowed stroke onset in hypertensive rats, which was associated with a reduction in oxidative stress in the PVN of the hypothalamus [18]. As the pathogenesis of SAH involves sympathetic nerve activation (catecholamine surge), our previous results encouraged us to explore whether RD exerted significant effects on the SAH prognosis. Although our current study did not observe significant effects on the primary endpoint, RD reduced arterial narrowing and endothelial cell damage induced by SAH. In addition, RD prevented the SAH-induced increase in the number of reactive astrocytes and DBH levels in the PVN and increased serum AT II levels. Based on the findings, we suggest that RD ameliorates CVS by inhibiting central sympathetic nerve activation.
The Effect of RD on CVS Via Central Sympathetic Nerve Activation
Numerous studies have examined the sympathetic neurogenic control of cerebrovascular contraction in SAH. In particular, catecholamine was mainly examined and shown to have a significant role in SAH pathogenesis, including EBI and CVS/DCI [7, 35]. However, researchers have not yet determined whether inhibition of increased catecholamine levels improves the prognosis of patients with SAH [10, 36]. On the other hand, some other peptides, including neuropeptide Y and AT II, which modulate sympathetic nerve activity, were reported to be involved in SAH pathogenesis [37, 38]. Thus, we hypothesized that catecholamines and other factors that modulate sympathetic nerve activity participate in the pathogenesis of SAH. Moreover, we speculated that the SAH prognosis would improve if those factors were regulated by a comprehensive treatment with a central intervention, such as RD.
The PVN and RVLM project to the sympathetic preganglionic motor neurons in the spinal cord, and neurons in the two regions influence sympathetic nerve activity [39, 40]. Thus, the PVN and RVLM are defined as the main nuclei of the central sympathetic nervous system. A previous study reported that hypothalamic injuries, including ischemic necrosis, macrohemorrhages, and microhemorrhages, were frequently seen in patients with SAH [41]. Further possible mechanisms underlying the injuries include direct damage to the fine perforating hypothalamic arteries by SAH, vasoconstriction resulting in ischemic damage, and the forced flow of subarachnoid blood up the sheaths of the perforating arteries that then ruptures out into the cerebral parenchyma [41, 42]. In addition, a continuous CBF reduction, which was observed in the acute phase after SAH in experiment 1, might participate in ischemic injury to the hypothalamus. Those injuries might cause carbon dioxide accumulation, a reduction in the partial pressure of oxygen, and excess glutamate levels, leading to activation of reactive astrocytes and central sympathetic nerves.
RD reduces oxidative stress and the number of FosB (neural activation marker)-positive cells in the PVN of hypertensive rats [18, 43]. In addition, RD inhibited SAH-induced increases in DBH and GFAP immunoreactivities in the PVN and also reduced serum AT II levels in the SAH rats in our current study. These findings support the hypothesis that RD prevents hypothalamic injury and reduces central sympathetic nerve activation in the PVN and subsequent efferent sympathetic nerve deactivation to regulate the renin-angiotensin system. Therefore, we postulate that RD stabilized activated sympathetic nerves and exerted protective effects on CVS after SAH.
The Underlying Mechanism of RD on CVS
CVS is presumed to be initiated by prolonged smooth muscle contraction, and the mechanism might be involved in SAH-specific pathogenesis [44]. The bioactive vasoconstrictors endothelin-1 and AT II have long been studied in SAH research [45, 46]. AT II induces arterial contraction via endothelin-1 signaling in endothelial cells [46] and also exerts direct effect on smooth muscle cells by binding to AT1R [47]. The blockage of those actions exerted beneficial effects on CVS in experimental studies and clinical trials, although clazosentan administration did not result in a significant protective effect on the SAH prognosis in patients with aneurysmal SAH [48].
The underlying cytotoxic responses, including the inflammatory response and oxidative stress, have been also reported to play pivotal roles in the pathogenesis of CVS. MAPKs may be one of the final common pathways for signal transduction in CVS, and we previously revealed MAPKs activation in injured endothelial cells and smooth muscle cells [24, 31, 49]. Moreover, phosphorylated MAPKs are associated with oxidative stress, the upregulation of TNF-α, and macrophage infiltration in the pathogenesis of SAH [50, 51]. In the present study, SAH significantly increased the number of pERK-positive endothelial cells and RD decreased the number of positively stained cells in the BA. In addition, RD significantly reduced pERK and COX-2 levels in major cerebral arteries and the number of infiltrated macrophages around the BA. COX-2 is associated with endothelin-1 expression and endothelial dysfunction in spastic arteries of an experimental SAH model [52]. Taken together with the result showing little effect of SAH and RD on AT1R expression, we speculate that the efferent sympathetic nerve deactivation induced by RD reduced serum AT II levels and endothelin-1 expression in endothelial cells to subsequently inhibit vasoconstriction via ERK-mediated endothelial cell damage in the SAH rats.
On the other hand, our present study did not reveal changes in CBF between the SAH + Sham and SAH + RD groups, while ERK activation is also associated with a reduced CBF in SAH [53]. Thus, we propose that ERK activation was associated with endothelial cell damage induced by SAH and ERK deactivation by RD was independent of the decrease in CBF, and the finding might support a role of RD in a different mechanism, such as the inhibition of sympathetic nerve activity.
Study Limitations
Although RD prevented CVS after SAH, some limitations must be acknowledged, which were mainly negative effects on the primary endpoint in this study. First, RD did not exert a protective effect on EBI and the potential explanation is the reduction in BP. Although RD is known to significantly reduce BP in patients with severe hypertension, the changes in BP occasionally produce harmful effects on severe brain injuries because of insufficient cerebral perfusion pressure (CPP) [54, 55]. In this study, we did not evaluate the changes of intracranial pressure, CPP, and electroencephalography within the first hour after SAH, which represent specific physiological effects of EBI [20]. Those findings might provide detailed information about the mechanism of RD in EBI. Next, RD did not exert beneficial effects on DCI in experiment 2, although RD prevented CVS. CVS plays relatively limited roles in the pathogenesis and clinical outcomes of DCI, and DCI should be defined as the presence of neurological deterioration and cerebral infarction identified on computed tomography/magnetic resonance imaging and functional deterioration in patients with SAH [56, 57]. In our current study, we evaluated the effect of RD on DCI by assessing neurological functions and brain edema in experiment 2 because good correlations were observed between these parameters [58] and brain edema is involved in the pathogenesis of cerebral infarction-induced cytotoxic and vasogenic edema. The protective role of RD was promising on experimental stroke studies; however, further studies are required to determine the effect before it is translated to patients with SAH in the clinic.
Conclusions
Our present study provided the first evidence that RD prevents CVS after SAH by stabilizing of central sympathetic nerve activation in experimental SAH animals. The novel insights into the potential role of RD also confirm that the efferent sympathetic nerve deactivation reduces serum AT II and endothelial cell damage in the SAH model.
References
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Caner B, Hou J, Altay O, Fujii M, Zhang JH. Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem. 2012;123(Suppl 2):12–21. https://doi.org/10.1111/j.1471-4159.2012.07939.x.
Shimamura N, Ohkuma H. Phenotypic transformation of smooth muscle in vasospasm after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2014;5(3):357–64. https://doi.org/10.1007/s12975-013-0310-1.
Takemoto Y, Hasegawa Y, Hashiguchi A, Moroki K, Tokuda H, Mukasa A. Predictors for functional outcome in patients with aneurysmal subarachnoid hemorrhage who completed in-hospital rehabilitation in a single institution. J Stroke Cerebrovasc Dis. 2019;28(7):1943–50. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.03.042.
Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int. 2014;2014:858496. https://doi.org/10.1155/2014/858496.
Lee VH, Oh JK, Mulvagh SL, Wijdicks EF. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5(3):243–9. https://doi.org/10.1385/ncc:5:3:243.
Naredi S, Lambert G, Eden E, Zall S, Runnerstam M, Rydenhag B, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901–6.
Gao C, Liu X, Shi H, Xu S, Ji Z, Wang C, et al. Relationship between sympathetic nervous activity and inflammatory response after subarachnoid hemorrhage in a perforating canine model. Auton Neurosci. 2009;147(1–2):70–4. https://doi.org/10.1016/j.autneu.2009.01.010.
Cameron MM, Haas RH. Adrenergic blockade in subarachnoid haemorrhage. Acta Neurochir. 1976;34(1–4):261–4.
Lambert G, Naredi S, Eden E, Rydenhag B, Friberg P. Sympathetic nervous activation following subarachnoid hemorrhage: influence of intravenous clonidine. Acta Anaesthesiol Scand. 2002;46(2):160–5.
Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. https://doi.org/10.1016/s0140-6736(10)62039-9.
Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61(2):457–64. https://doi.org/10.1161/hypertensionaha.111.00194.
Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. https://doi.org/10.1056/NEJMoa1402670.
Frame AA, Carmichael CY, Wainford RD. Renal afferents. Curr Hypertens Rep. 2016;18(9):69–7. https://doi.org/10.1007/s11906-016-0676-z.
Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens. 2011;24(6):635–42. https://doi.org/10.1038/ajh.2011.35.
Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39(2 Pt 2):519–24.
Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46(3):533–9. https://doi.org/10.1161/01.hyp.0000179088.57586.26.
Nakagawa T, Hasegawa Y, Uekawa K, Ma M, Katayama T, Sueta D, et al. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2(5):e000375. https://doi.org/10.1161/jaha.113.000375.
Hasegawa Y, Nakagawa T, Matsui K, Kim-Mitsuyama S. Renal denervation in the acute phase of ischemic stroke provides brain protection in hypertensive rats. Stroke. 2017;48(4):1104–7. https://doi.org/10.1161/strokeaha.116.015782.
Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26(6):1086–91; discussion 91-2. https://doi.org/10.1161/01.str.26.6.1086.
Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37(8):2140–6. https://doi.org/10.1161/01.str.0000229888.55078.72.
Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42(2):477–83. https://doi.org/10.1161/strokeaha.110.597344.
Kamp MA, Lieshout JHV, Dibue-Adjei M, Weber JK, Schneider T, Restin T, et al. A systematic and meta-analysis of mortality in experimental mouse models analyzing delayed cerebral ischemia after subarachnoid hemorrhage. Transl Stroke Res. 2017;8(3):206–19. https://doi.org/10.1007/s12975-016-0513-3.
Hasegawa Y, Suzuki H, Uekawa K, Kawano T, Kim-Mitsuyama S. Characteristics of cerebrovascular injury in the hyperacute phase after induced severe subarachnoid hemorrhage. Transl Stroke Res. 2015;6(6):458–66. https://doi.org/10.1007/s12975-015-0423-9.
Hasegawa Y, Uekawa K, Kawano T, Suzuki H, Kim-Mitsuyama S. Blockage of central sphingosine-1-phosphate receptor does not abolish the protective effect of FTY720 in early brain injury after experimental subarachnoid hemorrhage. Curr Drug Deliv. 2017;14(6):861–6. https://doi.org/10.2174/1567201813666160907094401.
Hasegawa Y, Nakagawa T, Uekawa K, Ma M, Lin B, Kusaka H, et al. Therapy with the combination of amlodipine and irbesartan has persistent preventative effects on stroke onset associated with BDNF preservation on cerebral vessels in hypertensive rats. Transl Stroke Res. 2016;7(1):79–87. https://doi.org/10.1007/s12975-014-0383-5.
Dong YF, Kataoka K, Tokutomi Y, Nako H, Nakamura T, Toyama K, et al. Beneficial effects of combination of valsartan and amlodipine on salt-induced brain injury in hypertensive rats. J Pharmacol Exp Ther. 2011;339(2):358–66. https://doi.org/10.1124/jpet.111.182576.
Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167(2):327–34. https://doi.org/10.1016/j.jneumeth.2007.08.004.
Wu CH, Chi JC, Jerng JS, Lin SJ, Jan KM, Wang DL, et al. Transendothelial macromolecular transport in the aorta of spontaneously hypertensive rats. Hypertension. 1990;16(2):154–61. https://doi.org/10.1161/01.hyp.16.2.154.
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41(2):368–74. https://doi.org/10.1161/strokeaha.109.568899.
Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol. 2010;68(5):650–60. https://doi.org/10.1002/ana.22102.
Fassot C, Lambert G, Gaudet-Lambert E, Friberg P, Elghozi JL. Beneficial effect of renin-angiotensin system for maintaining blood pressure control following subarachnoid haemorrhage. Brain Res Bull. 1999;50(2):127–32.
Howe PR, Rogers PF, Morris MJ, Chalmers JP, Smith RM. Plasma catecholamines and neuropeptide-Y as indices of sympathetic nerve activity in normotensive and stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1986;8(6):1113–21.
Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109(19):2357–62. https://doi.org/10.1161/01.cir.0000128695.49900.12.
Hansen-Schwartz J. Cerebral vasospasm: a consideration of the various cellular mechanisms involved in the pathophysiology. Neurocrit Care. 2004;1(2):235–46. https://doi.org/10.1385/ncc:1:2:235.
Luo H, Song WX, Jiang JW, Zhao JL, Rong WL, Li MH. Effects of preadmission beta-blockers on neurogenic stunned myocardium after aneurysmal subarachnoid hemorrhage: a meta- analysis. Clin Neurol Neurosurg. 2017;158:77–81. https://doi.org/10.1016/j.clineuro.2017.04.022.
Schebesch KM, Brawanski A, Kagerbauer SM, Martin J, Bele S, Herbst A, et al. The possible role of neuropeptide Y after spontaneous subarachnoid hemorrhage. Acta Neurochir. 2011;153(8):1663–8; discussion 8. https://doi.org/10.1007/s00701-011-1056-8.
Audibert G, Steinmann G, de Talance N, Laurens MH, Dao P, Baumann A, et al. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009;108(6):1922–8. https://doi.org/10.1213/ane.0b013e31819a85ae.
Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491(2):274–96.
Cruz JC, Flor AF, Franca-Silva MS, Balarini CM, Braga VA. Reactive oxygen species in the paraventricular nucleus of the hypothalamus alter sympathetic activity during metabolic syndrome. Front Physiol. 2015;6:384. https://doi.org/10.3389/fphys.2015.00384.
Crompton MR. Hypothalamic lesions following the rupture of cerebral berry aneurysms. Brain. 1963;86:301–14.
Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. Jama. 2007;298(12):1429–38. https://doi.org/10.1001/jama.298.12.1429.
Patel KP, Xu B, Liu X, Sharma NM, Zheng H. Renal denervation improves exaggerated sympathoexcitation in rats with heart failure: a role for neuronal nitric oxide synthase in the paraventricular nucleus. Hypertension. 2016;68(1):175–84. https://doi.org/10.1161/hypertensionaha.115.06794.
Hasegawa S, Hasegawa Y, Miura M. Current therapeutic drugs against cerebral vasospasm after subarachnoid hemorrhage: a comprehensive review of basic and clinical studies. Curr Drug Deliv. 2017;14(6):843–52. https://doi.org/10.2174/1567201813666160808100937.
Ansar S, Vikman P, Nielsen M, Edvinsson L. Cerebrovascular ETB, 5-HT1B, and AT1 receptor upregulation correlates with reduction in regional CBF after subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2007;293(6):H3750–8. https://doi.org/10.1152/ajpheart.00857.2007.
Wanderer S, Mrosek J, Vatter H, Seifert V, Konczalla J. Crosstalk between the angiotensin and endothelin system in the cerebrovasculature after experimental induced subarachnoid hemorrhage. Neurosurg Rev. 2018;41(2):539–48. https://doi.org/10.1007/s10143-017-0887-z.
Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639–72.
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10(7):618–25. https://doi.org/10.1016/s1474-4422(11)70108-9.
Hasegawa Y, Suzuki H, Altay O, Chen H, Zhang JH. Treatment with sodium orthovanadate reduces blood-brain barrier disruption via phosphatase and tensin homolog deleted on chromosome 10 (PTEN) phosphorylation in experimental subarachnoid hemorrhage. J Neurosci Res. 2012;90(3):691–7. https://doi.org/10.1002/jnr.22801.
Maddahi A, Povlsen GK, Edvinsson L. Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. J Neuroinflammation. 2012;9:274. https://doi.org/10.1186/1742-2094-9-274.
Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mitogen-activated protein kinases in cerebral vasospasm after subarachnoid hemorrhage: a review. Acta Neurochir Suppl. 2011;110(Pt 1):133–9. https://doi.org/10.1007/978-3-7091-0353-1_23.
Munakata A, Naraoka M, Katagai T, Shimamura N, Ohkuma H. Role of cyclooxygenase-2 in relation to nitric oxide and endothelin-1 on pathogenesis of cerebral vasospasm after subarachnoid hemorrhage in rabbit. Transl Stroke Res. 2016;7(3):220–7. https://doi.org/10.1007/s12975-016-0466-6.
Beg SA, Hansen-Schwartz JA, Vikman PJ, Xu CB, Edvinsson LI. ERK1/2 inhibition attenuates cerebral blood flow reduction and abolishes ET(B) and 5-HT(1B) receptor upregulation after subarachnoid hemorrhage in rat. J Cereb Blood Flow Metab. 2006;26(6):846–56. https://doi.org/10.1038/sj.jcbfm.9600236.
Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010;9(5):504–19. https://doi.org/10.1016/s1474-4422(10)70087-9.
Wijdicks EF, Vermeulen M, Murray GD, Hijdra A, van Gijn J. The effects of treating hypertension following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 1990;92(2):111–7.
Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–5. https://doi.org/10.1161/strokeaha.110.589275.
Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31(6):1443–51. https://doi.org/10.1038/jcbfm.2011.7.
Hasegawa Y, Suzuki H, Nakagawa T, Uekawa K, Koibuchi N, Kawano T, et al. Assessment of the correlations between brain weight and brain edema in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2016;121:89–92. https://doi.org/10.1007/978-3-319-18497-5_15.
Funding
This study was supported by JSPS KAKENHI Grant Number 15K10309 and 19K09459.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were conducted in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1.
Supplemental Fig. 1: Schematic illustrating the protocols used in the experiments (a) and the study profile (b) in experiment 1. Abbreviations used include CBF, cerebral blood flow; NF, neurological functions; Op., operation; RD, renal denervation; S, sham; SAH, subarachnoid hemorrhage; WB, western blot. Supplemental Fig. 2: Schematic illustrating the protocols used in the experiments (a) and the study profile (b) in experiment 2. Abbreviations used include BP, blood pressure; BWC, brain water content; CBF, cerebral blood flow; NF, neurological functions; Op., operation; RD, renal denervation; S, Sham; SAH, subarachnoid hemorrhage. Supplemental Fig. 3: Schematic illustrating the protocols used in the experiments (a) and the study profile (b) in experiment 3. Abbreviations used include BP, blood pressure; BWC, brain water content; CBF, cerebral blood flow; CVS, cerebral vasospasm; EBI, early brain injury; ELISA, Enzyme-linked immunosorbent assay; IHC, immunohistochemistry; NF, neurological functions; Op., operation; RD, renal denervation; S, Sham; SAH, subarachnoid hemorrhage; WB, western blot. Supplemental Fig. 4: Difference in the SBP (a), HR (b), modified Garcia neurological score (c), latency to fall in the accelerating rotarod test (d), and CBF (e) at 24 hours after SAH among the S+Sham, SAH+Sham, and SAH+RD groups. Values are presented as means ± SEM. Asterisk indicates P<0.05. Abbreviations used include CBF, cerebral blood flow; HR, heart rate; RD, renal denervation; S, Sham; SAH, subarachnoid hemorrhage; SBP, systolic blood pressure. (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Takemoto, Y., Hasegawa, Y., Hayashi, K. et al. The Stabilization of Central Sympathetic Nerve Activation by Renal Denervation Prevents Cerebral Vasospasm after Subarachnoid Hemorrhage in Rats. Transl. Stroke Res. 11, 528–540 (2020). https://doi.org/10.1007/s12975-019-00740-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00740-9