Abstract
Mesenchymal stem cells circulate between organs to repair and maintain tissues. Mesenchymal stem cells cultured with fetal bovine serum have therapeutic effects when intravenously administered after stroke. However, only a small number of mesenchymal stem cells reach the brain. We hypothesized that the serum from stroke patients increases mesenchymal stem cells trophism toward the infarcted brain area. Mesenchymal stem cells were grown in fetal bovine serum, normal serum from normal rats, or stroke serum from ischemic stroke rats. Compared to the fetal bovine serum group, the stroke serum group but not the normal serum group showed significantly greater migration toward the infarcted brain area in the in vitro and in vivo models (p < 0.05). Both C-X-C chemokine receptor type 4 and c-Met expression levels significantly increased in the stroke serum group than the others. The enhanced mesenchymal stem cells migration of the stroke serum group was abolished by inhibition of signaling. Serum levels of chemokines, cytokines, matrix metalloproteinase, and growth factors were higher in stroke serum than in normal serum. Behavioral tests showed a significant improvement in the recovery after stroke in the stroke serum group than the others. Stroke induces mesenchymal stem cells migration to the infarcted brain area via C-X-C chemokine receptor type 4 and c-Met signaling. Culture expansion using the serum from stroke patients could constitute a novel preconditioning method to enhance the therapeutic efficiency of mesenchymal stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell therapy is an emerging paradigm in the stroke treatment field, along with acute recanalization therapy and neuroprotective agents, and has recently been evaluated as a regenerative strategy for patients with fixed neurologic deficits [1,2,3,4,5,6]. In one randomized controlled trial of the intravenous application of mesenchymal stem cells (MSCs) culture expanded with fetal bovine serum (FBS) in subacute stroke patients, MSC treatment was found to be safe for stroke patients over a long-term period and to possibly improve recovery after stroke [3]. However, many patients in the MSC group remained significantly disabled. Therefore, further trials of methods to enhance the therapeutic effects of stem cells are required. While earlier clinical trials used autologous naïve MSCs, several recent trials have examined allogeneic or manipulated MSCs, using methods such as isolating functional subpopulations or preconditioning stem cells [7].
One of the main limitations of current stem cell therapies is the relatively small number of MSCs that migrate to ischemic brain lesions after transplantation, ranging from <1 to 21% [8, 9]. In order to participate in repair and regeneration after stroke, MSCs have to be mobilized and then migrate to the target sites after systemic administration. Cell migration to the injury region is an inherently inefficient process [10], and its enhancement could potentiate the effects of cell therapy.
It is widely accepted that adult stem cells, such as bone morrow MSCs, are able to circulate between organs to repair and maintain tissues. We hypothesized that the serum from stroke patients increases bone marrow MSC trophism toward ischemic damaged brain tissue. Thus, we tested whether culture expansion of MSCs using serum obtained during the acute phase of stroke enhances their migration ability toward ischemic damaged brain tissue compared to the traditional culture method using FBS or culture with serum from non-stroke subjects. In addition, we compared the characteristics of MSCs culture expanded with stroke serum, normal serum, or FBS and the levels of circulating factors between normal and stroke serum.
Materials and Methods
Animal Model
Anesthesia was induced using a facemask in male Sprague–Dawley rats (7–8 weeks, 250–300 g) with 4% isoflurane and maintained with 1.5% isoflurane in 70% N2O and 30% O2. Temperature was maintained between 37.0 and 37.5 °C (measured rectally) with heating pads. We induced a transient middle cerebral artery occlusion (tMCAo) using a previously described intraluminal vascular occlusion method modified in our laboratory [11]. Rats with 8–12 points of modified Neurological Severity Score (mNSS) at 1 day after stroke were used in this study. The mortality rate of rats was 25% during the operation. The procedures for the surgery and care of the rats were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Samsung Biomedical Research Institute (SBRI). SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) accredited facility and abide by the Institute of Laboratory Animal Resources (ILAR) guide. Studies were conducted and reported in accordance to the ARRIVE guidelines.
Preparation of Serum and Mesenchymal Stem Cells
Rat serum was prepared from tMCAo model animals (serum from stroke rats, SS) and normal rats (normal serum, NS). The blood was collected by cardiac puncture of a still-beating heart with a 5-ml syringe.
Human serum was collected from ischemic stroke patients (within 3 days of stroke onset) and healthy subjects, as previously described [12]. The serum was collected and filtered through a 0.2-μm membrane (Nalge Nunc International, Rochester, NY). Aliquots of the sterile serum were stored at −70 °C until use (Fig. 1a). The general clinical characteristics of the human subjects are provided in Supplementary Data-1. The protocol consent form was approved by the Korean FDA and the Institutional Review Board of the Samsung Medical Center.
Rat MSCs (rMSCs) were obtained from the femur and tibias of Sprague–Dawley rats (male, weighing 220–250 g), and human MSCs (hMSCs) were obtained from the iliac crest as previously described [11]. After culture, the cells were stored in liquid nitrogen until use. For stroke serum preconditioning, the MSCs were replated in 100-mm dishes at 3 × 105 cells/dish every 3 days from passage 2 to passage 4. MSCs were characterized by flow cytometry analysis; their expression levels of CD90 (positive surface marker) and CD11b (negative surface marker) were evaluated by flow cytometry (FACScan; BD Biosciences).
Migration Tests
-
1.
A novel in vitro migration system
In order to ensure standard experimental conditions, we developed an agarose-based migration assay by preparing chemotaxic-agarose blocks. The brain extracts agarose block mimicked infarcted brain regions. To prepare ischemic brain tissue extracts (IBE), ischemic brains were obtained 3 days after 90 min tMCAo. Ipsilateral hemispheres were homogenized by adding DMEM (150 mg/ml) on ice. After centrifugation for 10 min at 10,000×g at 4 °C, the supernatants were collected and stored at −70 °C until used again [13].
To prepare the chemotaxic-agarose blocks, Type II agarose (Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) in a microwave oven. The concentration of agarose solution was 1 g/100 ml PBS. After cooling to 37 °C in a water bath, the agarose solution was mixed with an equal volume of warm DMEM or 40% IBE in DMEM. Within this agarose chamber slide system, a 5-mm space between the cells and the agarose block containing the infarcted brain extracts was designed for the rapid diffusion of the chemoattractant molecules and fast induction of cell migration. The 1 ml agarose solution was poured into the four-well chamber slide (100 mm × 180 mm) and allowed to harden.
MSCs were plated at a density of 2 × 105 cells/well into poly-L-lysine-coated one-well chamber slides (Nalge Nunc International) using DMEM with (a) 10% FBS, (b) 10% NS, or (c) 10% SS. Some MSCs were removed with a cell scraper (Nalge Nunc International) within 150 mm from the lateral side of the chamber to locate the agarose block in the MSC culture slide. Before removing the MSCs, a start line was marked around the edge of the agarose block at the bottom side of the chamber slide. The MSCs were incubated for 2 days with serum-free knockout DMEM during the migration assay. Figure 2a shows a schematic of the agarose block used for the migration experiments. With this method, we obtained reproducible well configurations that were consistent in size from experiment to experiment. Furthermore, this agarose chamber slide system facilitates cell observation.
In vitro and in vivo migration tests. a Schematic illustration of the vertical migration system using a chamber slide and representative images of migrating cells with toluidine blue staining. The black line denotes the starting line. b The migrating MSCs were counted, and the results are presented in the bar graph. c Quantification of migrating human MSCs in the ischemic boundary zone (IBZ) at 1 and 5 weeks after transplantation (6 fields/section). Representative images of human nuclei immunoreactivity (blue, DAPI; red, human nuclei) in the IBZ of rats that received FBS-MSCs or SS-MSCS. d Bar graph presenting the number of human nuclei-positive cells (*p < 0.05, **p < 0.01, vs. FBS-MSC). Data are expressed as the mean ± SEM (n = 4, *p < 0.05, **p < 0.01, vs. FBS-MSC, independent t test)
Migrated MSCs were stained with Toluidine Blue (Sigma-Aldrich) for visualization and quantitation. The number of migrating cells (N) was calculated according to the difference in the moving distance within 10 μm from the start line between the chemotactic agarose block [chemotaxis (CT)] and the control agarose block [chemokinesis (CK)]: N = CT − CK. MSCs were counted in eight consecutive fields of 0.9 × 100 μm2. For each measured parameter, the value corresponds to the mean of three independent experiments.
-
2.
Immunohistochemistry to assess in vivo migration
A total of 1 × 106 human MSCs or PBS only was injected via the tail vein at 1 day after tMCAo. Seven days after treatment, the animals were killed and subjected to transcardial perfusion with PBS and 4% paraformaldehyde. The brains were removed, stored in 4% paraformaldehyde at 4 °C overnight, and immersed in 30% sucrose for 3–4 days at 4 °C. The brains were then frozen rapidly in powdered dry ice and stored at −70 °C. Frozen brains were sectioned coronally between 3 and 4 mm posterior to the bregma at a thickness of 18 μm using a Cryocut Microtome (Leica Microsystems) and stored at −70 °C until use.
Monitoring of implanted MSCs migration in vivo was performed by immunofluorescence staining, as previously described [14]. Migrated MSCs were calculated using immune-staining with mouse anti-human nuclei monoclonal antibody (1:200, Chemicon). Finally, sections were mounted using Vectashield with DAPI (Vector Laboratories). All fluorescence images were taken by EVOS microscope. The number of human nuclei-positive cells was counted in the six areas in the ischemic boundary zone (IBZ).
CXCR4, c-Met, and HIF-1α Expression
-
1.
Immunocytochemistry
Immunocytochemistry assays were performed on MSCs subjected to NS or SS conditioning, as previously described. Samples were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-CXCR4 (diluted 1:500, Abcam), rabbit anti-c-met (diluted 1:500, Santa Cruz Biotechnology), and mouse anti-HIF-1ɑ (diluted 1:50, Novus Biologicals). The signals were detected using Dylight-labeled anti-mouse IgG (diluted 1:200, Abcam) or Dylight-labeled anti-rabbit IgG (diluted 1:200, Abcam). Samples were mounted with Vectashield mounting medium (Vector Laboratories) and viewed using a microscope (EVOS, Advanced Microscopy Group).
-
2.
Western blotting
Western blot was performed on MSCs subjected to NS or SS conditioning, as previously described [15]. The signals were detected using Chemiluminescent HRP substrates (Thermo Scientific) and then analyzed using Agfa X-ray film and Image Gauge version 4.0 (Fuji Photo Film Co. Ltd). The CXCR4 rabbit polyclonal antibody (1:1000, Abcam) recognizes a 45-kDa region of the N-terminus of CXCR4. The rabbit c-met polyclonal antibody (1:1000, Santa Cruz Biotechnology) recognizes a 145–170-kDa C-terminal cytoplasmic domain of Met. The HIF-1ɑ monoclonal antibody (1:500, Novus Biologicals) recognizes a 100–120-kDa region of human HIF-1ɑ encompassing amino acids 432–528.
Protein Analysis of Serum from Patients with Acute Ischemic Stroke
To compare the serum levels of proteins between stroke patients and healthy controls, antibody array analysis was performed using the RayBio Biotin Label-based Human Antibody Array (AAH-BLG-1-4, RayBiotech, Inc.) according to recommended protocols, detecting 507 different human proteins, including cytokines, chemokines, growth factors, differentiation factors, angiogenic factors, adipokines, adhesion molecules, matrix metalloproteases (MMPs), binding proteins, inhibitors, and soluble receptors. The signals were scanned with a GenePix 4000B laser scanner (Agilent). Normalization was performed using the signal of internal controls on each array chip. To determine significance, t tests were used, and the fold change was cutoff at 1.5.
Behavioral Testing
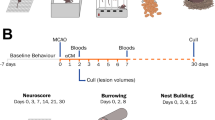
For all animals, a battery of behavioral tests was performed before tMCAo and 1, 3, 7, 14, 21, 28, and 35 days after tMCAo by an investigator who was blinded to the experimental groups. mNSS were calculated as a measure of motor, sensory, and reflex functions and balance using a modified version of sensory tests, as described previously [16]. The schematic procedure of in vivo studies was provided in Fig. 1b.
Statistical Analysis
For all assays, a total of three to six independent and separate experiments were performed to evaluate the different serum source supplements, and the results are expressed as the mean ± SEM. Statistical differences between groups were evaluated using one-way analysis of variance (ANOVA) and Tukey post-hoc analysis or independent t tests. A p value <0.05 was considered statistically significant. Statistical analyses were performed using a commercially available software package, SPSS version 20 (SPSS Inc.). Graphs were drawn using Graph Pad Prism (Graph Pad Software).
Results
Conditioning of MSCs Promotes Cell Migration In Vitro
Cellular morphology and expression levels of stem cell markers were not different among the groups (Supplementary Data II). The results of the in vitro cell migration assay are shown in Fig. 2a, b. The number of rat MSCs that migrated toward the brain extracts agarose block that mimicked an infarcted brain region was significantly higher in the SS group than in the FBS group (p = 0.033). No significant difference in the number of migrated cells was observed between the NS and FBS groups (p > 0.05). An in vivo analysis of the number of human MSCs in the IBZ (Fig. 2c) was performed to confirm the in vitro results. Compared to the FBS group, the SS group showed a significantly higher number of human nuclei monoclonal antibody-positive cells in the IBZ at 7 days (p = 0.037) and 5 weeks (p = 0.0005) after injection (Fig. 2d).
CXCR4 and c-Met Mediate the Increased Migration of MSCs after Conditioning with Stroke Serum
We then compared the characteristics of MSCs cultured with FBS, NS, or SS. The immunocytochemistry results showed that the expression levels of the SDF-1 receptor CXCR4 and the hepatocyte growth factor (HGF) receptor c-Met in MSCs were much more intense in MSCs cultured with SS than in MSCs cultured with FBS or NS (Fig. 3a). In addition, the mRNA expression level of HGF increased in MSCs cultured in SS (Fig. 3b). Western blot analysis showed that the expression levels of CXCR4 and the mature forms of c-Met increased in MSCs cultured in SS for 48 h in a dose-dependent manner (p < 0.05 for 10% SS vs. FBS, NS, or 5% SS and p < 0.001 for 20% SS vs. FBS, NS, or 5% SS) (Fig. 3c, d). The expression of CXCR4 and c-Met did not change significantly in MSCs cultured in NS conditions over the different concentrations.
a Immunofluorescence analysis of the expression of CXCR4 or c-Met in MSCs cultured with FBS, NS, or SS (upper, anti-CXCR4; lower, anti-c-met). b RT-PCR results for determination of mRNA expression levels of hepatocyte growth factor (HGF) in FBS-MSCs, NS-MSCs, or SS-MSCs. Western blot analysis of the expression of CXCR4 (c) or c-Met (d) in MSCs cultured with different concentrations of FBS, NS, or SS. Bar graphs present the quantitative results. Data are expressed as the mean ± SEM (n = 4, *p < 0.05, **p < 0.01, vs. FBS-MSC, independent t test)
To confirm whether the enhancing effect of SS conditioning on MSC migration is mediated via CXCR4 and c-Met, their inhibitors were administered during an in vitro cell migration assay. The number of migrating MSCs was significantly higher in SS-cultured MSCs than in FBS- or NS-cultured MSCs (p < 0.01), but this enhanced MSC migration was completely abolished by treatment with either AMD3100 (a CXCR4 inhibitor, 20 μM) or PHA665752 (c-Met-specific inhibitor, 0.25 μM) (Fig. 4).
a Representative images of stained chambers showing MSCs that migrated toward the ischemic brain extracts agarose block following treatment with FBS, NS, or SS with/without AMD3100 (20 μM) or PHA66752 (0.25 μM). b Bar graph presenting the average number of migrating cells. Data are expressed as the mean ± SEM (n = 4, **p < 0.01, vs. CTRL, one-way ANOVA, Tukey post-hoc test)
HIF-1a Contributes to HGF-Mediated CXCR4 Upregulation
Because the expression levels of CXCR4, HIF-1a, and both HGF and c-Met were upregulated in SS-cultured MSCs, we tested whether there is crosstalk among the SDF-1/CXCR4, HIF-1a, and HGF/c-Met axes relevant to MSC migration (Fig. 5). Blocking c-Met inhibited the induction of HIF-1a and CXCR4 expression in SS-cultured MSCs. Inhibition of CXCR4, however, did not inhibit the induction of c-Met and HIF-1a. These results suggest that the activation of c-Met mediated CXCR4 induction via HIF-1a.
Western blot analysis to assess a CXCR4; b c-Met; and c HIF-1ɑ expression in FBS-MSCs, NS-MSCs, or SS-MSCs with/without AMD3100 (20 μM) or PHA 665752 (0.25 μM). Quantitative data are presented in bar graphs. Data are expressed as the mean ± SEM (n = 4, *p < 0.05, **p < 0.01, vs. CTRL, one-way ANOVA, Tukey post-hoc test). d The proposed mechanism of migration by stroke serum preconditioning
Circulating Factor Levels in Serum from Acute Ischemic Stroke Patients
We then compared the circulating factors in SS and NS. Antibody array analysis showed that several chemokines and cytokines related to MSC mobilization were increased in the serum from stroke patients relative to the serum from normal human subjects (Fig. 6). Specifically, the levels of chemokines (e.g., CCL14, MCP-1, and MIP-3α), MMPs (e.g., MMP-9), inflammatory cytokines (e.g., IFN-γ), and growth factors (e.g., brain-derived neurotrophic factor, basic fibroblast growth factor, epidermal growth factor, and vascular endothelial growth factor [VEGF]) were significantly increased in the SS group compared with the NS group (p < 0.05 in all cases).
Effects of Preconditioning with Stroke Serum on Recovery after Stroke
Behavioral testing showed a significant improvement in recovery after stroke in the SS group compared with the other groups (Fig. 7). Immunohistochemistry data showed enhanced neurogenesis in the subventricular zone and angiogenesis in the IBZ, and diffusion tensor imaging using 7.0 T animal MRI showed increased synaptogenesis in the SS group (Supplementary Fig. II).
Discussion
Main findings of this study are (a) preconditioning of MSCs using serum obtained at the acute phase of stroke enhances the migration of MSCs toward infarcted brain areas, (b) this enhanced migration occurs by the upregulation of CXCR4 expression in MSCs via the HGF/c-Met and HIF-1ɑ pathways, and (c) enhanced migration facilitates the recovery from neurologic deficits in a rat model of stroke.
Efforts to Increase the Number of MSCs That Migrate to Infarcted Brain Areas
The success rate of localizing transplanted cells to the brain after stroke is quite low, with <1% of infused bone marrow-derived cells detected 24 h after infusion in recent clinical studies [17]. Moreover, most stem cells die in the infarcted brain within several weeks [18]. Therefore, strategies to increase stem cell homing and trophism to target regions may improve the therapeutic efficacy of MSC therapy. There have been efforts to increase the number of MSCs that migrate to infarcted tissue [19]. For example, an intra-arterial approach, in which pulmonary circulation can be bypassed, results in greatly improved delivery of stem cells to the ischemic brain [9]. However, an arterial approach may cause arterial occlusion, resulting in stroke; hence, this approach was not found to be a better option than an intravenous approach for recovery after stroke [9, 20]. In addition, enhancing the chemotactic SDF-1-CXCR4 signaling axis has been used to augment stem cell homing and retention; this approach includes genetic modification (adenoviral transfection of CXCR4 to MSCs) [21], ex vivo treatment (administration of a stable analog of prostaglandin E2 to hematopoietic stem cells) [22], and strategies to increase of the level of SDF-1α in infarcted brain areas, such as target delivery using polymers [14] and direct injection of a small molecule antagonist of CXCR4 (e.g., dipeptidyl peptidase-4 inhibitors) [23], nitric oxide donor (diethylenetriamine-NONOate) [24], or VEGF [25]. However, most of these approaches are not feasible for stroke patients.
Preconditioning with Stroke Serum as a Strategy for Enhancing MSC Migration Toward Infarcted Brain Areas
Presenting the appropriate stimuli to cells may promote a transient adaptive response (preconditioning). Various methods of preconditioning, including hypoxia [26,27,28,29] and SDF-1 treatment [30], have been reported to enhance stem cell migration and survival in infarcted myocardium or brain tissues. Hypoxic preconditioning unregulated the levels of HIF-1a and growth factors, chemokine receptors, and anti-apoptotic gene expression [26, 28].
We examined the effect of stroke serum conditioning on MSC migration in both in vitro and in vivo systems. Our present study first demonstrates the effects of preconditioning using stroke serum on MSC migration in stroke models. Stroke serum preconditioning may particularly be helpful in the setting of allogeneic MSC transplantation (“absence of request”) or autologous MSC transplantation, which requires prolonged ex vivo culture expansion (“declined request”). To analyze the migration ability toward target tissues, we designed a vertical migration system, with which we achieved improvements in preciseness and reproducibility. Various methods were used to assess chemotaxis, including the modified Boyden chamber assay [31], Zigmond chamber assay [32], and under-agarose assay (mainly used for studying blood cells such as neutrophils) [33]. One advantage of our in vitro migration system is that it is possible to observe the cells at any time and analyze cellular interactions. In addition, the system is simple to make, can be modified to specific experimental needs, and is quite inexpensive compared to other commercial devices.
Molecular Mechanisms Underlying Increased MSC Migration Following Preconditioning with Stroke Serum
To potentiate the effects of MSC therapy, an understanding of the intracellular signaling pathways underlying MSC migration is crucial. Our present results showed that both CXCR4 and c-Met were upregulated in MSCs by preconditioning with stroke serum, leading to enhanced migration. Our results are in line with very recent findings that CXCR4 was upregulated in MSCs after treatment with serum from rats with acute liver failure [34]. The homing capacities of MSCs are greatly influenced by cell culture conditions. Culturing MSCs for more than two passages is associated with decreased adhesion molecule expression and loss of chemokine receptors, including CXCR4 [35, 36].
The SDF-1α/CXCR4 axis and HGF/c-Met axis may play important roles in tissue repair because SDF-1α and HGF are activated at the sites of ischemic injury. Moreover, MSCs express CXCR4, the receptor for SDF-1α, and therefore, the SDF-1α/CXCR4 axis has been implicated in the migration of MSCs in a series of studies [37, 38]. Several growth factors and their receptors may also be involved in MSC migration. Following myocardial ischemia and reperfusion in a rat model, HGF and its high-affinity receptor c-Met were upregulated [39]. In addition, MSCs are known to secrete several trophic factors, including HGF [11, 40], and CXCR4 intracellular storage has been previously described in response to cytokines. A study on breast cancer cells reported that HGF was able to regulate the expression and activity of HIF-1α and contribute to tumor cell survival [41, 42].
HIF-1a has been previously shown to be affected by hypoxia and be associated with cell motility [43, 44]. In the present study, we tested whether HIF-1a played a role in the induction of CXCR4 in response to HGF during the stroke serum preconditioning process. Our cytokine analysis comparing SS and NS revealed that several cytokines and growth factors related to HIF-1a expression were increased in serum from stroke patients relative to serum from control subjects. An inflammatory cytokine mixture containing IFN-y, TNF-ɑ, IL-1β, and IL-17 family members markedly upregulated the expression of HIF-1a mRNA and protein in normoxic conditions [45, 46]. In addition, some growth factors, such as insulin-like growth factor, brain-derived neurotrophic factor, epidermal growth factor, fibroblast growth factor, and transforming growth factor-β, may regulate VEGF expression via HIF-1a [47,48,49,50,51]. Specially, HGF was directly associated with proinflammatory cytokines. IFN-y, IL-1, and transforming growth factor-β can stimulate HGF expression in carcinoma cells or human skin fibroblasts [52,53,54].
Our results suggest that although CXCR4 is upregulated by HIF-1a during stroke serum preconditioning, HIF-1a induces CXCR4 only after HGF stimulation. Upregulation of CXCR is therefore likely due to the activity of transcriptional activators such as HIF-1a [26, 55]. Collectively, our data indicate crosstalk between two ligand/receptor systems essential for MSC migration (Fig. 5d).
Enhanced Migration of MSCs Facilitates Recovery after Stroke
In the present study, behavioral recovery was more pronounced in rats that received SS-cultured MSCs. This effect could be related to increased neurogenesis, angiogenesis, and possibly synaptogenesis associated with the enhanced migration of MSCs to infarcted brain regions. In addition to the enhanced migration of MSCs, preconditioning with stroke serum may facilitate neurogenesis and neurogenesis via HIF-1a because HIF-1a may play role in neuroectoderm differentiation [56] and the HIF-1a signaling pathway can switch on an angiogenesis process in ischemic disease [57]. In addition, our unpublished data showed that preconditioning with stroke serum increased trophic factor release, decreased senescence, and increased MSC survival under ischemic brain conditions via circulating signals from the infarcted brain area.
Limitations and Conclusions
This study has some limitations. First, we compared only the differential effects of culturing MSCs in FBS, normal serum, and stroke serum on migration. Enhanced mobility toward ischemic regions in a concentration-dependent manner, accompanied by elevated levels of chemokines and trophic factor expression levels, was not assessed quantitatively. Further studies are needed to identify the mechanisms underlying the effects of circulating factors on MSC trophism. Second, the present study did not evaluate the blood-brain barrier, which could also affect the number of cells that migrate into ischemic brain areas.
In conclusion, our results indicate that culture expansion of MSCs with serum obtained during the acute phase of stroke is an important step in the application of MSCs to treat ischemic stroke because it can enhance the therapeutic efficacy of stem cell therapy. Our novel approach of preconditioning MSCs with autologous serum obtained during the acute phase of stroke is a feasible strategy to enhance therapeutic efficacy while meeting the FDA regulations for stem cells in clinical applications. Based on the results of the present study, the STARTING-2 trial (a prospective, randomized clinical trial evaluating the efficacy of intravenous autologous MSCs culture expanded with autologous stroke serum) is currently ongoing in patients with ischemic stroke (clinical trial identifier NCT01716481) [12].
References
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82. doi:10.1002/ana.20501.
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–807. doi:10.1093/brain/awr063.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106. doi:10.1002/stem.430.
Savitz SI, Dinsmore J, Wu J, Henderson GV, Stieg P, Caplan LR. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: a preliminary safety and feasibility study. Cerebrovasc Dis. 2005;20(2):101–7. doi:10.1159/000086518.
Savitz SI, Misra V, Kasam M, Juneja H, Cox CS Jr, Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. doi:10.1002/ana.22458.
Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092). Stroke. 2006;37(12):2979–83. doi:10.1161/01.STR.0000248763.49831.c3.
Bang OY. Clinical trials of adult stem cell therapy in patients with ischemic stroke. J Clin Neurol. 2016;12(1):14–20. doi:10.3988/jcn.2016.12.1.14.
Lindvall O, Kokaia Z. Recovery and rehabilitation in stroke: stem cells. Stroke. 2004;35(11 Suppl 1):2691–4. doi:10.1161/01.STR.0000143323.84008.f4.
Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41(9):2064–70. doi:10.1161/STROKEAHA.109.575993.
Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47(8):1295–301.
Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, et al. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17(9):1045–59.
Kim SJ, Moon GJ, Chang WH, Kim YH, Bang OY. Intravenous transplantation of mesenchymal stem cells preconditioned with early phase stroke serum: current evidence and study protocol for a randomized trial. Trials. 2013;14(1):317. doi:10.1186/1745-6215-14-317.
Shin JH, Park YM, Kim DH, Moon GJ, Bang OY, Ohn T, et al. Ischemic brain extract increases SDF-1 expression in astrocytes through the CXCR2/miR-223/miR-27b pathway. Biochim Biophys Acta. 2014;1839(9):826–36. doi:10.1016/j.bbagrm.2014.06.019.
Kim DH, Seo YK, Thambi T, Moon GJ, Son JP, Li G, et al. Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1alpha using a dual ionic pH-sensitive copolymer. Biomaterials. 2015;61:115–25. doi:10.1016/j.biomaterials.2015.05.025.
Moon GJ, Shin DH, Im DS, Bang OY, Nam HS, Lee JH, et al. Identification of oxidized serum albumin in the cerebrospinal fluid of ischaemic stroke patients. Eur J Neurol. 2011;18(9):1151–8. doi:10.1111/j.1468-1331.2011.03357.x.
Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, Lu M, et al. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41(3):524–30. doi:10.1161/STROKEAHA.109.568881.
Rosado-de-Castro PH, Schmidt Fda R, Battistella V, Lopes de Souza SA, Gutfilen B, Goldenberg RC et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen Med 2013;8(2):145–155. doi:10.2217/rme.13.2.
Rosenblum S, Wang N, Smith TN, Pendharkar AV, Chua JY, Birk H, et al. Timing of intra-arterial neural stem cell transplantation after hypoxia-ischemia influences cell engraftment, survival, and differentiation. Stroke. 2012;43(6):1624–31. doi:10.1161/STROKEAHA.111.637884.
Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20(8):814–21. doi:10.1038/nm.3627.
Yang B, Migliati E, Parsha K, Schaar K, Xi X, Aronowski J, et al. Intra-arterial delivery is not superior to intravenous delivery of autologous bone marrow mononuclear cells in acute ischemic stroke. Stroke. 2013;44(12):3463–72. doi:10.1161/STROKEAHA.111.000821.
Bang OY, Jin KS, Hwang MN, Kang HY, Kim BJ, Lee SJ, et al. The effect of CXCR4 overexpression on mesenchymal stem cell transplantation in ischemic stroke. Cell Med. 2012;4:65–76.
Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074–81. doi:10.1182/blood-2013-05-503177.
Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4(4):313–23. doi:10.1016/j.stem.2009.02.013.
Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25(11):2777–85. doi:10.1634/stemcells.2007-0169.
Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, et al. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376(2):419–22. doi:10.1016/j.bbrc.2008.09.003.
Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun. 2010;401(4):509–15. doi:10.1016/j.bbrc.2010.09.076.
Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi:10.1038/cddis.2009.22.
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi:10.1016/j.jtcvs.2007.07.071.
Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–70. doi:10.1016/j.expneurol.2007.12.020.
Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77(1):134–42. doi:10.1093/cvr/cvm025.
Ancelin M, Chollet-Martin S, Herve MA, Legrand C, El Benna J, Perrot-Applanat M. Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Lab Investig. 2004;84(4):502–12. doi:10.1038/labinvest.3700053.
Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137(2):387–410.
Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, et al. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol. 2006;80(1):117–24. doi:10.1189/jlb.1205744.
Deng C, Qin A, Zhao W, Feng T, Shi C, Liu T. Up-regulation of CXCR4 in rat umbilical mesenchymal stem cells induced by serum from rat with acute liver failure promotes stem cells migration to injured liver tissue. Mol Cell Biochem. 2014; doi:10.1007/s11010-014-2147-7.
Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24(5):1254–64. doi:10.1634/stemcells.2005-0271.
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–41. doi:10.1634/stemcells.2005-0319.
Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356(3):780–4. doi:10.1016/j.bbrc.2007.03.049.
Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovas Revasc Med. 2006;7(1):19–24. doi:10.1016/j.carrev.2005.10.008.
Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997;95(11):2552–8.
Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22(4):275–9.
Tacchini L, De Ponti C, Matteucci E, Follis R, Desiderio MA. Hepatocyte growth factor-activated NF-kappaB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis. 2004;25(11):2089–100. doi:10.1093/carcin/bgh227.
Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22(9):1363–71.
Kohler T, Reizis B, Johnson RS, Weighardt H, Forster I. Influence of hypoxia-inducible factor 1alpha on dendritic cell differentiation and migration. Eur J Immunol. 2012;42(5):1226–36. doi:10.1002/eji.201142053.
Choi JH, Lee YB, Jung J, Hwang SG, Oh IH, Kim GJ. Hypoxia inducible factor-1alpha regulates the migration of bone marrow mesenchymal stem cells via integrin alpha 4. Stem Cells Int. 2016;2016:7932185. doi:10.1155/2016/7932185.
Hot A, Zrioual S, Lenief V, Miossec P. IL-17 and tumour necrosis factor alpha combination induces a HIF-1alpha-dependent invasive phenotype in synoviocytes. Ann Rheum Dis. 2012;71(8):1393–401. doi:10.1136/annrheumdis-2011-200867.
Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G373–84. doi:10.1152/ajpgi.00076.2002.
Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66(8):4249–55. doi:10.1158/0008-5472.CAN-05-2789.
Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, et al. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J Pathol. 2005;205(4):530–6. doi:10.1002/path.1734.
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–5.
Slomiany MG, Rosenzweig SA. IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 alpha expression and activity in retinal pigment epithelial cell line D407. Invest Ophthalmol Vis Sci. 2004;45(8):2838–47. doi:10.1167/iovs.03-0565.
McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281(34):24171–81. doi:10.1074/jbc.M604507200.
Chu SH, Ma YB, Zhang H, Feng DF, Zhu ZA, Li ZQ, et al. Hepatocyte growth factor production is stimulated by gangliosides and TGF-beta isoforms in human glioma cells. J Neuro-Oncol. 2007;85(1):33–8. doi:10.1007/s11060-007-9387-2.
Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90(4):822–32. doi:10.1038/sj.bjc.6601611.
Takami Y, Motoki T, Yamamoto I, Gohda E. Synergistic induction of hepatocyte growth factor in human skin fibroblasts by the inflammatory cytokines interleukin-1 and interferon-gamma. Biochem Biophys Res Commun. 2005;327(1):212–7. doi:10.1016/j.bbrc.2004.11.144.
Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Investig. 2006;86(12):1221–32. doi:10.1038/labinvest.3700482.
Zhao Y, Matsuo-Takasaki M, Tsuboi I, Kimura K, Salazar GT, Yamashita T, et al. Dual functions of hypoxia-inducible factor 1 alpha for the commitment of mouse embryonic stem cells toward a neural lineage. Stem Cells Dev. 2014;23(18):2143–55. doi:10.1089/scd.2013.0278.
Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–4.
Acknowledgements
This study was supported by a grant from the Korea Health Technology R&D Project, the Ministry of Health & Welfare (HI14C1624).
STem cell Application Researches and Trials In NeuroloGy (STARTING)-2 collaborators: Oh Young Bang, MD, PhD; Ji Hyun Lee; Gyeong Joon Moon, PhD; Yeon Hee Cho, MS; Ji Hee Sung; Soo Yoon Kim, MS; Jeong Pyo Son, MS; Dong Hee Kim, MS; Jong-Won Chung, MD; Mi Ji Lee, MD; Suk Jae Kim, MD; Soo Kyoung Kim, MD; Yoon Mi Kang, MS; Yong Man Kim, PhD; Hyun Soo Kim, MD, PhD; Jun Ho Jang, MD, PhD; Won Hyuk Chang, MD, PhD; Yun-Hee Kim, MD, PhD.
Author information
Authors and Affiliations
Consortia
Contributions
O.Y.B.: conception and design, manuscript writing, financial support, collection and/or assembly of data, data analysis and interpretation, administrative support, final approval of manuscript; D.H.K.: provision of study material, collection and/or assembly of data, data analysis and interpretation; J.H.L.: collection and/or assembly of data, data analysis and interpretation, provision of study material; S.Y.K.: collection and/or assembly of data, data analysis and interpretation, provision of study material; J.P.S.: collection and/or assembly of data, data analysis and interpretation, provision of study material; Y.H.C.: collection and/or assembly of data, data analysis and interpretation, provision of study material; J.M.C.: collection and/or assembly of data, provision of study material; W.H.C.: provision of study material or patients, administrative support; Y.H.K.: provision of study material or patients, administrative support; G.J.M: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to report.
Funding
This study was supported by a grant from the Korea Health Technology R&D Project, the Ministry of Health & Welfare (HI14C1624).
Ethical Approval
In this study, all human subject research was approved by the local institutional review board (Samsung Medical Center Institutional Review Board, Approval No. SMC 2011-10-047-047). All patients or guardians of patients provided written informed consent to participate in this study. All animal experiments were approved by Institutional Animal Care and Use Committee (IACUC) of Samsung Biomedical Research Institute (SBRI, Approval No. 201300117002) and performed under the Institute of Laboratory Animal Resources (ILAR) guidelines. All animals were maintained in compliance with the relevant laws and institutional guidelines of Laboratory Animal Research Center (LARC; AAALAC International approved facility, No. 001003) at the Samsung Medical Center.
Electronic Supplementary Material
ESM 1
(PDF 314 kb).
Rights and permissions
About this article
Cite this article
Bang, O.Y., Moon, G.J., Kim, D.H. et al. Stroke Induces Mesenchymal Stem Cell Migration to Infarcted Brain Areas Via CXCR4 and C-Met Signaling. Transl. Stroke Res. 8, 449–460 (2017). https://doi.org/10.1007/s12975-017-0538-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0538-2