Abstract
Pd-modified Ni foam electrodes were prepared by a spontaneous deposition method. Ni foam samples were immersed in acid PdCl2 solutions for different durations (t SD). The Pd loading and the surface area of the Pd deposits were determined as a function of [PdCl2] and t SD. SEM–EDX showed that Pd deposits were homogeneously formed on the walls of both the outer and the inner cells of the Ni foam. Pd-modified Ni foam electrodes were used as anodes for the oxidation of methanol, ethanol, ethylene glycol, and glycerol in basic media. Voltammetric curves for the oxidation of alcohols showed that the peak current increased with increasing Pd loading in a sub-linear way. For the lowest loading explored (ca. 1 mg of Pd in a 1 cm3 foam volume), the peak current per unit Pd mass was of the order of 650 A g−1 for 0.5 M methanol and ethanol, ca. 1,500 A g−1 for glycerol and higher than 2,000 A g−1 for ethylene glycol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel foams have been commercially available for more than two decades. Owing to the good electrocatalytic activity of Ni and to foam properties like high specific surface area, high porosity that favors reactant mass transfer, very good mechanical strength, electrical conductivity, and corrosion resistance, their use as electrodes was envisaged. Foams with different grade, conventionally measured as number of pores per linear inch (ppi), were carefully characterized, with special attention to their specific surface area, mass transfer rate, and potential distribution under both flow-by and flow-through conditions [1–4].
More recently, in order to enhance the catalytic activity of Ni, several groups investigated the modification of Ni foams by deposition of noble metals and other chemicals, with the aim of producing electrodes for fuel cells [5–13], water electrolysers [14], hydrogen storage [15], degradation of pollutants [16]. Two main strategies were employed: spontaneous deposition [8, 11, 13–16], in which a metal nobler than Ni displaces some of the foam material in a metal exchange reaction, and electrodeposition [5–7, 9, 10, 12, 14]. In spite of the fairly open structure of the foams, with pore diameters of the order of hundreds of micrometers, significant current distribution must be expected in electrodeposition experiments [3], possibly leading to inhomogeneous coverage of the foam surface. The composition data in [14], showing that deposition onto the inner cells wall is less effective than onto the outer cells wall, probably reflect current distribution effects. Spontaneous deposition, being an open circuit process, is not prone to similar problems. An apparent advantage of electrodeposition over spontaneous deposition is the possible control of the mass of deposited material through the electrolysis charge [6, 7, 9, 12]. Such an advantage may be significant in some case, but should not be overemphasized: when a noble metal is deposited at low current densities, the electrode potential may not be negative enough to completely prevent spontaneous deposition, i.e., not negative enough to avoid Ni oxidation by the noble metal cations. When spontaneous deposition accompanies electrodeposition, the use of Faraday’s law leads to underestimate the noble metal loading and thus to overestimate the catalyst activity per unit mass of active material.
Several reports demonstrated that Ni foams modified by noble metal deposition are effective electrocatalysts [5–7, 9, 11–13, 15, 16]. Data on the noble metal loading [7–9, 12, 16] and/or the noble metal surface area [6, 8, 9] were reported in some studies. However, the relationships linking noble metal loading, noble metal surface area, and catalytic activity of the electrodes were not studied in detail.
In the present work, we report on a study on the spontaneous deposition of Pd onto Ni foam, on the characterization of the Pd-modified Ni foam electrodes and on their use as electrocatalysts for the oxidation of methanol, ethanol, ethylene glycol, and glycerol. In order to characterize the electrodes, the specific surface area of the Ni foam was estimated by cyclic voltammetry (CV) and by electrochemical impedance spectroscopy (EIS), and its variation upon etching in acid media was assessed. The Pd loading, the surface area of Pd deposits, the residual surface area of Ni not coated by Pd and the morphology of the Pd deposits formed onto the Ni foam were investigated as a function of the experimental variables of the spontaneous deposition process. The catalytic activity of Pd-modified Ni foam electrodes with respect to the oxidation of alcohols was analyzed, mainly in terms of peak current per unit Pd loading (i p,Pd ), and the electrode stability was tested.
Experimental
Materials and Equipment
The Ni foam electrodes were produced from grade 50 INCOFOAMTM sheets 0.17 cm thick. The apparent density of the foam was 0.22 g cm−3, corresponding to a void volume fraction of 0.975. In most cases, the electrode exposed to the solution was a 0.5 × 0.5 × 0.17 cm parallelepiped (volume 0.0425 cm3); in some tests 0.25 × 0.25 × 0.17 cm or 1.0 × 1.0 × 0.17 cm electrodes were used. Before use in spontaneous deposition or in electrochemical experiments, the Ni foam was successively washed with acetone and dichloromethane, dried in an air stream, and then etched in an acid solution (typically 2 M HCl at 60 °C for 15 min). Other working electrodes used in voltammetric experiments included a Ni disc electrode (0.317 cm2) and a Pd wire electrode (0.322 cm2).
Voltammetric experiments aimed at electrode characterization or alcohol oxidation were performed in a two-compartment cell; the working electrode and a Pt wire counter electrodes were placed in the main compartment; an Hg|HgO|1 M KOH reference electrode was placed in the lateral compartment connected to the main one through a Luggin capillary. All potentials are referred to this reference electrode.
The electrochemical equipment consisted of an Autolab PGSTAT 100 and an Autolab PGSTAT 302N. SEM images were obtained with an FEI Quanta 200 FEG ESEM instrument, equipped with a field emission gun, operating in high vacuum condition at an accelerating voltage variable from 5 to 30 keV, depending on the observation needs. PdCl2 solutions were analyzed with a Metrohm 850 Professional IC liquid chromatographer equipped with a Metrohm 887 UV-visible detector and a METROSEP A SUPP 15–50 anionic column.
Procedures
The spontaneous deposition of Pd was performed by immersing the Ni foam samples in acid, deaerated PdCl2 solutions kept at 25 °C with a thermostat. The PdCl2 concentrations used in this study were 0.001, 0.005, and 0.03 M; the pH was adjusted at 1.0 with HCl. The duration of the spontaneous deposition reaction (t SD) was varied between 5 and 120 min.
The amount of Pd deposited for different t SD values was estimated by measuring the decrease in the Pd2+ concentration caused by each deposition experiment [15, 17], using ionic chromatography according to the method proposed by Bruzzoniti et al. [18]. Reasons for preferring this method, which assumes that all the Pd2+ that underwent reduction was deposited on the Ni foam as Pd(0), to an alternative approach based on the measurement of the electrode weight variation, were explained in [19].
The Pd-modified Ni foam electrodes were submitted to CV in 1 or 3 M KOH, over an extended potential range encompassing both the oxidation of Pd and the reduction of Pd surface oxides [20], as well as the Ni(OH)2/NiOOH inter-conversion [21]. The voltammetric charges associated to the reduction of Pd surface oxides (henceforth denoted Q PdO disregarding the real composition of the Pd oxides which depends on the formation potential) were used to estimate the true surface area of the Pd deposits.
The catalytic activity and the stability of the Pd-modified Ni foam electrodes towards the oxidation of alcohols were studied by recording cyclic voltammograms in stirred, deaerated 1 M KOH solutions containing methanol, ethanol, ethylene glycol, or glycerol at a 0.5 M concentration, kept at 25 °C with a thermostat. Cyclic voltammograms were recorded without ohmic drop compensation. However, the electrolyte resistance (R e) was measured by electrochemical impedance spectroscopy at E = −0.5 V, a potential at which no faradaic process takes place, and selected voltammetric data were corrected for the ohmic drop R e × i.
Quantities and Units
The specific surface area of a Ni foam sample (a FOAM) is defined as the true surface area of 1 cm3 volume of foam; it is expressed in cm−1, i.e., square centimeter per cubic centimeter. The true surface area of the Pd deposit present in 1 cm3 volume of Ni foam is henceforth denoted a Pd and is also expressed in cm−1. The Pd loading per unit volume (\( L_{\text{Pd}}^V \)) is the Pd mass in 1 cm3 volume of modified foam, and is expressed in milligrams per cubic centimeter. The Pd loading per unit Ni true surface area (\( L_{\text{Pd}}^S \)) is the Pd mass deposited on 1 cm2 of Ni true surface area, expressed in milligrams per square centimeter, and is calculated as
The current densities relevant to disc and wire electrodes (j) are expressed in Amperes per square centimeter and refer to the geometric area of the electrodes. Instead, for the foam electrodes the current (i) is reported and the relevant electrode volume (V FOAM) is mentioned in the text and/or in the figure captions. For foam electrodes with dimensions between 0.25 × 0.25 × 0.17 cm and 0.5 × 0.5 × 0.17 cm, i and V FOAM were found to be proportional to each other; significantly larger electrodes yielded somewhat lower i/V FOAM ratios. The alcohol oxidation peak current per unit Pd mass (i p,Pd) is expressed in Amperes per gram, and is calculated as
The peak current density per unit Pd true surface area (j p,Pd) is expressed in Amperes per centimeter, and is calculated as
Results and Discussion
Surface Area of Ni Foams; Effect of Etching in Acid Media
Figure 1 shows cyclic voltammograms recorded with a polished Ni disc electrode (a) and with a Ni foam electrode (b), in aqueous 3 M KOH, at variable scan rates v in the range 1 to 200 mV s−1. The ordinate axis reports the current density j for the disc and the current i for the foam electrode which had a volume of 0.17 cm3. The redox system observed in both cases is due to the Ni(OH)2/NiOOH inter-conversion [21]. At the highest scan rate, the oxidation peak is centered at 0.46 V for both electrodes; the reduction peak is at 0.375 V for the disc and at 0.31 V for the foam. Both oxidation and reduction peaks are significantly broader for the foam than for the disc electrode. The peak current measured with the Ni disc is proportional to the scan rate in the whole v range, whereas, in the case of the foam, a direct proportionality is observed only for v ≤ 10 mV s−1. Deviations observed at higher v values may be ascribed to the porous nature of the electrode [22]. At v ≤ 10 mV s−1, the redox charges of the Ni(OH)2/NiOOH system were independent of v for both electrodes and the ratio between the charge measured with the foam (Q Ni-foam) and that measured with the disc (Q Ni-disc) could be used to estimate the specific surface area of the foam as
where Q Ni-foam refers to a foam volume of 1 cm3 and Q Ni-disc refers to a disc geometric area of 1 cm2, assuming the true area of the carefully polished disc to be identical to the geometric area.
The impedance of a Ni foam electrode and of a polished Ni disc electrode was measured in 3 M KOH. In a frequency range between 10 mHz and 300 Hz the imaginary part of the impedance varied as f −α, with α = 0.90 ± 0.01 for both the Ni foam and the Ni disc. Although neither electrode exhibited an ideally capacitive behavior, the difference between the α values being very small, the specific surface area of the foam could be estimated from the ratio of the imaginary parts, i.e., as
where Z Ni-disc refers to a disc geometric area of 1 cm2 and Z Ni-foam refers to a foam volume of 1 cm3.
The specific surface area values obtained by CV and by EIS were respectively 235 and 220 cm−1 (i.e., areas of 235 and 220 cm2 were estimated for a 1-cm3 volume of Ni foam). The numerical values obtained by CV and EIS are quite close to each other and intermediate between the values reported by Langlois and Coueret [1], measured by BET method and by a pressure drop method with Ni foam samples very similar to those used in this work. Taking into account the apparent density of the Ni foam (0.22 g cm−3), a surface area per unit Ni foam mass of the order of 1,000 cm2 g−1 can be estimated.
The electrochemical methods were used to assess the effect of an etching treatment on the Ni foam specific surface area. The etching treatment currently used in this work (15 min in 2 M HCl at 60 °C) typically caused the specific surface area to increase to 285 ± 15 cm−1, i.e., by a factor of 1.2 to 1.3.
Electrodeposition of Pd vs. Spontaneous Deposition
Palladium was electrodeposited onto Ni foam electrodes, at different current densities, according to Harrison et al. [23], using a bath containing 0.025 M PdCl2, 0.2 HCl, and 3.8 M HClO4. The Pd-modified electrodes were then submitted to cyclic voltammetry in 1 M KOH. Figure 2 shows, for example, the voltammograms recorded with electrodes obtained by depositing Pd at −0.1 and −0.2 A cm−2 (referred to the foam electrode geometric surface area) for 60 and 30 s, respectively. These voltammograms are compared to those recorded with electrodes obtained by leaving Ni foam samples of the same shape and size immersed in the same solution, at open circuit, for the same durations. A voltammogram recorded with an unmodified Ni foam electrode is also presented.
Cyclic voltammograms recorded in 1 M KOH with Ni foam electrodes submitted to different treatments (etching, Pd electrodeposition, immersion in PdCl2 solution at open circuit) as indicated on the figure. Volume of the Ni foam electrodes is 0.17 cm3. Scan rate is 10 mV s−1. In each case, the tenth consecutive voltammogram is shown
The following features are evident
-
For all electrodes except the Ni foam, there is a reduction peak, centered around −0.3 V, which is ascribed to the reduction of Pd oxides [20]; its intensity is higher for the electrodeposits than for the samples left at open circuit, but is significant also for the latter; for the open circuit samples the peak intensity increases with the duration of the immersion; for the electrodeposited samples it slightly increases with the deposition current.
-
The redox system associated to the Ni(OH)2/NiOOH transition is much more intense for all Pd-modified samples than for the pristine Ni foam electrode, as it was already observed in the spontaneous deposition of Ru [24], Ir [24], and Pd [25] onto porous Ni.
These results clearly suggest that, at open circuit, spontaneous deposition of Pd takes place through the usual metal exchange reaction
most probably accompanied by the acid corrosion of Ni
Both processes induce a roughening of the Ni surface, with an increase in its surface area [25]. When a cathodic current is applied to the foam, Ni(0) is stabilized and both reactions 6 and 7 are likely to become slower. However, the large increase in the intensity of the Ni(OH)2/NiOOH redox system shows that the oxidation of Ni by Pd2+ and its corrosion are not entirely prevented by imposing a current of −0.1 or −0.2 A cm−2 (respectively corresponding to ca. −4.1 and −8.2 mA cm−2, if referred to the true surface area of the foam electrodes). Under such conditions, Pd is likely to be simultaneously deposited via two parallel routes: electrodeposition and spontaneous deposition, and so the total amount of Pd deposited may be well in excess of that predicted by the Faraday’s law.
On the basis of the above data, and of the fact that Pd electrodeposition onto Ni foams had already been studied by Wang et al. [12], our study was focused on Pd spontaneous deposition, an approach recently used by Cheng et al. [13] to prepare anodes for the oxidation of 2-propanol.
Spontaneous Deposition of Pd
Deaerated acid PdCl2 solutions were used for the spontaneous deposition of Pd onto Ni foam. PdCl2 concentration was varied between 0.001 and 0.030 M, and the deposition time (t SD) was varied between 5 and 120 min. The SEM images in Fig. 3 summarize the effect of [PdCl2] and t SD on the deposit morphology. The images of the left column, relevant to [PdCl2] = 0.005 M, show only sparse Pd nuclei which become larger and more numerous as t SD increases; grain boundaries, which become much more evident after acid etching than in as-purchased Ni foams, are also visible. A rougher surface and a much higher surface density of Pd nuclei, which increases for increasing t SD values, are visible in the images of the right column, relevant to [PdCl2] = 0.03 M. Figure 4 shows SEM images of Pd-modified Ni foams compared with maps of the same samples recorded by selectively detecting the Pd-L radiation. In these maps, the presence of Pd is highlighted by the light spots. Clearly, a high density of these spots is observed in the positions occupied by micrometer-size Pd nuclei. However, even for a sample prepared in 0.005 M PdCl2 solution with t SD as low as 5 min, the presence of Pd is evident over the whole sampled area. Of course, the dimension of the light spots is defined by the resolution of the image and must not be confused with the dimension of the nuclei. For identical Pd-L radiation collection times, the surface density of light spots is much higher for the longer t SD, to testify the increased Pd loading.
EDX analyses were performed by selectively sampling the walls of either inner or outer cells of the foam. Comparable Pd/Ni ratios were obtained, suggesting that an effective deposition of Pd took place also in the inner parts of the foam.
Figure 5 shows the dependence of the Pd loading \( L_{\text{Pd}}^V \)on t SD, for a 0.005 M PdCl2 solution. In spite of some scattering, a linear dependence can be recognized, with a constant deposition rate close to 0.16 ± 0.01 mg cm−3 min−1. Additional data reported in Table 1 show that the deposition rate measured in 0.001 and 0.030 M PdCl2 solutions were respectively ca. two times lower and 20–30 times higher than in 0.005 M solution. The \( L_{\text{Pd}}^V \) values were converted to values of Pd loading per unit Ni true surface area (\( L_{\text{Pd}}^S \)) using Eq. 1 and the typical a FOAM value of 285 cm−1. The \( L_{{Pd}}^S \) values may be useful to compare the 3D electrodes discussed in this work with other Pd-based systems described in the literature (see below).
Figure 6 shows cyclic voltammograms recorded with a Pd-modified Ni foam electrode, at a constant scan rate and progressively increasing anodic limits (E A). The redox system consisting of an oxidation peak at 0.475 V and a reduction peak at 0.405 V is much less intense than for a pristine Ni foam electrode, but testifies that part of the Ni surface is still in contact with the electrolyte. The intensity of these peaks is little sensitive to E A. The reduction peak located at a potential in the range −0.19 to −0.27 V, and moving to more negative potential values as EA becomes more positive, is due to the reduction of Pd surface oxides [20], formed during the anodic sweep, to metallic Pd. Its intensity markedly increases as EA becomes more positive, in agreement with the results in the literature [26–28]. The Pd oxides reduction charge (Q PdO) was used to quantify the surface area of Pd deposits by comparing it to the reduction charge measured with a smooth Pd electrode (Q° PdO). This charge was measured at a conveniently low scan rate, e.g., v = 10 mV s−1, with various anodic limits. The Q PdO /Q° PdO ratio was found to be essentially independent of E A, and therefore, a Pd was computed as
where Q PdO refers to a foam volume of 1 cm3 and Q° PdO to a geometric area of 1 cm2. Table 1 reports a Pd data obtained for some combinations of [PdCl2] and t SD. Predictably, larger a Pd values are obtained with more concentrated PdCl2 solutions and at longer deposition times. By using 0.005 or 0.03 M PdCl2 solutions and sufficiently prolonging the spontaneous deposition, a Pd values larger or much larger than the specific surface area of the etched foam (ca. 285 cm−1) were obtained. For example, the Pd surface areas of electrodes obtained with [PdCl2] = 0.005 M and t SD = 120 min and with [PdCl2] = 0.03 M and t SD = 60 min were larger than the specific surface area of an etched Ni foam by factors of ca. 3.5 and ca. 18, respectively. Such results agree with the roughness of the Pd deposits shown by SEM (compare Fig. 3).
The last column in Table 1 shows values of the Pd true surface area per unit Pd mass; these values are an index of how effectively the deposited Pd mass was used to generate a catalytically active Pd surface. From this point of view, the most effective deposition was obtained with [PdCl2] = 0.005 M, especially at low t SD values. The values of Pd surface area per unit mass in Table 1 are about 1 order of magnitude lower than those of Pd/C electrocatalysts described by Xu et al. [29].
The effect of [PdCl2] and t SD on the properties of Pd-modified Ni foam electrodes is also illustrated in Fig. 7, where cyclic voltammograms recorded with a fixed E a value, using electrodes prepared under different experimental conditions are shown. Besides the progressive increase in the intensity of the Pd oxide reduction peak, Fig. 7 shows that, upon increasing t SD, the Ni(OH)2/NiOOH system became increasingly less evident and virtually disappeared for the longest spontaneous deposition durations. This suggests that, for sufficiently high t SD values, almost the whole Ni foam surface was coated by Pd, in agreement with SEM images.
Cyclic voltammograms recorded in 3 M KOH with Ni foam electrodes modified by spontaneous deposition of Pd, in either 0.005 M PdCl2 solution (a) or 0.030 M PdCl2 solution (b), for different durations indicated on the figure. The voltammogram recorded with an unmodified Ni foam electrode is reported for comparison. Volume of the Ni foam electrodes: 0.0425 cm3. Scan rate is 10 mV s−1. In each case, the tenth consecutive voltammogram is shown
The Pd surface area is reported in Fig. 8 as a function of \( L_{\text{Pd}}^V \) for Pd-modified Ni foam electrodes prepared in 0.005 M PdCl2 with variable spontaneous deposition durations; the data suggest that a rapid increase of a Pd occurred upon deposition of very low Pd masses, and then the a Pd-\( L_{\text{Pd}}^V \)loading dependence was roughly linear in the investigated range.
Oxidation of Methanol at Pd-Modified Ni Foam Electrodes
The oxidation of methanol was studied by recording cyclic voltammograms in 1 M KOH, 0.5 M CH3OH solutions. Figure 9 collects the curves obtained with electrodes prepared by spontaneous deposition in 0.005 M PdCl2, for t SD values in the range 5 to 120 min. All voltammograms have the shape reported by many authors (for a review see [30]): an oxidation peak followed by passivation during the anodic scan and a sharp depassivation during the reverse scan, were observed. Higher t SD values produced electrodes with stronger activity. The marked shift in the peak potential towards higher values for increasing Pd loadings is largely due to uncompensated ohmic drop, and is greatly attenuated when the ohmic drop is corrected. The activity of unmodified Ni foam electrodes was completely negligible in the explored potential range (data not shown). An enhancement of the electrocatalytic activity of Pd by transition metal oxides, including Ni oxide has been reported [30]. The data in this work neither support nor contradict those reports.
Cyclic voltammograms recorded with Pd-modified Ni foam electrodes in 1 M KOH, 0.5 M CH3OH. For each curve, the duration of the Pd spontaneous deposition is indicated on the figure. Volume of the Ni foam electrodes: 0.0425 cm3. Scan rate is 10 mV s−1. For each t SD, the tenth consecutive voltammogram is shown
Figure 10a shows the peak currents measured in cyclic voltammetry (i p ) plotted as a function of the Pd loading: a sub-linear dependence was observed. As a result, the peak current per unit Pd mass (i p,Pd) was higher at lower loading, as shown in Fig. 10b. The i p,Pd value measured at low Pd loading in this study, 650 A g−1, was more than six times higher than those reported by our group for Pd-modified porous Ni electrodes [25], and comparable to the i p,Pd value which can be calculated from the data in [12] where, however, the Pd loading is likely to be underestimated, and therefore, the catalytic activity is likely to be overestimated.
The oxidation charge transferred during a voltammetric cycle is fairly large, at high Pd loading; for example, for the most active electrode in Fig. 9 (t SD = 120 min), it is of the order of 2 C. Taking into account that the total amount of methanol initially present within the cells of a 0.0425 cm3 Ni foam electrode is of the order of 2 × 10−5 moles, a significant methanol depletion must be expected, paralleled by some local pH decrease due to H+ release upon alcohol oxidation. On the other hand, the time necessary for a methanol molecule to diffuse across half of the foam electrode thickness may be estimated at ca. 700 s, a time about four times higher than that necessary to complete a voltammetric cycle. Therefore, one should expect methanol oxidation at Pd-modified Ni foam electrodes to be partially under mass transport control, as already observed with Pd-modified porous Ni or Co electrodes [19] although mass transfer should be more efficient in the foam comparatively large cells than in the much narrower pores of the Ni or Co electrodeposits.
The peak current density referred to the true surface area of Pd, j p,Pd defined by Eq. 3, was calculated for several Pd-modified Ni foam electrodes with variable Pd loading and was found to be of the order of 5 ± 1 mA cm−2. The electrodes stability is discussed in section Oxidation of Higher Alcohols: Ethanol, Ethylene Glycol, and Glycerol.
Oxidation of Higher Alcohols: Ethanol, Ethylene Glycol, and Glycerol
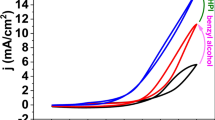
The oxidation of ethanol, ethylene glycol, and glycerol at Pd-modified Ni foam electrodes was studied under the same experimental conditions employed for methanol oxidation. Figure 11 compares the cyclic voltammograms obtained with Pd-modified Ni foam electrodes, prepared with [PdCl2] = 0.005 M and t SD = 15 min, in 1 M KOH containing the different alcohols at a 0.5-M concentration. The shape of the curves is similar for all alcohols, in agreement with results in the literature [25, 29–35]. The lowest onset potential is that of ethanol followed by ethylene glycol, glycerol, and methanol. The peak current varies in the order ethylene glycol>glycerol>ethanol≈methanol. Such an order essentially agrees with the results of other authors who employed Pd-based electrocatalysts, although the differences between the oxidation current of polyols and alcohols were larger in our study than in previous studies.
Cyclic voltammograms recorded with Pd-modified Ni foam electrodes in 1 M KOH, 0.5 M alcohol (methanol, ethanol, ethylene glycol, or glycerol, as indicated on the figure). The electrodes were prepared in 0.005 M PdCl2 with t SD = 15 min. Volume of the Ni foam electrodes is 0.0425 cm3. Scan rate is 10 mV s−1. For each alcohol, the tenth consecutive voltammogram is shown
Table 2 summarizes the peak current per unit Pd mass obtained for the oxidation of methanol, ethanol, ethylene glycol, and glycerol with electrodes prepared with three different t SD values. For all alcohols, i p,Pd decreased upon increasing t SD, i.e., upon increasing the Pd loading. The i p,Pd values obtained in the oxidation of ethylene glycol and glycerol with low loading electrodes (\( L_{\text{Pd}}^S \)<10 μg cm−2) were comparable to those reported for ethanol and ethylene glycol by Bambagioni et al. [35, 36] for Pd-(Ni–Zn)/C electrodes with 20–30 μg cm−2 Pd loading. Table 2 also reports the current values measured at E = −0.2 V (corrected for ohmic drop), a potential much more negative than the peak potential, at which the current remained quite stable upon prolonging the polarization.
The peak current density referred to the true surface area of Pd, j p,Pd, was calculated for each alcohol and for several Pd-modified Ni foam electrodes with variable Pd loading. The values obtained for ethanol, ethylene glycol, and glycerol were 5 ± 1, 12 ± 2, and 9 ± 1.5 mA cm−2, respectively. Alcohol and polyol oxidation at Pd-based electrocatalysts is known to produce mainly (poly)carboxylates, because C–C bond cleavage is not effectively promoted [30]. Assuming (poly)carboxylates to be the sole reaction products, the oxidation of methanol, ethanol, ethylene glycol, and glycerol would require the removal of 4, 4, 8, and 10 electrons, respectively. The fact that the measured j p,Pd values are very similar for methanol and ethanol, and about twice larger for the polyols than for alcohols, may be a direct consequence of the different number of electrons exchanged in the process.
Figure 12 compares the evolution of i p,Pd with the number of successive voltammetric cycles, for the oxidation of methanol, ethanol, ethylene glycol, and glycerol at Pd-modified Ni foam electrodes, prepared with [PdCl2] = 0.005 M and t SD = 60 min. In all cases, the stability is quite good, better for the methanol and ethanol than for the polyols, for which part of the decline may be ascribed to the stronger consumption of the substrate due to higher oxidation currents.
Conclusions
Spontaneous deposition of Pd was used as a method for activating Ni foams towards the oxidation of alcohols and polyols. The Pd loading of the modified foam electrodes could be easily controlled by acting on the PdCl2 concentration and on the duration of the deposition treatment. The conditions leading to the highest values of Pd true surface area per unit Pd loading involved intermediate PdCl2 concentration and short deposition time. Not surprisingly, the electrodes prepared under these experimental conditions were the most active in the oxidation of alcohols. The peak currents per unit Pd mass measured with low Pd loading electrodes (650 A g−1 for 0.5 M methanol and ethanol, ca. 1,500 A g−1 for glycerol and higher than 2,000 A g−1 for ethylene glycol) were comparable to those reported for the most active Pd-based electrocatalysts. The very good coverage of the Ni foam surface by tiny Pd particles and the rather effective mass transport ensured by the Ni foam geometry were probably at the origin of these good results.
References
S. Langlois, F. Coeuret, Journal of Applied Electrochemistry 19, 43 (1989)
S. Langlois, F. Coeuret, Journal of Applied Electrochemistry 19, 51 (1989)
S. Langlois, F. Coeuret, Journal of Applied Electrochemistry 20, 740 (1990)
S. Langlois, F. Coeuret, Journal of Applied Electrochemistry 20, 749 (1990)
J.M. Skowronski, A. Wazny, Journal of Solid State Electrochemistry 9, 890 (2005)
W. Yang, S. Yang, W. Sun, G. Sun, Q. Xin, Journal of Power Sources 160, 1420 (2006)
W. Yang, S. Yang, W. Sun, G. Sun, Q. Xin, Electrochimica Acta 52, 9 (2006)
Y. Yamauchi, M. Kumatsu, A. Takai, R. Sebata, M. Sawada, T. Momma, M. Fuziwara, T. Osaka, K. Kuroda, Electrochimica Acta 53, 604 (2007)
F. Bidault, D.J.L. Brett, P.H. Middleton, N. Abson, N.P. Brandon, International Journal of Hydrogen Energy 34, 6799 (2009)
F. Bidault, D.J.L. Brett, P.H. Middleton, N. Abson, N.P. Brandon, International Journal of Hydrogen Energy 35, 1783 (2010)
D. Cao, Y. Guo, G. Wang, R. Miao, Y. Liu, International Journal of Hydrogen Energy 35, 807 (2010)
Y.-L. Wang, Y.-Q. Zhao, C.-L. Xu, D.-D. Zhao, M.-W. Xu, Z.-X. Su, H.-L. Li, Journal of Power Sources 195, 6496 (2010)
Y. Cheng, Y. Liu, D. Cao, G. Wang, Y. Gao, Journal of Power Sources 196, 3124 (2011)
H. He, H. Liu, F. Liu, K. Zhou, Surface and Coating Technology 201, 958 (2006)
J.M. Skowronski, A. Czerwinski, T. Rozmanowski, Z. Rogulski, P. Krawczyk, Electrochimica Acta 52, 5677 (2007)
B. Yang, G. Yu, D. Shuai, Chemosphere 67, 1361 (2007)
L.I. Elding, Inorganica Chimica Acta 6, 647 (1972)
M.C. Bruzzoniti, C. Mucchino, E. Tarasco, C. Sarzanini, Journal of Chromatography 1007, 93 (2003)
L. Vázquez-Gómez, E. Verlato, S. Cattarin, N. Comisso, P. Guerriero, M. Musiani, Electrochimica Acta 56, 2237 (2011)
M. Grdeń, M. Łukaszewski, G. Jerkiewicz, A. Czerwiński, Electrochimica Acta 53, 7583 (2008)
M.A. Sattar, B.E. Conway, Electrochimica Acta 14, 695 (1969)
B. Conway, E. Barsoukov, J.R. Macdonal (eds.), Impedance spectroscopy (Wiley Interscience, Hoboken, 2005), pp. 469–497
J.A. Harrison, H.B. Sierra Alcazar, J. Thompson, Journal of Electroanalytical Chemistry 53, 145 (1974)
L. Vázquez-Gómez, S. Cattarin, P. Guerriero, M. Musiani, Electrochimica Acta 53, 8310 (2008)
E. Verlato, S. Cattarin, N. Comisso, P. Guerriero, M. Musiani, L. Vázquez-Gómez, Electrochemistry Communications 12, 1120 (2010)
T. Chierchie, C. Mayer, W.J. Lorenz, Journal of Electroanalytical Chemistry 135, 211 (1982)
A.N. Correia, L.H. Mascaro, S.A.S. Machado, L.A. Avaca, Electrochimica Acta 42, 493 (1997)
O. Ghodbane, L. Roué, D. Bélanger, Chemistry of Materials 20, 3495 (2008)
C. Xu, Z. Tian, P.K. Shen, S.P. Jiang, Electrochimica Acta 53, 2610 (2008)
C. Bianchini, P.K. Shen, Chemical Reviews 109, 4183 (2009)
P.K. Shen, C. Xu, Electrochemistry Communications 8, 184 (2006)
C. Coutanceau, L. Demarconnay, C. Lamy, J.-M. Léger, Journal of Power Sources 156, 14 (2006)
Z. Wang, F. Hu, P.K. Shen, Electrochemistry Communications 8, 1764 (2006)
V. Bambagioni, C. Bianchini, A. Marchionni, J. Filippi, F. Vizza, J. Teddy, P. Serp, M. Zhiani, Journal of Power Sources 190, 241 (2009)
V. Bambagioni, C. Bianchini, J. Filippi, W. Oberhauser, A. Marchionni, F. Vizza, R. Psaro, L. Sordelli, M.L. Foresti, M. Innocenti, ChemSusChem 2, 99 (2009)
V. Bambagioni, M. Bevilacqua, C. Bianchini, J. Filippi, A. Marchionni, F. Vizza, L.Q. Wang, P.K. Shen, Fuel Cells 10, 582 (2010)
Acknowledgments
The authors acknowledge the financial support of the Italian Ministry for Economic Development (MSE), MSE-CNR Agreement on National Electrical System. The authors are indebted to FILA INDUSTRIA CHIMICA SPA, San Martino di Lupari, Padova, Italy, owner of the Fei-ESem FEI Quanta 200 FEG instrument, for allowing its use for the research work described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verlato, E., Cattarin, S., Comisso, N. et al. Preparation of Pd-Modified Ni Foam Electrodes and Their Use as Anodes for the Oxidation of Alcohols in Basic Media. Electrocatal 3, 48–58 (2012). https://doi.org/10.1007/s12678-011-0075-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-011-0075-9