Abstract
The development of nanoparticles derived from noble metals has garnered significant attention in recent years, particularly with gold and silver being identified as prominent options for production of nanoparticles. The increasing interest in silver nanoparticles synthesis is due to its multidimensional applications such as water treatment, biosensor, food packaging and preservation, anti-microbial, anti-cancer and anti-inflammatory. Based on its diverse applications, the present study aimed to synthesize silver nanoparticles (SAgNPs) using Spirulina platensis extract as a green source and evaluate their antibacterial, antioxidant, and biocompatible properties. Techniques including UV spectroscopy, scanning electron microscopy (SEM) and Fourier transform-infrared spectroscopy (FTIR) were used to characterize the synthesized SAgNPs. The antioxidant activity was determined through DPPH radical scavenging activity, while the antibacterial activity was assessed using disk diffusion method. Results from UV- spectroscopy revealed a peak absorption at the range of 400–500 nm, with a prominent peak at 423 nm, which confirms the successful biosynthesis of SAgNPs. SEM analysis indicated an average particle size ranging from 50–70 nm. Results from FTIR analysis identified different functional groups (OH-, carbonyl and amino acids) which stabilized the nanoparticles synthesis. Hemolysis assay demonstrated less than 5% hemolysis at all the concentrations studied (100–12.5 µM) that represents the biocompatible nature of SAgNPs. The synthesized SAgNPs showed potent antibacterial activity against both S. aureus and E. coli. Furthermore, the nanoparticles displayed notable antioxidant activity, as evidenced by the antioxidant assay. In conclusion, the biosynthesized SAgNPs derived from Spirulina platensis demonstrated potential antibacterial, antioxidant, and biocompatible properties. These findings highlight the potential of utilizing Spirulina platensis for the biosynthesis of AgNPs with diverse applications in biomedical and pharmaceutical fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The term nanotechnology, as defined by the Royal Society, is “the design, characterization, production and application of structures, devices and systems by controlling shape and size at the nanometer scale”[1]. It is a fast-growing multidisciplinary field because of its wide scope of uses in various areas of science and technology. The applications of nanoparticles are developing quickly on multiple fronts including, biomedical, transportation, biosensors, drugs, vehicles, catalysis, drug delivery, food technology, medical services, horticulture, antimicrobial, and water purification [2].
Inorganic metals such as Ag, Au, Fe, Se, Si etc. have diverse roles in nanotechnology. Among these, silver nanoparticles (AgNPs) offer significant interest in accelerating scientific progress in modern nanotechnology research [3]. Silver nanoparticles (AgNPs) have broad utility across multiple domains including drug discovery, luminescence, renewable energy technologies, textiles, food industries, electronics, optics, cosmetics, catalysis, wound dressing, dentistry, and agriculture [4]. Green-synthesized AgNPs have been documented to possess diverse biological properties such as anti-inflammatory, antidiabetic, antiplasmodial, anticancer, antibacterial, antiviral and antioxidant attributes [3, 5].
There are several chemical and physical methods available for conventional synthesis of AgNPs. However, these conventional methods holds a range of disadvantages such as high energy consumption, toxic chemicals usage, and often yield particles in non-polar organic solutions, thus making them undesirable for biomedical applications [6]. The recent advancements in green synthesis of nanoparticles deliver an effective approach to overcome issues related to conventional approaches. They provide cost-effective, eco-friendly methods and also allow easy scale-up for large scale production of NPs. Green synthesis of NPs can be achieved by using microorganism and plants without the need of any toxic chemical use [7, 8]. The biological entities found in these biological systems serve as both reducing and capping agents in biosynthesis of nanoparticles. For instance, certain intracellular or extracellular proteins, functional groups and peptides reduce precursor salts into nanoparticles thereby acting as a stabilizing agent [9, 10].
Among various living organisms, algae have been widely used for the green synthesis of several metallic and metal oxide nanoparticles, because of their rapid growth, easy handling and fast biomass growth as compared to higher plants. To date, various algal strains have been investigated to synthesize different types of nanoparticles using the green approach. Blue-green algae (order: Chroococcales) has been classified into two families, Chroococcaceae and Entophysalidaceae. Both these families are distinguished based on their growth habitat forming colonies [11]. Spirulina platensis, a filamentous, blue-green algae has been widely used for the synthesis of AgNPs. These are free floating in nature and contains multicellular trichomes with one open end and left-handed helix [12]. S. platensis has high protein content (~ 60–70%), rich in essential amino acids, fatty acids, antioxidants, and vitamins. As most of these molecules are involved in stabilizing nanoparticles, S. platensis is widely used for green synthesis of nanoparticles and also serves as a major contributor for the synthesis of AgNPs [13].
Based on literature and to the best of our knowledge limited studies on Spirulina platensis biomass extract mediated AgNPs have reported the biocompatibility and antioxidant analysis. Therefore, the present study reported the green synthesis and optimization of silver nanoparticles using Spirulina platensis biomass extract (cell free extract) and evaluated its biocompatibility, antioxidant and antibacterial activity. The characterization of these synthesized SAgNPs was carried out through UV–Vis spectroscopy for determining the surface plasma resonance peak of the nanoparticles, scanning electron microscopy and energy dispersive X-ray spectroscopy to determine the shape, size as well as elemental analysis of the synthesized nanoparticles.
2 Material and Methods
2.1 Materials
Silver nitrate (AgNO3) and ascorbic acid were acquired from Daejung Chemical Co., Korea. 2,2-diphenyl-1-picrylhydrazyl (DPPH) was obtained from Sigma-Aldrich. Ethanol was obtained from BDH Laboratory Supplies. Spirulina platensis (biomass) was obtained from the culture collection in America. The chemicals and reagents used were all analytical grade.
2.2 Preparation of Spirulina Biomass Extract
The algal biomass extract was prepared by following the procedures mentioned by Muthusamy et al. [14] with slight modifications. The culture was maintained in conical flasks with Zarrouk’s media [15] under 30 W fluorescent lights for 16:8 h light and dark cycles. Subculturing was done at regular intervals to maintain the growth potential of microalgae. Through filtration method Spirulina was harvested and washed thoroughly to remove any extraneous material. 5 g of wet biomass was mixed with 50 ml deionized water in 100 ml Erlenmeyer flask and was kept in shaker incubator for 1 h at 50 ℃. The resulting extract was filtered with Whatman filter No. 1 and the filtrate was stored at 4 ℃ for further use.
2.3 Biosynthesis of Spirulina Mediated AgNPs
The spirulina extract mediated silver nanoparticles (SAgNPs) were prepared using 1 ml of Spirulina platensis extract (SPE) mixed with 5 ml of 1 mM silver nitrate aqueous solution. The resulting solution was kept in sunlight for 10 min and then kept in dark in shaking incubator at 30℃ for 24 h. Synthesis of SAgNPs from spirulina extract was indicated by color change of solution. (light green to yellow brown) [14].
2.4 Characterization of SAgNPs
2.4.1 UV–Visible Spectroscopy
The bio-reduction of silver ions by Spirulina biomass extract and formation of SAgNPs was observed by taking absorbance in the range of 300–700 nm using Epoch 2 microplate UV–Vis spectrophotometer (Agilent, Santa Clara, USA). The Spirulina biomass extract absorbance was also measured for comparison. The spectrum was plotted between wavelength and absorbance on X and Y axis.
2.4.2 Scanning Electron Microscopy (SEM) Analysis
The size and morphology of synthesized SAgNPs was examined by Scanning electron microscope (JSM-IT 100, Tokyo, Japan) at Dow Dental College, Dow University of Health Sciences, Karachi, Pakistan. The elemental composition of SAgNPs was determined via energy dispersive X-ray spectroscopy (EDX). The sample for SEM–EDX was prepared by placing a small amount of SAgNPs sample on carbon tape mounted on aluminium stub followed by gold sputtering for 30 s.
2.4.3 Fourier-Transform Infrared (FT-IR) Spectroscopy
Fourier-transform infrared (FT-IR) spectroscopy was performed for the identification of functional groups present on the surface of SAgNPs. Briefly SAgNPs solution was centrifuged for 20 min at 10,000 rpm then washed twice using deionized water and once with absolute ethanol. The Pellet was then left to air-dry and then it was ground to make fine powder to be used for FTIR analysis. The FT-IR analysis was done within the range of 3500–800 cm−1 using Cary 630 FTIR spectrometer (Agilent, Santa Clara, USA).
2.5 Antioxidant Activity Analysis
Identification of free radical scavenging activity of SAgNPs was done using DPPH method as described by Blois with slight modification [16]. Firstly, a 0.1 mM DPPH solution was prepared using 95% ethanol. Then from the prepared DPPH solution, 100 µL was mixed with 100 µL SAgNPs solution at different concentrations (25 µM-6.25 µM). In ethanol solution, DPPH gives violet/purple coloration which in the presence of antioxidants decolorizes to shades of yellow. The reaction was performed using a 96-well plate and was left in dark for 30 min at room temperature and pressure. The absorbance was measured with a microplate reader at 517 nm wavelength. In the control, the sample was substituted with deionized water. Antioxidant activity of spirulina extract, ascorbic acid and silver nitrate solution at equivalent concentration was also evaluated. To calculate the scavenging activity, the following equation was used:
where Ac is the absorbance of control and As is the absorbance of sample/extract/standard.
2.6 Hemolysis Assay
The hemolysis test was performed by the method described by Luna-Vázquez-Gómez, R., et al. [17] with slight modifications. Erythrocyte’s suspension was prepared using fresh blood samples taken from healthy individuals after getting informed consent. The blood sample was centrifuged for 5 min at 500 rcf and then washed three times with normal saline (0.9%). After washing, 2% (v/v) RBCs suspension was prepared in normal saline. The samples for hemolysis test were prepared by adding SAgNPs at different concentrations (100 µM, 50 µM, 25 µM and 12.5 µM) to 2% RBCs suspension. The SAgNPs were substituted with water in positive control and normal saline in negative control respectively. The contents were mixed by gently inverting the sample and control containing microcentrifuge tubes. After mixing, all the tubes were incubated at 37℃ for 1 h in water bath. After incubation, the tubes were centrifuged at 500 rcf for 5 min. Finally, 200 µL of supernatant was added carefully in a 96 well plate, without disturbing the pellet and absorbance was taken spectrophotometrically at the wavelength of 540 nm. To calculate the percentage hemolysis of RBCs, the following equation was used:
where, As, An and Ap indicate the absorbance of S-AgNPs sample, negative control, and positive control, respectively.
2.7 Antibacterial Activity Analysis
Antibacterial activity of SAgNPs was investigated using well diffusion method. Briefly the fresh cultures of Staphylococcus aureus (ATCC No:25923) and Escherichia coli (ATCC No:35218) were used in this experiment to prepare the inoculum in Nutrient Broth incubated for 24 h at 37℃. The turbidity of both bacterial suspensions was adjusted to 0.5 McFarland standard. Each bacterial suspension was then spread uniformly on the Mueller–Hinton agar (MHA) using a sterile glass spreader. After spreading, wells were prepared in the agar plate and 50 µl of sterile water as control, 1 mM SAgNPs, 0.5 mM SAgNPs, silver nitrate solution, and spirulina extract were added in the wells. Then the plates were incubated for 24 h at 37℃ and antibacterial activity of SAgNPs was evaluated by measuring the zone of inhibition.
2.8 Statistical Analysis
Data was analyzed using IBM SPSS Statistics software version 26. For comparison analysis, the One-Way ANOVA test was performed followed by Tukey and Games-Howell post hoc tests in case of equal and unequal variance, respectively. P-value 0.05 or less was considered statistically significant.
3 Results and Discussion
The green synthesis of nanoparticles, employing biological components such as plant extracts and microorganisms, represents a significant domain in nanotechnology. This method is an environmentally safe option for nanoparticles synthesis compared to the chemical and physical synthesis methods. Green synthesis operates under mild reaction conditions, eliminating the need for high-energy inputs and harsh conditions, thereby reducing environmental impact. Furthermore, the resulting nanoparticles exhibit favorable biocompatibility and non-toxic characteristics, making them suitable for diverse biomedical applications. Overall, the green synthesis of nanoparticles using biological elements presents a promising and sustainable alternative to traditional methods [18].
3.1 Synthesis of SAgNPs
A straightforward, affordable, and ecologically friendly strategy was chosen for the preparation of SAgNPs. The current study demonstrated the formation of AgNPs using S. platensis biomass extract as reducing agent. During nanoparticles synthesis, the mixing of the Spirulina extract (SPE) with aqueous silver nitrate resulted in yellowish brown coloration indicating the formation of Spirulina extract mediated silver nanoparticles (SAgNPs) as shown in Fig. 1. Since silver NPs are known for their propensity to stimulate surface plasmon vibrations, this gives them a yellowish-brown color in aqueous solution [19]. Similar color change has also been reported extensively in literature [20, 21].
3.2 Characterization of SAgNPs
3.2.1 UV–Vis Spectroscopy of SAgNPs
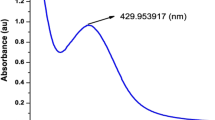
UV–Visible spectra of the synthesized nanoparticles were taken in the range of 300 nm to 600 nm. The synthesized SAgNPs showed peak in the range of 400 nm to 500 nm with maximum absorbance at 423 nm as shown in Fig. 2. According to earlier research, AgNPs can be discovered in the 400–550 nm region of the UV–visible spectrum. As a result, the formation of more SAgNPs was primarily responsible for the rise of the peak intensity [22]. Studies on green synthesis of AgNPs reported by Ahmed et al., and El Rabey et al., also demonstrated the AgNPs absorbance in the similar range [23, 24].
3.2.2 SEM and EDX Analysis
The size and shape of SAgNPs was observed through scanning electron microscopy (SEM) which gave a clear image of SAgNPs confirming the synthesis of nanoparticles Fig. 3a. The identified particles were roughly spherical shaped with the size range from 50 to 70 nm. In the sample, the presence of silver was confirmed by EDX reading in its desired state. The EDX graph also showed the presence of silicon (Si) and calcium (Ca). This is probably due to the presence of substrate (coverslip) over which the SAgNPs sample was placed during SEM microscopy Fig. 3b. The findings of the present study were in accordance with the previous studies that reported a similar size range of AgNPs [25, 26].
3.2.3 FT-IR Analysis of SAgNPs
The presence of functional groups responsible for stability and synthesis of silver nanoparticles was investigated by FT-IR spectroscopic analysis. The peaks obtained in FT-IR spectra confirmed the presence of Spirulina extract mediated silver nanoparticles as observed in Fig. 4. Upon the formation of AgNPs, significant changes were observed in the spectrum of spirulina extract mediated AgNPs. FTIR analysis is commonly employed to identify functional groups in compounds. The vibrational frequency of the OH groups in the FTIR spectrum became broader, indicating the presence of hydrogen bonding and the involvement of amine functionalities in the stabilization of the nanoparticles. Additionally, the carbonyl stretching vibration became more intense, suggesting the participation of amino acid groups in the stabilization process. Previous investigations have also shown the involvement of hydroxyl and amino acid functionalities in nanoparticle stabilization [27,28,29]. Phytochemicals present in microalgae serve as both metal-reducing agents and strong coating agents on nanoparticles. These phytochemicals contain amino, carboxyl, and hydroxyl functional groups. The presence of these groups likely contributes to silver ions reduction and their subsequent stabilization on the surface of the nanoparticles [30]. These findings further support the understanding of the underlying mechanisms involved in the green synthesis and stabilization of nanoparticles through biological extracts.
3.3 Antioxidant Activity
The antioxidant activity of SAgNPs at different concentrations (25 µM, 12.5 µM and 6.25 µM) was determined with DPPH free radical scavenging assay. As shown in Fig. 5, SAgNPs demonstrated 46.6%, 43.7% and 42.4% antioxidant activity at 25, 12.5 and 6.25 µM concentration. The SAgNPs antioxidant activity was significantly high in comparison to both silver nitrate and spirulina biomass extract (p ≤ 0.000). The standard ascorbic acid at the same concentrations mentioned above showed non-significant difference in the radical scavenging activity compared to SAgNPs. The DPPH radical is a stable nitrogen-centered radical. The ability to scavenge these DPPH radicals indicates the efficacy of sample for antioxidant activity. Previously done research by Ismail, G. A. et al., and Chakraborty eta l., also support our data [21, 31]. The results indicated that SAgNPs exhibited higher antioxidant activity compared to the S. platensis extract. It is important to note that the reducing activity found in each case is influenced by the structural characteristics of the studied antioxidant (AgNPs) [32, 33].
3.4 Hemolysis Assay
The emergence of silver nanoparticle-based therapeutics has prompted concerns regarding their potential toxicity. It is hypothesized that the observed membrane damage and cell death associated with silver nanoparticles may be attributed to their strong affinity for thiol groups present in biological constituents, such as proteins and phospholipids within the cellular membrane [34, 35]. It is essential to look at how nanoparticles affect blood, specifically erythrocytes, to determine whether or not they are biocompatible with the human body. Using a blood hemolysis test, the biocompatibility of our synthesized SAgNPs was estimated. For this purpose, various concentrations of SAgNPs were tested against hemolysis of RBCs using suspended RBCs in normal saline as negative control whereas suspended RBCs in distilled water as positive control. According to Figs. 6 and 7, hemolysis of 4.39% and 0.28%, was seen at concentrations of 100 µM and 50 µM SAgNPs and at 25 µM and 12.5 µM concentration 0.1% hemolysis was observed. All SAgNPs concentration significantly reduced RBCs hemolysis as compared to positive control (P ≤ 0.000). The hemolysis percentage decreased as the concentration of SAgNPs decreased. Our results are in agreement with the research studies related to biocompatibility analysis of AgNPs using hemolysis assay [36, 37]. According to the E2524-08 standard of American Society for Testing and Materials (ASTM), the hemolysis rate less than 5% fits within the biocompatible range [38]. SAgNPs were found to be biocompatible with erythrocytes in our investigation at various concentrations. The biocompatible characteristics of SAgNPs is mostly due to the biogenic synthesis of nanoparticles by cyanobacteria.
3.5 Antibacterial Activity
Antibacterial activity of SAgNPs was performed with two different concentrations (1 mM and 0.5 mM) against two human pathogenic bacteria, S. aureus (gram-positive) and E. coli (gram-negative). In the case of E. coli, the zone of inhibition (ZOI) was observed at both 1 mM and 0.5 mM concentration of SAgNPs with a diameter of 15.5 mm and 15 mm respectively. Ciprofloxacin demonstrated 28.7 mm ZOI which was significantly higher than both the concentrations of SAgNPs (P ≤ 0.000) (Figs. 8, 9 and 10). Similarly in the case of S. aureus, only 1 mM SAgNPs concentration showed 14.5 mm ZOI which was significantly high (P ≤ 0.010) in comparison to Ciprofloxacin (10.5 mm ZOI). However, the difference was non-significant between penicillin (16 mM ZOI) and SAgNPs. Similar observations have also been reported using Penicillium brasilianum NP5 Plumeria alba mediated AgNPs synthesis [39, 40]. Silver nitrate and Spirulina extract didn’t show any antibacterial activity (Figs. 8, 9 and 10). The results obtained are summarized in Table 1. Among the tested bacteria, the variations in sensitivity towards SAgNPs may be attributed to the differences in the composition of bacterial cell wall. Gram-positive bacteria possess a thick peptidoglycan layer (20–30 nm) between the cytoplasmic membrane and the non-lipid outer membrane, whereas Gram-negative bacteria have a thin peptidoglycan layer (approximately 3 nm) and an outer lipid membrane. This disparity could explain the heightened antibacterial activity of SAgNPs against Gram-negative strains, such as E. coli. The antibacterial activity of AgNPs is attributed to their small size, limited volume area, and crystalline structure, which can disrupt the bacterial cell wall by creating perforations or pits. This disruption leads to the release of crucial membrane proteins and lipopolysaccharide molecules. Additionally, the interaction between sulfur- and phosphorus- containing compounds, (like proteins and DNA) with the released silver ions (Ag+), is proposed to cause cell damage, resulting in the inhibition of cellular metabolic activities. Furthermore, AgNPs can induce antibacterial effects by releasing Ag+ ions upon contact with bacteria, which consequently modulate the intracellular signaling pathways of microbial cells potentially leading to apoptosis. This occurs through a series of events including interruption of intracellular oxygen reduction, interaction with respiratory chain proteins on cell membranes, generation of free radicals and reactive oxygen species (ROS) production that leads to cellular oxidative stress. Overall, these findings provide substantial support for the antibacterial activity of SAgNPs synthesized using S. platensis extract, which is mediated by multiple mechanisms including physical disruption of the cell wall, interaction with cellular components, and induction of oxidative stress [41,42,43,44].
4 Conclusion
In conclusion, the biosynthesis of silver nanoparticles (SAgNPs) using S. platensis extract demonstrated remarkable stability, antibacterial efficacy, antioxidant potential, and biocompatibility. These findings highlight the promising applications of SAgNPs in various fields, including medicine and pharmaceuticals. The use of sustainable and environmentally friendly cyanobacterial cells as a green resource for nanoparticle production holds significant potential for future advancements in nanotechnology. However, further research is required to fully explore the therapeutic potential of SAgNPs and evaluate their safety and efficacy for human use. Overall, this study contributes to the growing body of knowledge regarding the utilization of biological sources for the synthesis of nanoparticles with diverse biomedical applications.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Pidgeon, N., Porritt, J., Ryan, J., Seaton, A., Tendler, S., Welland, M., & Whatmore, R. (2004). Nanoscience and nanotechnologies: Opportunities and uncertainties. The Royal Society, The Royal Academy of Engineering, 29(07), 2004.
Helland, A. (2004). Nanoparticles: A closer look at the risks to human health and the environment. Perceptions and precautionary measures of industry and regulatory bodies in Europe. International Institute for Industrial Environmental Economics (IIIEE). http://lup.lub.lu.se/student-papers/record/1329339
Burdușel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials, 8(9), 681.
Konappa, N., Udayashankar, A. C., Dhamodaran, N., Krishnamurthy, S., Jagannath, S., Uzma, F., Pradeep, C. K., De Britto, S., Chowdappa, S., & Jogaiah, S. (2021). Ameliorated Antibacterial and Antioxidant Properties by Trichoderma harzianum Mediated Green Synthesis of Silver Nanoparticles. Biomolecules, 11(4), 535. [Google Scholar] [CrossRef].
Jogaiah, S., Kurjogi, M., Abdelrahman, M., Hanumanthappa, N., & Tran, L.-S.P. (2019). Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arabian Journal of Chemistry, 12, 1108–1120. [Google Scholar] [CrossRef].
Jain, N., et al. (2011). Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale. https://doi.org/10.1039/c0nr00656d
Kalaiarasi, R., Jayallakshmi, N., & Venkatachalam, P. (2010). Phytosynthesis of nanoparticles and its applications. Plant Cell Biotechnology and Molecular Biology, 11(1), 1–16.
Sinha, S. N., et al. (2015). Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Applied Nanoscience. https://doi.org/10.1007/s13204-014-0366-6.
Pillai, Z. S., & Kamat, P. V. (2004). What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? The Journal of Physical Chemistry B. https://doi.org/10.1021/jp037018r
Dhillon, G. S., et al. (2012). Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Critical Reviews in Biotechnology. https://doi.org/10.3109/07388551.2010.550568
Mosulishvili, L. M., et al. (2007). Neutron activation analysis for studying Cr uptake in the blue-green microalga Spirulina platensis. Journal of Neutron Research. https://doi.org/10.1080/10238160601025138
Mukherjee, P., et al. (2002). Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chembiochem : A European Journal of Chemical Biology. https://doi.org/10.1002/1439-7633(20020503)3:5%3c461::Aid-cbic461%3e3.0.Co;2-x
Doshi, H., Ray, A., & Kothari, I. L. (2007). Bioremediation potential of live and dead Spirulina: Spectroscopic, kinetics and SEM studies. Biotechnology and Bioengineering. https://doi.org/10.1002/bit.21190
Muthusamy, G., et al. (2017). Biosynthesis of silver nanoparticles from Spirulina microalgae and its antibacterial activity. Environmental Science and Pollution Research International. https://doi.org/10.1007/s11356-017-9772-0
Zarrouk, C. (1966). Contribution a l'etude d'une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina mixima (Thesis, PhD), University of Paris, France.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature. https://doi.org/10.1038/1811199a0
Luna-Vázquez-Gómez, R., et al. (2021). Hemolysis of Human Erythrocytes by Argovit™ AgNPs from Healthy and Diabetic Donors: An In Vitro Study. Materials (Basel, Switzerland). https://doi.org/10.3390/ma14112792.
Reddy, G. A. K., Joy, J. M., Mitra, T., Shabnam, S., & Shilpa, T. (2012). Nano silver–a review. Internationa Journal Advances Pharmacology, 2(1), 09–15.
Shankar, S. S., et al. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of Colloid and Interface Science. https://doi.org/10.1016/j.jcis.2004.03.003
Ritthichai, T., & Pimpan, V. (2019). Ammonia sensing of silver nanoparticles synthesized using tannic acid combined with UV radiation: Effect of UV exposure time. Journal of King Saud University-Science, 31(2), 277–284.
Chakraborty, B., Bhat, M. P., Basavarajappa, D. S., Rudrappa, M., Nayaka, S., Kumar, R. S., ... & Perumal, K. (2023). Biosynthesis and characterization of polysaccharide-capped silver nanoparticles from Acalypha indica L. and evaluation of their biological activities. Environmental Research, 225, 115614.
Stamplecoskie, K. G., & Scaiano, J. C. (2010). Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles. Journal of the American Chemical Society. https://doi.org/10.1021/ja910010b
Ahmad, A., Muhammad, J., Dinislam, K., Maria, M., Elizaveta, V., Madina, D., ... & Victoria, P. (2024). Green Synthesis Of Antibacterial Silver Nanoparticles Using Grewia Asiatica Leaf Extract: Characterization And Potential For Combatting Antibiotic-Resistant Bacteria. Migration Letters, 21(S5), 111–126.
El Rabey, H. A., Alamri, E. S., Alzahrani, O. R., Salah, N. M., Attia, E. S., & Rezk, S. M. (2023). Silymarin and vanillic acid silver nanoparticles alleviate the carbon tetrachloride-induced nephrotoxicity in male rats. International Journal of Polymer Science, 2023. https://doi.org/10.1155/2023/4120553
Eswaran, A., Muthukrishnan, S., Mathaiyan, M., Pradeepkumar, S., Mari, K. R., & Manogaran, P. (2023). Green synthesis, characterization and hepatoprotective activity of silver nanoparticles synthesized from pre-formulated Liv-Pro-08 poly-herbal formulation. Applied Nanoscience, 13(3), 2315–2327.
Ali, F., Younas, U., Nazir, A., Hassan, F., Iqbal, M., Mukhtar, S., ... & Ishfaq, A. (2022). Biosynthesis and characterization of silver nanoparticles using strawberry seed extract and evaluation of their antibacterial and antioxidant activities. Journal of Saudi Chemical Society, 26(6), 101558.
Attia, M. S., El-Sayyad, G. S., Saleh, S. S., Balabel, N. M., & El-Batal, A. I. (2019). Spirulina platensis-polysaccharides promoted green silver nanoparticles production using gamma radiation to suppress the expansion of pear fire blight-producing Erwinia amylovora. Journal of Cluster Science, 30, 919–935.
Makarov, V. V., Love, A. J., Sinitsyna, O. V., Makarova, S. S., Yaminsky, I. V., Taliansky, M. E., & Kalinina, N. O. (2014). “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae, 6(1), 35–44.
Li, S., et al. (2007). Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chemistry. https://doi.org/10.1039/B615357G.
Mahdavi, M., et al. (2013). Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules (Basel, Switzerland). https://doi.org/10.3390/molecules18055954.
Ismail, G. A., et al. (2021). Antimicrobial, Antioxidant, and Antiviral Activities of Biosynthesized Silver Nanoparticles by Phycobiliprotein Crude Extract of the Cyanobacteria Spirulina platensis and Nostoc linckia. BioNanoScience. https://doi.org/10.1007/s12668-021-00828-3
Kumar, S., et al. (2018). Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packaging and Shelf Life. https://doi.org/10.1016/j.fpsl.2018.03.008
Lee, H.-Y., et al. (2010). Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. Journal of Nanoparticle Research. https://doi.org/10.1007/s11051-009-9666-2
Chen, L. Q., et al. (2015). Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chemical Research in Toxicology. https://doi.org/10.1021/tx500479m
Lin, Y. S., & Haynes, C. L. (2010). Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. Journal of the American Chemical Society. https://doi.org/10.1021/ja910846q
Nagarajaiah, S., Shivanna Giresha, A., Gopala Krishna, P., Manikrao Gadewar, M., Praveen, M., Nanda, N., ... & Venkatesh Yatish, K. (2024). Anti‐oncogenic Potential and Inflammation Modulatory Response of Green Synthesized Biocompatible Silver Nanoparticles. Chemistry & Biodiversity. https://doi.org/10.1002/cbdv.202301533.
Gul, A., Fozia, Shaheen, A., Ahmad, I., Khattak, B., Ahmad, M., ... & Mahmood, H. M. (2021). Green synthesis, characterization, enzyme inhibition, antimicrobial potential, and cytotoxic activity of plant mediated silver nanoparticle using Ricinus communis leaf and root extracts. Biomolecules, 11(2), 206.
ASTM E2524-08 (2013). Standard test method for analysis of hemolytic properties of nanoparticles. ASTM International. Available online at: https://www.astm.org/
Rudrappa, M., Kumar, R. S., Nagaraja, S. K., Hiremath, H., Gunagambhire, P. V., Almansour, A. I., ... & Nayaka, S. (2023). Myco-nanofabrication of silver nanoparticles by Penicillium brasilianum NP5 and their antimicrobial, photoprotective and anticancer effect on MDA-MB-231 breast cancer cell line. Antibiotics, 12(3), 567.
Rudrappa, M., Rudayni, H. A., Assiri, R. A., Bepari, A., Basavarajappa, D. S., Nagaraja, S. K., ... & Nayaka, S. (2022). Plumeria alba-mediated green synthesis of silver nanoparticles exhibits antimicrobial effect and anti-oncogenic activity against glioblastoma U118 MG cancer cell line. Nanomaterials, 12(3), 493.
Sharma, V. K., Yngard, R. A., & Lin, Y. (2009). Silver nanoparticles: Green synthesis and their antimicrobial activities. Advances in Colloid and Interface Science. https://doi.org/10.1016/j.cis.2008.09.002
Singh, L., et al. (2017). The role of nanotechnology in the treatment of viral infections. Therapeutic Advances in Infectious Disease. https://doi.org/10.1177/2049936117713593
El-Naggar, N. E., Hussein, M. H., & El-Sawah, A. A. (2018). Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Scientific Reports. https://doi.org/10.1038/s41598-018-27276-6
Long, Y. M., et al. (2017). Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. International Journal of Nanomedicine. https://doi.org/10.2147/ijn.S132327
Acknowledgements
The authors are grateful to Dow University of Health Sciences (DUHS), Karachi, Pakistan for all the support and assistance to conduct this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
A.G: Conceptualization, Methodology, Investigation, Data curation, Writing—original draft, Writing—review & editing. M.N.B: Methodology, Investigation, Data curation, D.A: Methodology, Investigation, Data curation, Writing—original draft. Z.N: Investigation, Data curation, Writing—original draft. T.A: Data curation, Writing—original draft. S.A: Investigation, Methodology, Data curation.
Corresponding author
Ethics declarations
Ethical Approval
The blood sample was taken from the healthy individuals with their consent following declaration of Helsinki guidelines after taking approval from the Institutional Review Board of Dow University of Health Sciences, Karachi Pakistan.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gul, A., Baig, M.N., Ahmed, D. et al. Green Synthesis of Silver Nanoparticles from Spirulina platensis Extract: Antibacterial and Antioxidant Potential. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01473-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01473-2