Abstract
During the production of stainless steel, dust of size less than 50 μm is produced. It has been estimated that the production of one ton stainless steel can produce l8–33 kg dust, which contains a large amount of Fe, Cr, Ni and other valuable metals. In this study, according to the composition requirements of iron-based powder metallurgy friction materials on raw materials, the physicochemical properties of 400 series stainless steel dust were analyzed. The dust was magnetically selected, and the low-medium reduction magnetic separation was adopted, which was explored as a new process for the preparation of powder metallurgy friction material. Considering the magnetic material yield, TFe grade and decalcification effect, the suitable magnetic field strength ranges from 60 to 90 mT and the reduction temperature is from 800 to 900 °C. The main phases of the primary magnetic material after reduction include Fe, FeCr2O4, chromium carbides (Cr3C2 and Cr7C3), MgO and graphite (C), indicating that Fe3O4 is reduced to elemental Fe and FeCr2O4 and MgO are still present in material. Only a small part of the material reduces to chromium carbide at medium and low temperature (800–900 °C).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the beginning of the twenty-first century, the world stainless steel production has been increasing by 6%. According to the latest statistics released by BBS (ISSF), the global output of raw stainless steel in 2019 reaches 52.1 million tons, an increase in 3.3% per year [1, 2]. It is estimated that the production of one ton stainless steel can produce l8–33 kg of dust [3, 4]. The amount of dust in the electric furnace is about 1–2% of the furnace charge [5, 6] and among which is about 0.7–1% produced in AOD furnace charge [7, 8]. The particle size distribution of 400 series stainless steel dust ranges from 0.69 to 36.08 μm, with a diameter of 4.42 μm [9, 10]. The dust contains a lot of valuable metals such as Fe, Cr, Ni and some trace elements, such as Pb, C, Si, Mg, Mn, Ca and Zn. [11,12,13]. These metals are mostly in the form of oxides, with Fe in the form of Fe2O3, Cr in the form of CrO and Ni in the form of NiO [14,15,16]. The chemical composition and physical properties of the steel making dust are different due to the difference in smelting raw materials, smelting temperature, blowing amount. In general, these dusts with small particle size (more than 60% of < 0.05 mm) has a high iron content (50%), the content of Cr and Ni is high (Cr content is usually between 8 and 15%; Ni content is between 3 and 9%) and a little amount of impurities (such as C, SiO2, MnO, MgO, Al2O3, Zn and Pb.) [17,18,19,20].
In iron-based powder metallurgy friction materials, the matrix composition is mainly iron, and added Ni, Co, Cr, Mn, W, Mo elements, to achieve alloying so as to further improve the thermal stability and mechanical strength of iron-based friction materials [21]. Silicon dioxide, corundum, zirconia, other metal oxides as well as other metal carbides and metal nitrides are added as friction components to improve the friction coefficient [22]. Graphite, metal sulfide, fluoride and low melting point metal are added as solid lubricant to protect the dual stable friction coefficient [23]. Powder metallurgy friction material is developed on the basis of porous bearing of powder metallurgy, whose composition is similar to that of stainless steel dust [24]. By comprehensive use of modern analysis and test technology, pattern recognition and other data processing software, the preparation of powder metallurgy friction material stainless steel dust separation process conditions are optimized, seeking the best process parameters.
Reasonable recovery and utilization of stainless steel dust can alleviate the contradiction between raw material supply and demand to a certain extent. More importantly, with the improvement of waste recovery rate, the pollution to the environment will be further reduced. A large number of stainless steel components produce stainless steel dust particles containing Fe, Cr and Ni. In this paper, the stainless steel dust was reduced by magnetic separation for the preparation of raw materials for powder metallurgical process. The particle size, morphology, initial characteristics of substance equality of stainless steel dust and the distribution law of Fe, Cr, Ni, Ca, Mg and other elements in the dust were characterized by various test methods. The parameters of reduction and magnetic separation were obtained. The stainless steel powder metallurgical raw materials were prepared directly by removing the Ca, Mg, O and other impurities in the dust. It was a novel method for utilization of stainless steel dust.
2 Stainless Steel Dust Characterization
The raw materials used in this study are 400 series stainless steel arc furnace smelting dust of the iron and steel industry. The dust is reddish-brown and contains small white granule material, and there are also large particles gathered by small particles. The qualitative and semiquantitative analysis of stainless steel dust was carried out by PW2404 X-ray fluorescence spectrometer. The analysis results are shown in Table 1. The content of Fe, Cr, Ca and Mg in the 400 series stainless steel dust is high. Among them, the TFe in stainless steel dust is about 20% or more, the content of Cr reaches 13.19%, which has great recycling value, while the content of O and Ca is up to 30.94% and 28.70%, respectively, and the Mg content is about 3.71%.
The particle size of stainless steel dust was characterized with laser particle size analyzer. The particle distribution is shown in Fig. 1. It can be seen that the particle size distribution of the 400 series stainless steel dust is between 0.69 and 36.08 μm.
The phase composition of stainless steel powder was determined using D/max2550 X-ray diffraction (10°–90°) for stainless steel powder XRD scanning. The XRD pattern of 400 series stainless steel dust is shown in Fig. 2. The XRD pattern shows that stainless steel dust is the oxide of metal iron and chromium, in which iron exists in the form of Fe3O4 and FeO·Cr2O3 and chromium in the form of FeO·Cr2O3, MgCr2O4 and CaCrO4. It indicates that the small white particles in the dust that can be observed by the naked eye are CaO powder. The content of Mg, Si, Ti, Al, Ni and other elements in the dust is very low according to chemical composition analysis, and there is no obvious corresponding characteristic peak in the XRD pattern. According to the formation mechanism analysis of stainless steel dust, these elements all exists in the dust in the form of oxide. The microscopic morphology of stainless steel dust under scanning electron microscopy shows that the stainless steel dust particles are small, mostly spherical and with rough and uneven surface.
3 Experimental Methods and Procedures
Through literature review and preliminary experiments, it could be found that the direct magnetic separation of 400 series stainless steel dust raw materials effectively reduce impurities such as calcium (Ca) and magnesium (Mg) in the dust, and the oxygen (O) in the dust could be partly removed by the reduction process. Secondary magnetic separation was performed on the reduced dust and the impurities in the dust could be further removed. Powder metallurgy friction material samples were prepared with the selected dust and other suitable pure chemical substances to test the comprehensive properties of the samples.
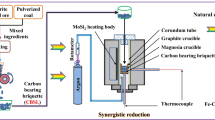
The experimental procedure is shown in Fig. 3. A certain amount of experimental samples was laid up in a corundum crucible, placed in a box-type atmosphere furnace, the air tightness of the device was checked, and vacuum pump was used for vacuuming. When the temperature was raised to the required experiment temperature, the reducing gas was introduced. After a certain time of heat preservation, the sample was cooled to room temperature. When using solid reducing agent, initially the dust and the reducing agent were mixed evenly according to the established proportion. During the experiment, argon gas was continuously injected until the end of the experiment, and the reduced samples were sealed and tested in time to prevent the samples from being oxidized.
4 Results
4.1 Magnetic Separation Experiment Results
As the strength of the magnetic field increases, the main chemical composition changes in the magnetic material obtained by magnetic separation of stainless steel dust are shown in Fig. 4. It can be seen that in the range of magnetic field strength of 40–250 mT, the content of Cr, Mg, Si and Al in the magnetic material does not change significantly with the increase in the magnetic field strength. In addition to Cr, the content of Mg, Si and Al is all below 2.5%, and the content of Fe is decreased, and the content of Ca and O is increased. This is because the magnetic field is enhanced and even the weak magnetic substance is also selected. Although changing the magnetic field strength cannot further reduce impurities such as Mg and Al in the dust, selecting a suitable magnetic field strength is advantageous for increasing the Fe grade and reducing the content of the main impurity Ca.
After magnetic separation of stainless steel dust, the relationship between magnetic material yield and TFe grade and magnetic field intensity is shown in Fig. 5. As seen from Fig. 5a, the productivity of magnetic substance increases gradually with the increase in magnetic field intensity in the range of 40–250 mT. At 40 mT, it is almost impossible to select magnetic substances. The productivity of magnetic substances is the highest, reaching 65% at 250 mT. However, with the increase in the magnetic field intensity, the grade of TFe in the magnetic material first rises and then gradually decreases. When it is near 60 mT, the TFe grade is the highest, which is 58.56%, and the yield is 8.7%.
The stainless steel dust of magnetic separation is shown in Fig. 5b, which shows the relationship between magnetic material yield and calcium content and magnetic field intensity. As seen from Fig. 5b, it can be deduced that with the increase in magnetic field intensity in the range of 40–250 mT, the yield of magnetic substances gradually increases, while the percentage of Ca first decreases slightly and then gradually increases. At 60 mT, the content of Ca is about 3%. When the magnetic field intensity increases to 120 mT, the content of Ca increases rapidly. Based on the above analysis, the appropriate magnetic field strength range should be 60–90 mT, taking into account the yield of magnetic substance, TFe grade and decalcalization effect.
4.2 Mineral Phase Characterization After Primary Magnetic Separation
The main purpose of dust magnetic separation is to remove nonmagnetic substances such as CaO and MgO. When the magnetic field intensity is 150 mT, the magnetic separation effect is the best, and all the iron oxide reduces to iron. The stainless steel dust contains magnetite (Fe3O4) phase, so the material is directly magnetically selected under the magnetic field strength of 150 mT. The analysis results are shown in Table 2 and Fig. 6.
The major phases of magnetic substances are Fe3O4, FeO·Cr2O3 and Fe2O3 according to the XRD pattern. The composite of nonmagnetic substances is CaCO3, Fe2O3, CaCrO4 and MgCr2O4. The yield of magnetic substances after magnetic separation reaches 48.27%, and the contents of Fe, Cr, Ca, Mg and O in magnetic and nonmagnetic substances change significantly as shown in Table 2. Most of Fe and Cr are concentrated in magnetic materials, while Ca, Mg and O are enriched mainly in nonmagnetic materials. The result indicates that it is feasible to use magnetic separation technology to reduce the content of Ca, Mg, O and other impurities in stainless steel dust. A small part of silicon contained in magnetic materials is a beneficial component, and its oxide can be used as a wear-resistant component in powder metallurgy friction materials.
The morphology analysis of magnetic materials is shown in Fig. 7. The magnetic materials are mainly rough spherical particles, bright irregular blocks and sharp blocks with obvious edges and corners. Bright color particles are iron with content of 42.32%, and the iron content of spherical particles is about 40%, while the iron content in sharp block particles was about 20%. The content of Fe in these particles significantly increases compared with the raw material of stainless steel dust, and the content of Ca and Mg is greatly reduced, which is consistent with the results of X-ray fluorescence spectrum analysis.
4.3 Secondary Magnetic Separation Experiment Results
The matrix component of the iron-based powder metallurgy friction material is iron, with its mass percentage generally 50% to 80% [25]. It is required to reduce the content of harmful impurities such as Ca, Mg, O as much as possible. The full process experiment of strong magnetic separation-reduction-weak magnetic separation has been carried out during the experimental procedure in order to further reduce the content of Ca, Mg, O and other impurities in dust.
A magnetic field strength of 150 mT is used for magnetic separation of the stainless steel dust. A suction filter is used for collection of the magnetic material, which is then dried and placed in a box-type furnace for reduction with H2 for 2 h under 800 °C. After cooling to room temperature, the reduced sample is ground to below 100 μm and is then subjected to secondary magnetic separation under a magnetic field strength of 50 mT. The phase change before and after the reduction of the magnetic substance is shown in Fig. 8. For magnetic materials after the reduction, Fe3O4 peak disappear, but the strong Fe peak appear, peak FeCr2O4 has no obvious change. For H2 as reducing agent under the condition of 800 °C, iron chromium spinel cannot be restored and the magnetite phase can be completely reduced to elemental iron.
The sample morphology of stainless steel dust after magnetic separation and reduction is shown in Figs. 9 and 10. The morphology of magnetic and nonmagnetic substances after grinding and magnetic separation is shown in Fig. 9b, c, respectively. The reduced stainless steel dust particles are considered as two categories according to the morphological characteristics: spherical particles with smooth surface and irregularly shaped particle with a rough, uneven surface. It is found that the spherical particles with smooth surface are highly magnetic materials. Nonmagnetic materials are mainly rough surfaces with uneven irregular shaped particles. After the magnetic separation reduction EDS spectrum analysis (see Fig. 11), the contents of Fe and Cr in magnetic materials are very high, the Fe content in some regions reaches 73.82 wt%, and the Cr content reaches 17.33 wt%. The content of Fe in nonmagnetic materials is significantly reduced, and the main components are Ca or Cr. The content of Ca in some areas reaches 71.79 wt%, and the content of Cr reaches 47.3 wt%.
The scanning and energy spectrum analysis for magnetic materials obtained are shown in Fig. 11. The surface of the magnetic particles are smooth, in the shape of a strip or dark round ball and are scattered with small bright ball particles. The energy spectrum analysis shows that in the strips and dark large spherical particles, the Fe content reaches about 98 wt%. It is considered as the Fe element formed by reduction, which is required for the powder metallurgy friction material. In the highlighted spherical particles, the content of Fe is relatively low, while the content of Cr and O are relatively high, which is because of the unreduced Cr2O3 in ferrochrome spinel.
5 Discussion
The main chemical composition changes of the stainless steel dust materials after the magnetic separation, reduction and magnetic separation process are shown in Fig 12. With the progress of the experiment, the contents of Mg, Si and Al in the magnetic material does not change significantly, the content of Cr increases slightly, the content of Fe increases significantly, and the contents of Ca and O decrease significantly. The changes in the content of these elements are exactly consistent with the requirements of powder metallurgy friction materials.
After the stainless steel, dust is subjected to a series of processes such as magnetic separation → reduction → grinding → magnetic separation. The chemical composition of the magnetic substance and the nonmagnetic substance is shown in Table 3. The TFe grade in the magnetic material is 53.66%, and the Cr content is 19.79%, Ca content and Mg content are lower than 4%. After conversion to oxide, (CaO + MgO) the content is lower than 11.61%. The yield of magnetic material reaches 37.39%, and the recovery rates of Fe, Cr and Ni are 71.42%, 41.58% and 80.47%, respectively. Combined with the commonly used formulation of iron-based powder metallurgy friction materials [26], with the addition of Fe powder, Cu powder and graphite, the magnetic material can be used as the raw material for the preparation of powder metallurgy friction materials to reduce the cost.
The yield of magnetic materials and the recovery rate of important metals are shown in Table 3. The total yield rate of magnetic materials obtained is 37.39%, among which the recovery rates of Fe and Ni are higher (71.42% and 80.47%, respectively), while the recovery rate of Cr is low (only 41.58%), indicating that there is still a lot of Cr in nonmagnetic materials, which needs further treatment.
6 Conclusion
This work conducted the study on the direct magnetic separation, reduction and magnetic separation process of 400 series stainless steel dust for the preparation of powder metallurgy friction materials in detail. The conclusions were as follows:
-
(1)
It is feasible to reduce the content of Ca, Mg, O and other elements in 400 series stainless steel dust by magnetic separation and reduction process and to improve the TFe grade.
-
(2)
The stainless steel dust was treated as follows: magnetic separation → reduction → grinding → magnetic separation. The TFe grade of final material could reach 56.66%, Cr content was 19.79%, Ca content and Mg content were lower than 4%. The content of main impurities CaO and MgO was lower than 11.61%, which could be used as raw material for preparing powder metallurgy friction materials.
-
(3)
The magnetic material was reduced by H2 at 800 °C for 2 h, then underwent magnetic separation at 150 mT. The yield of magnetic material obtained by secondary magnetic separation was 37.39%. After adding a certain amount of Fe powder, Cu powder and graphite, it could be used as a raw material for preparing powder metallurgy friction materials, and the cost of the powder metallurgy friction material could be reduced.
References
Zhang H, Dong J, Xiong H, Wang Z, and Lu Y, J Alloys Compd 699 (2017) 408.
Rosales J, Cabrera M, and Agrela F, Constr Build Mater 142 (2017) 444.
Zhang H W, and Hong X, Conserv Recycl 55 (2011) 745.
Nolasco-Sobrinho P J, Espinosa D C R, and Tenório, J A S, Ironmak Steelmak 30 (2003) 11.
Omran M, and Fabritius T, Powder Technol 308 (2017) 47.
Salihoglu G, and Pinarli V, J Hazard Mater 153 (2008) 1110.
Stefanova A, Aromaa J, and Forsen O, Physicochem Probl Miner Process 49 (2013) 37.
Majuste D, and Mansur M B, J Hazard Mater 153 (2008) 89.
Rubio-Cintas M D, Barnett S J, Perez-García F, and Parron-Rubio M E, Constr Build Mater 204 (2019) 675.
Fernández Pereira C, Luna Y, Querol X, Antenucci D, and Vale J, Fuel 88 (2008) 1185.
Oustadakis P, Tsakiridis P E, Katsiapi A, and Agatzini-Leonardou S, J Hazard Mater 179 (2010) 1.
de Araújo J A, and Schalch V, J Mater Res Technol 3 (2014) 274.
Drissen P, Ehrenberg A, Kuhn M, and Mudersbach D, Steel Res Int 80 (2009) 737.
Geldenhuis J M A, Miner Eng 5 (2002) 95.
Moroni B, and Viti C, Aerosol Sci 40 (2009) 938.
Denton G M, Barcza N A, Scott P D, and Fulton T, EAF Stainless Steel Dust Processing, John Floyd International Symposium on Sustainable Developments in Metals Processing, 2005, July 3–6, Melbourne, Australia, p 273.
Liu X, Zhang J, Xiao Q, and Li Q, Characterization of wastes generated during stainless steel production, TMS Annual Meeting, Characterization of Minerals, Metals, and Materials, 2014, February 16–20, San Diego, America, p 343.
Bing P, Ji P, Kozinski J A, Lobel J, Chai L-y, Zhang C-f, and Chen W-l, J Centre South Univ Technol 10 (2003) 20.
Zhang Y-l, Guo W-m, and Jia X-l, Int J Miner Metall Mater 22 (2015) 573.
Kim G, and Sohn I, J Hazard Mater 359 (2018) 174.
Talijan N M, Trifunović D S, and Trifunović D D, Mater Lett 46 (2000) 255.
Mansour R, Amir M R Z, Mohammad R R, Reza K, Mohsen O S, and Rahim Y R, Ceram Int 37 (2011) 443.
He G, and Chen Q, J Alloys Compd 797 (2019) 213.
Boillot P, and Peultier J, Procedia Eng 83 (2014) 309.
Babets N V, Vasil’ev B N, and Ismailov M A, Metallurgist 56 (2012) 462.
Materials Standards for PM Structural Parts 35, 2007, Metal Powder Industries Federation, Princeton, NJ.
Acknowledgements
This work is supported by National Natural Science Foundation of China (Grant Nos. 51974185, U1760109). This work is supported by Engineering Technology Research Center of Shanghai (No. 19DZ2252900). The authors also acknowledge CAS Interdisciplinary Innovation Team.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Li, Q., Yang, F. et al. Experimental Study on Stainless Steel Dust by Reduction and Enrichment for Preparation Raw Material of Powder Metallurgy. Trans Indian Inst Met 74, 119–127 (2021). https://doi.org/10.1007/s12666-020-02121-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02121-5