Abstract
Substantial quantities of openly dumped slag contribute to the absence of recovery and utilization of valuable metals as well as potential environmental pollution to water and soil. Owing to the excessively high arsenic content present in iron extracted from copper smelting slag, we propose a new arsenic removal process in this paper. The results indicate that arsenic sulfides in copper smelting slag can be significantly removed at 1100 °C in an N2 atmosphere, leading to a 0.15% residual arsenic content. Furthermore, undecomposed arsenate in the slag can be reduced in an N2–CO atmosphere. During a coal-based direct reduction process, under optimal conditions, the removal efficiency of arsenic is relatively low. Subsequently, under optimal conditions, the arsenic content drops significantly from 0.18 to 0.080%. This new removal process engenders lower arsenic content in iron extracted from slag than the standards of pig iron and direct reduced iron in steelmaking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Copper smelting slag is an important by-product in the pyrometallurgical processing of copper. It has been estimated that the production of 1 ton of copper generates approximately 2.2 tons of copper smelting slag [1]. At least 17 million tons of slag is annually generated from copper production in China. According to the growth rate in recent years, it is predicted that copper smelting slag production will exceed 24 million tons by 2020 [2,3,4]. Disposing such substantial quantities of copper smelting slag poses environmental and spatial challenges. Consequently, the Chinese government has implemented policies that mandate mining and metallurgical companies to be responsible for reducing the volume of solid waste deposits, as well as enhancing its reutilization [5].
Copper smelting slag contains a high number of valuable metals, as lower comprehensive utilization rate; the vast majority of copper slag were open-air accumulated, occupying a substantial portion of land. In addition, the slag contains non-biodegradable matter such as arsenic, lead, and other highly toxic substances, which will go deep into the soil and flow into rivers during the accumulation process and accumulate in the biological body, leading to several diseases and health risks. The usage of copper slag to relieve the pressure on mineral resources and establish a profitable relationship between the economy and environment has become an urgent problem. Recent investigations on the reclamation and recovery of copper smelting slag have been burgeoning. Quite a few researchers have attempted to recover Cu or Fe from copper smelting slag through oxidation, magnetic separation, and smelting reduction technologies [6,7,8,9,10]. However, it is noteworthy that few of the studies focus on the removal of arsenic from copper smelting slag. The arsenic content in iron extracted from copper smelting slag can be as high as 0.3%, which far exceeds the value required in ordinary low-carbon steel (As = 0.025–0.045 wt%) [11]. The high arsenic content significantly affects properties of steel, such as reducing the impact toughness, welding performance, and thermoplastic quality under high temperature [12]. In addition, the arsenic content in iron products extracted from copper slag significantly reduces the sales price. Consequently, the iron can only be sold to interested companies at low prices, thereby affecting the economic efficiency of the industry. This challenge in sales and low profitability significantly erodes the enthusiasm of enterprises to contribute to the development and utilization of copper slag.
Considering the importance of removing arsenic from copper smelting slags, a new process known as the two-step arsenic removal process is demonstrated in this study. In this process, an additional arsenic removal process is added prior to the existing direct reduction smelting process. The main objective of this study is to report the mechanisms of arsenic removal during iron extraction.
2 Materials and Methods
2.1 Materials
The composition of copper smelting slag used in this study is listed in Table 1. The slag sample contained significant levels of FeO (42.69 wt%) with high arsenic content of approximately 0.3 wt%, although the specific form of arsenic could not be determined using XRD (X-ray diffraction) analysis.
Table 2 reveals that the chemical phase composition of arsenic mainly comprised of 55.17% arsenic sulfides (AsxSy), 22.99% arsenate, and 2.53% arsenic oxides.
Figure 1 illustrates the SEM (scanning electron microscope) image of the arsenic-bearing phase, whereas the relevant chemical compositions of selected phases are presented in Table 3. Figure 1 further illustrates that there are no noticeable independent arsenic phases in the slag, and the arsenic elements are randomly distributed in the sulfides and glassy phases are represented by points one and two, respectively.
2.2 Experimental Procedure
The experimental apparatus is schematically illustrated in Fig. 2. An Al2O3 boat (7 cm × 3 cm × 2 cm) was used as the vessel of the reduction reaction, whereas a MoSi2 heating element high-temperature horizontal furnace, with a heating and atmosphere control system, was used as the heater.
The copper smelting slag was first crushed, ground, and oven-dried at 105 °C for 2 h until the weight remained constant. The Al2O3 boat laden with the copper slag was then placed in the furnace upon attaining the predetermined heating rate of 10 °C min−1. In addition, nitrogen was introduced into the furnace to prevent the gasified form of arsenic from being reoxidized. When the temperature and atmosphere reached the pre-established point, the furnace was maintained at a N2/N2–CO gas setting for different predesigned durations and temperatures, and the copper slag was roasted. Subsequently, the Al2O3 boat with its roasted content was taken out of the furnace and cooled down to room temperature (23–25 °C). This substance was finally ground into appropriate particle sizes to obtain products that were compatible under different experimental conditions.
Based on the procedure described above, the arsenic removal rate (γ %) was used as a function to determine the optimal arsenic removal parameters, such as reduction time, temperature, and atmosphere. The arsenic removal rate was defined as follows:
where γ denotes the ratio of arsenic removal from slag,%; β represents the arsenic content of the original sample,%; α denotes the arsenic content of the roasted slag,%; and ε% represents the weight loss rate.
3 Results and Discussion
3.1 De-Arsenic Experiment in Different Atmospheres
In this study, copper slag was treated using a high-temperature reduction method, and the high-temperature decomposition experiment was protected by an inert gas (N2). As the arsenate is relatively stable in the copper slag, it is difficult to decompose it in a high-temperature inert atmosphere. To further remove arsenic, a reduction roasting method was used in the atmosphere of N2–CO to remove high-priced arsenic from arsenate.
-
(1)
Arsenic removal in N2 atmosphere
The arsenic removal rate is significantly dependent on the thermal decomposition of arsenic sulfides [13] because arsenic sulfides are the main components (Table 2) derivable from copper slag. The specific forms of arsenic sulfides are AsS, As2S2, As2S3, As4S4, and As4S6. From the thermodynamic database, the gasification reaction temperatures of As2S2, As2S3, and As4S4 are approximately 350 °C, 350 °C, and 400 °C, respectively. The gasification reaction of As4S4 can be described using Eq. 1.
In addition, arsenic sulfide undergoes decomposition reactions in inert atmospheres, thereby forming specific products, such as As2(g), As3(g), As4(g), AsS(g), and S2(g). The overall reaction formula is shown in Eq. 2 [14] as follows:
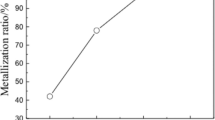
Samples of copper slag (the thickness of copper slag of 75–100 μm was made up more than 90%) have been prepared and roasted at 500 °C, 700 °C, 900 °C, and 1100 °C for 30 min in an N2 atmosphere. The roasted products are thereafter sampled to detect the arsenic content. The analyses of the results of arsenic removal rates and arsenic contents are illustrated in Fig. 3.
The results indicate that as the temperature increases, the arsenic removal rate increases slowly at the initial stage of 500 °C to 700 °C before rapidly increasing after 900 °C, while the arsenic content exhibits a decreasing trend. Maximum arsenic removal rate (76.17%) is attained for a roasting temperature of 1100 °C.
An appropriate roasting temperature is necessary to produce ideal results because temperature (T) is directly proportional to the value of P and diffusion coefficient (De). The equilibrium for instantaneous evaporation rate (v) and De is represented by Eq. 3 as follows:
From Eq. 3, a positive correlation between v and De is established, thereby indicating that change in De with temperature will directly affect the arsenic removal rate. Rearranging the equation for the equilibrium constant results in Eq. 4 as follows:
Since the values of \( \sigma_{AB}^{2} \) and \( \varOmega_{D} \) remain fairly constant for insignificant changes in roasting temperature, and MA (molecular weight of arsenic) and MB (nitrogen molecular weight) are constant, the relationship between De and T can be represented by Eq. 5.
From Eq. (5), it can be concluded that De increases exponentially with increase in T based on the previously established direct proportionality between the arsenic removal rate and temperature.
Rational temperature and abundant time are important parameters that can guarantee thorough gasification of arsenic. To determine the rational roasting time, four samples are prepared and roasted at 1100 °C for 20 min, 60 min, 80 min, and 120 min, while other experimental conditions are maintained as stated above. The results are presented in Fig. 4.
Figure 4 illustrates that in the early stage, sufficient time facilitates arsenic removal, while longer durations (exceeding 1 h) lead to adverse results. At the 60 min mark, arsenic removal rate reaches its maximum value (82.03%) and arsenic content decreases to 0.10%. Thus, 60 min can be regarded as the rational time.
-
(2)
Arsenic removal in N2–CO atmosphere
Based on the results in the N2 atmosphere provided above, the arsenic exists in the form of arsenic sulfides, from which arsenic oxides can be easily removed. However, the arsenate does not decompose under similar experimental conditions as described above and is still in the copper slag, thereby it requires to undergo reducing conditions to extract it. Using the HSC chemistry program, the Ellingham graph (Fig. 5) is plotted from Fe2SiO4, Fe3O4, arsenate, and CO.
As illustrated in Fig. 5, the Gibbs free energy curves of CO intersect those of Fe3O4 and Fe2SiO4 at 600 °C and 1200 °C, respectively. At a temperature of 600–1200 °C, the arsenate can be reduced using CO, whereas the reduction of fayalite is inhibited, which can lead to the prevention of the formation of FeAs and Fe2As. Therefore, the use of CO is more conducive to reduction of arsenate in slag in contrast to coal-based reduction.
In this section, samples of the copper slag used are obtained from the final product of the previous copper slag roasting procedure, and certain amounts of CO are introduced during the experiment at 1.5%, 2.5%, 3.5%, 5.0%, and 6.5% for 60 min in the N2–CO atmosphere. The gas-based reduction arsenic removal experimental results are illustrated in Fig. 6.
Figure 6 indicates that when the proportion of CO increases from 1.5 to 2.5%, the arsenic content decreases significantly and the arsenic content reaches a minimum value at 2.5% (0.054% As). When the proportion of CO exceeds 2.5%, steady addition of CO can lead to an increase in arsenic content, and the arsenic removal rate gradually decreases. Therefore, the ideal proportion of CO is 2.5%, when the arsenic content decreases to 0.054%, and the arsenic removal rate reaches 70.71%. The use of the CO-N2 gas mixture results in the removal of over 70.71% of the arsenic in less than one hour, which is similar to the result reported by Liu et al. [15].
From the results above, the optimal condition is obtained at a CO concentration of 2.5%, temperature at 1100 °C, and reduction time of 60 min. According to the above-mentioned conditions, two validation experiments are conducted to verify the feasibility of this process, and the results are expressed in Table 4.
Table 4 indicates that the results of the aforementioned validation experiments are consistent and approximate to those of the single factor experiment. This verifies that the optimal conditions are operable and repeatable. However, the remnant arsenic in the slag after roasting needs further removal in the subsequent reduction smelting process.
3.2 Arsenic Removal in Reduction Smelting Process
-
(1)
Arsenic removal in coal-based direct reduction process
The stable phase of arsenic in the roasted slag is mainly in the metallic stage such as FeAs, Fe2As, and CuAs2. To determine the behavior of arsenic in the coal-based direct reduction experiment, the effects of the reducing agent, reduction time, and temperature, as well as CaO addition are discussed. The results are illustrated in Fig. 7.
Figure 7 indicates that the arsenic content increases from 0.082 to 0.12% in the pellets, whereas iron recovery increases from 15.18 to 74.15% between 1150 and 1300 °C. The weight loss of pellets is responsible for this increment in arsenic content. However, the arsenic content decreases from 0.12 to 0.092% as the mass ratio of calcium-to-silicon (Ca/Si) increases while the variation trend of the arsenic content for different carbon contents is constant, although the absolute value of arsenic is low (0.082–0.073%). The reduction time has insignificant impact on the arsenic content, which is stable throughout the reduction process. The arsenic contents in slag are recorded to be 0.071%, 0.073%, 0.073%, and 0.074%.
In summary, it is confirmed that the removal efficiency of arsenic in the coal-based direct reduction process is relatively low under optimal conditions, indicating arsenic content in the pellets to be 0.071%. Therefore, coal-based direct reduction process is unsuitable for arsenic removal.
-
(2)
Arsenic removal in smelting process
Pellets produced in the coal-based direct reduction have been prepared for the subsequent smelting process. These experiments are conducted in a graphite crucible, utilizing the prepared pellets with the mixture of CaO and CaF2 between 1400 and 1500 °C. The distribution behavior of arsenic has been studied from the change in arsenic content in the gas–slag–metal phase relative to holding time and temperature, as well as the quantities of CaO and CaF2 added. The specific experimental results are illustrated in Fig. 8.
As illustrated in Fig. 8, the white background indicates the gas phase, whereas the white-line and gray-line backgrounds represent the slag phase and metal phase, respectively. During the smelting process, most of the arsenic is distributed into the iron and gas phases while only a small portion enters into the slag phase. It has been discovered that the arsenic content (approximately 0.18% mass) in the iron does not change with an increase in the holding temperature. However, continuous CaO addition drops the arsenic content from 0.18 to 0.12%. This is because Ca2As3 may be formed during the smelting process. The specific reaction is shown in the Eq. (6) as follows:
At the holding temperature of 1480 °C, \( \Delta G^{\theta } \) is − 114,272.83 J/mol, which indicates that Eq. (6) will shift to the right-hand side. Thus, arsenic is preferentially dissolved into the slag phase. However, increasing CaO results in it reacting with the SiO2 dissolved in iron to form a layer of CaSiO4 that hinders further dissociation of CaO, thereby slowing down the rate of arsenic removal. Thus, it is necessary for CaF2 to be added to reduce the viscosity of the slag. It is clear from Fig. 8 that as CaF2 addition increases from 0 to 1%, the arsenic content decreases significantly from 0.12 to 0.091%. When holding duration extends from 20 to 30 min, the arsenic content drops significantly from 0.091 to 0.080%, and later increases slightly when the duration is more than 30 min.
In summary, the optimal conditions from the above tests include the preparation of the reduced pellets by blending with 4% CaO and 1% CaF2; the pellets are smelted at 1480 °C for 30 min. The composition of iron and slag are provided in the next section.
-
(3)
Characterization of experimental products under optimal conditions
Two groups of comparative experiments have been conducted under the same conditions to determine the effectiveness of the new process used in this study. The product has been characterized by XRD and SEM. The main components of iron are presented in Table 5.
As presented in Table 5, the direct reduced iron (DRI) (De-As), comprising of 92.00% Fetotal, 0.082% As, 0.0081% S, and 0.089% P, has been manufactured. In particular, the arsenic content in DRI manufactured without the arsenic removal process is as high as 0.57%, which clearly indicates that the new process described in this paper has a splendid arsenic removal effect. The morphology of the product is illustrated in Fig. 9.
The SEM and EDS (energy-dispersive X-ray spectroscopy) images of the iron after the de-arsenic treatment is illustrated in Fig. 10. The section structure of iron is flat, smooth, and pure, except for a low number of microcracks (Fig. 10a). According to EDS analysis (Fig. 10b), Fe and C are the main components of iron ingot, where Fe accounts for 94.95% of the total content of the element, and C constitutes 5.05%. As can be seen in Fig. 11, the main component of slag is CaSi2O5, owing to the addition of CaO.
Through the new arsenic removal process described above, the arsenic content of iron is lower than the standard value present in steelmaking pig iron (YB/T5296-2006) and DRI (YB/T 4170-2008). The pig iron is used in steelmaking (C ≥ 3.5 wt%, Mn ≤ 0.45 wt%, P ≤ 0.1 wt%, S ≤ 0.02 wt%). This process achieves the purpose of ultimate harmless and reduction in the amount treatment.
4 Conclusions
-
(1)
In the original copper slag, the chemical phase composition of arsenic is mainly represented by arsenic sulfides, which account for 55.17%, together with arsenate and arsenic oxides comprising of 22.99% and 2.53%, respectively.
-
(2)
From the de-arsenic experiments, it has been discovered that arsenic sulfide (AsxSy) can be substantially removed by decomposition at 1100 °C in an N2 atmosphere, thereby allowing the reduction from 0.3 to 0.15% arsenic content. Arsenate can be reduced by the addition of CO, and the use of CO-N2 gas mixtures result in the removal of more than 70.71% of the arsenic in less than an hour.
-
(3)
During the coal-based direct reduction process, the arsenic removal efficiency is very low under optimal conditions, leading to a 0.071% arsenic content in the pellets. In the smelting process, a product of iron with 0.080% arsenic content can be extracted from slag under the conditions of CaO 4%, CaF2 1%, as well as smelting temperature and time of 1480 °C and 30 min, respectively.
References
Zhang J, Qi Y H, and Yan D L, J Iron Steel Res Int 22 (2015) 396.
Wang Y, Nonferrous Mines 32 (2003) 19.
Li L, Hu J H, and Wang H, Chin J Process Eng 11 (2011) 65.
Bipra G, Jana R K, and Prem C, Conserv Recycl 39 (2003) 299.
Shen H, Forssberg E, Waste Manag 23 (2003) 933.
Yang T, Hu J H, and Wang H, Chin J Process Eng 11 (2011) 77.
Huang Z L, Luo F, and Li M, Conserv Util Min Resour 10 (2009) 54.
Liu G, Zhu R, and Wang C A, China Nonferrous Metall 02 (2009) 71.
Guo Z Q, Improving Separation of Cu Fe from Copper Slag by Modifying Melted Copper Slag, Central South University, Chang Sha (2014).
Yang H F, Jing L L, and Dang C G, Chin J Nonferrous Metal 21 (2011) 1165.
Wan X Y, Qi Y H, Gao J J, J Iron Steel Res 28 (2016) 22.
Lv Q, Huang H H, Liu X J, Iron Steel Van Titan 36 (2015) 83.
Wan X Y, Zhao K, and Qi Y H, Min Metall Eng 37 (2017) 73.
Wan X Y, Qi Y H, and Gao J J, Nonferrous Metals Eng 04 (2017) 32.
Liu L C, Res Iron Steel 02 (1977) 01.
Funding
Item Sponsored by National Science Foundation of China (51404075); Natural science foundation of Hebei Province (E2019209160) and Natural science foundation of Hebei Province (E2018209284).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wan, Xy., Hong, Lk., Qi, Yh. et al. Removal of Arsenic During Iron Extraction from Waste Copper Slag. Trans Indian Inst Met 73, 2683–2691 (2020). https://doi.org/10.1007/s12666-020-02056-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02056-x