Abstract
The Mahallat hot springs are located in the city of Mahallat, central Iran. This region is famous for its balneotherapy and health tourism attractions. They are located in the transitional zone between the Sanandaj–Sirjan (mainly metamorphosed zone) and the Orumieh–Dokhtar (mainly volcanic zone) structural zones. The host rocks of the region include sedimentary to volcanic rocks (Permian to Quaternary), but Quaternary-aged alluvium and travertine layers are the main outcrops. Additionally, these features reveal that thermal and shallow ground waters had been mixed before hot springs exposure. The chemistry data show that these waters are enriched in Ca2+, Mg2+, Na+, Cl−, HCO3−, and SO42− as well as U and NO3−. The chemistry of thermal and shallow groundwater samples are graphed in the Cl−–SO42−–HCO3− ternary and other diagrams. In addition, hot springs having high NO3−-concentrations indicate that thermal waters mix with shallow groundwater, which are attributed to agricultural and other anthropogenic activities. The hot springs have high U concentration that may result in shallow groundwater mixing with andesitic tuff, granodiorite, shale, and schist of the region. Therefore, the local farms and agricultural crops as well as local residents’ health could be at risk of exposure to the U and NO3− pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are many hot springs in Iran along a belt from Turkey to Pakistan (Shakeri et al. 2015). Many surveys were carried out to assess various aspects of this belt, including: environmental, geological, petrological, geochemical, hydrothermal, water chemistry, energy resources, and hydrogeological aspects (Gansser 1971; Mehdizadeh et al. 2002; Shakeri et al. 2008; Saadat and Charles 2011; Esmaeili-Vardanjani et al. 2013; Yazdi et al. 2014; Shakeri et al. 2015; Esmaeili-Vardanjani et al. 2016; Yazdi et al. 2016). The region is also famous for its balneotherapy and health tourism. This research aims to assess the hot springs origin and to establish hydrogeochemical characteristics of hot springs in Mahallat region (Fig. 1) as well as their adverse impacts on local environment. The study region is located at transitional zone between Sanandaj–Sirjan and Orumieh–Dokhtar structural zones (Aghanabati 2004). These hot springs are used for recreational purposes as well as balneotherapy and the study region is developed as a domestic and international tourist attraction.
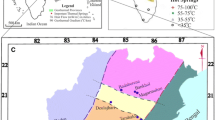
Geology map of the study area (Sheikholeslami 2005)
Geology of study region
The host rocks of the region include sedimentary to volcanic rocks (Permian to Quaternary). Lithologic outcrops include (Fig. 1):
-
1.
Quaternary alluvium.
-
2.
Quaternary travertine that overlays Eocene sedimentary rocks (angular unconformity).
-
3.
Marl, sandstone, and conglomerate of the Upper Red Formation (Miocene).
-
4.
Marl and limestone of Qom Formation (Oligocene–Miocene).
-
5.
Upper Eocene–Oligocene intrusive igneous rocks (mainly granodiorite).
-
6.
Middle Cretaceous andesitic tuff, shale, and sandstone.
-
7.
Oolitic limestone of Badamu Formation (Upper Jurassic).
-
8.
Shale and sandstones of Shemshak Formation (Lower Jurassic).
-
9.
Dolomite of Shotori Formation (Triassic).
-
10.
Limestone and dolomite of Jamal Formation (Permian).
Field observations show that Quaternary-aged alluvium and travertine layers are the main outcrops in the region of the hot springs. The travertine layers have been pervasively affected by regional structures. Many faults and fractures provide conduits for the hot springs outflow from travertine and alluvium beddings (Fig. 1).
Materials and methods
A total of six hot-spring water samples were collected from the region (Table 1). To minimize dilution of the hot-springs water with infiltrated meteoric water and to investigate the trace element concentrations in dry season, samples were collected during the summer (dry period). The locations of the water samples are shown in Fig. 1. Some in situ measurements such as pH, electrical conductivity (EC), and temperature were conducted during sampling. All samples were collected as two filtered batches into 250-ml polyethylene containers. One untreated batch was taken for anion analyses and the second treated with 2.5-ml ultrapure HNO3 (1 N) for cation analyses. The samples were analyzed using a standard method suggested by the American Public Health Association (APHA 1992) in the Geological Survey of Iran (GSI) laboratory. The ICP-OES method was applied to analyze major and trace elements and cations measured by flame photometry. Sulfate concentration was measured by spectrophotometry. Chloride and bicarbonate concentrations were measured by titration methods. The TDS values were also analyzed. The term TDS (Total dissolved solids) describes the inorganic salts and colloids present in solution in water. We used long-term annual data of cold springs of the region from Iran Ministry of Energy database (Table 2) for comparison of the hot and cold springs’ chemical characteristics.
Results and discussion
There are several hot springs in different sizes in the Mahallat region. These hot springs are located in an arid zone and average annual precipitation is low and about 250 mm. The region gains its most precipitation during winter, while the summer is extremely hot and dry. The long-term maximum and minimum air temperatures are 36 °C (July) and − 6 °C (January), respectively. However, the six hot springs are the major water supply for some agricultural and recreational purposes even in the dry season. Our research was focused on these six hot springs. We compared the hot springs’ chemical characteristics with that of cold groundwater (springs) of the region too. It is very important to show the chemical changes in the two types of springs.
Table 1 shows the hot springs’ geochemical analyses results, the main physical and chemical characteristics of cold groundwater are shown in Table 2. Pentecost et al. (2003) believe that the conventional temperature for considering a spring as a thermal spring is 36.7 °C. The thermal springs’ temperature of the study region ranges from 35 °C (sample S4) to 47.7 °C (sample S6). pH values were between 6.7 (sample S4) and 8.09 (sample S5). TDS contents range from 1789 to 2214 mg l−1 (Table 1), but the TDS values for cold groundwater samples range from 636 to 1131 mg l−1 (Table 2). The hot spring waters have high EC values as well as high Ca2+, K+, Mg2+, Na+, Cl−, HCO3−, and SO42− as well as high U and nitrate concentrations. Cations and anions show the following order of abundance, respectively: K+ < Na+ < Mg2+< Ca2+ and Cl− < HCO3− < SO42− (Table 1).
We used Cl−–SO42−–HCO3− ternary plot (Giggenbach 1988; Marini 2000) for an initial classification of these geothermal waters (Fig. 2). The chemical values are plotted toward the SO42− corner of the plot. This field is labeled where “hydrothermal vapors and steam-heated waters” are dominant, but that classification may not be appropriate for these waters, as discussed below. Also, we used Piper diagram to compare the results with Fig. 2. The results show that the samples fields were labeled in the corner of SO42− (Fig. 3). These data support the fact that these geothermal waters are mainly of sulfate type.
Relative Cl−, SO42− and HCO3− contents of the hot and cold water samples (Giggenbach 1988)
The groundwater samples, apparently, tailed from peripheral and shallow waters field toward the SO42− end member, representing that the shallow groundwater has potentially mixed with thermal waters. The K/100–Na/100–√Mg ternary plot (Giggenbach 1988) was applied to evaluate the state of water–rock chemical equilibrium (Fig. 4). The model shows that none of the samples have attained a water–rock chemical equilibrium. Additionally, all the points are aggregated at the √Mg2+ end of the plot and reveal that the water samples are strongly influenced by Mg-bearing host rocks such as dolomitic and/or marly formations as well as andesitic tuff volcanic rocks. It is important to note that there are no evaporite rocks in the region. So, high sulfate and the other ion concentrations could not have resulted from interaction between groundwater and evaporites. The plot of %Mg/(10 Mg + Ca) versus %K/(10 K + Na) (Giggenbach 1988) indicates that the these water samples do not attain the rock–water equilibrium condition (Fig. 5). Furthermore, the dissolved chemicals of the water samples in the region have not been originated through simple rock leaching or mixing processes and/or dissolution of average crustal rock. Result suggests that although the selected springs’ temperature is low and very similar to low-temperature metamorphism conditions, they are exposed from low-temperature hydrothermal processes; and regional and/or contact metamorphism shall be the heat source in the study area. However, some minor chemical weathering is suspected.
Graphical evaluation of the water-rock equilibration temperatures (Giggenbach 1988) using relative Na, K and Mg concentrations of the hot and cold water samples
Plot of 10 K/(10 K + Na) vs. 10 Mg/(10 Mg + Ca) (Giggenbach 1988) of the hot and cold water samples. The numbers on the curve are temperature in °C
Uranium was one the most important element in our research, because of its environmental impacts. Natural uranium, distinct from artificially enriched uranium, typically exists in schists, black shales, granodiorites and granites. Under reducing conditions, the uranium solubility is low, but is high under oxidizing condition (Milvy and Cothern 1990). USEPA suggested 30 μg L−1 of dissolved uranium as the maximum allowed concentration (MAC) within the drinking water (USEPA 2009). The uranium concentration ranges from approximately 200 to 550 μg L−1 in the hot springs of the region (Table 1). But peripheral and shallow waters may dilute the uranium concentration, as they mix with thermal waters. However, the study region is at risk of high uranium concentration in both groundwater and soil, especially during drought periods, when there is less peripheral and shallow waters to dilute hot springs.
Nitrate (NO3−) was another important element in our research, because of its environmental impacts. Nitrate is an anthropogenic contaminant in drinking water that is normally derived from sewage and livestock manure or from the use of artificial or natural fertilizers. According to USA, MAC standard, the NO3− normal value is 10 mg L−1 expressed as NO3−–N or 44 mg L−1 expressed as NO3−. Nitrate concentration in the hot springs of the region ranges from < 5 to 252 mg L−1 (Table 1). The data suggest that the hot springs water may partially mixed with infiltrating water from rural and urban zones in the study region.
Conclusion
Relatively high concentrations of Ca2+, Mg2+, HCO3−, and SO42− resulting from rock–water interaction have been observed in the hot springs water of the Mahallat region. The widespread structural geology structure (such as faults) in the study region may enhance the groundwater circulation and mixing that results in significant increase of elements concentration. High nitrate concentrations confirm that thermal waters mix with shallow groundwater, which are attributed to anthropogenic and agricultural activities.
The presence of uranium in these hot spring waters is of considerable concern, especially where the uranium is over the MAC standard values. High U concentration may result in shallow groundwater mixing with andesitic tuff, granodiorite, shale, and schist of the region. The problem becomes even greater, if we consider the diluting impact of peripheral and shallow groundwater. Therefore, the groundwater and soil toxicity may increase during drought periods. In the Mahallat region, the diffusion and dispersion of uranium from thermal waters into shallow aquifers could contaminate the groundwater. Also, the hot springs discharge into the local streams and rivers and could affect downstream farms and crops. Therefore, the shallow aquifers are vulnerable and the local residents’ health is at the risk of contamination with toxic and radioactive pollutants.
References
Aghanabati A (2004) Geology of Iran. GSI Publication, Tehran
American Public Health Association (APHA) (1992) Standard method for the examination of water and wastewater. APHA, Washington, DC
Esmaeili-Vardanjani M, Shamsipour-Dehkordi R, Eslami A, Moosaei F, Pazand K (2013) A study of differentiation pattern and rare earth elements migration in geochemical and hydrogeochemical environments of Airekan and Cheshmeh Shotori regions (Central Iran). Environ Earth Sci 68:719–732
Esmaeili-Vardanjani M, Rasa I, Yazdi M, Pazand K (2016) The hydrochemical assessment of groundwater resources in the Kadkan basin, Northeast of Iran. Carbonates Evaporites 31(2):129–138. https://doi.org/10.1007/s13146-015-0248-3
Gansser A (1971) The Taftan volcano (SE Iran). Eclogae Geol Helv 64:319–334
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Ca–Mg geoindicators. Geochim Cosmochim Acta 52:2749–2765
Iran Water Resources Managaement Co. (IWRM) online database. Accessed: September 2018. http://www.wrm.ir/#Info
Marini L (2000) Geochemical techniques for the exploration and exploitation of geothermal energy, Universita` degli Studi di Genova. Dipartimento per lo Studio del Territorio e delle sue Risorse, Genova
Mehdizadeh H, Liotard JM, Dautria JM (2002) Geochemical characteristics of an intracontinental shoshonitic association: the example of the Damavand volcano, Iran. C R Geosci 334:111–117
Milvy P, Cothern CR (1990) Scientific background for the development of regulations for radionuclides in drinking water. In: Cothern CR, Rebers P (eds) Radon, radium and uranium in drinking water. Lewis Publishes, Chelsea, pp 1–16
Pentecost A, Jones B, Renaut RW (2003) What is a hot spring? Can J Earth Sci 40:1443–1446
Saadat S, Charles RS (2011) Petrochemistry and genesis of olivine basalts from small monogenetic parasitic cones of Bazman stratovolcano, Makran arc, southeastern Iran. Lithos 125:607–619
Shakeri A, Moore F, Kompani-zare M (2008) Geochemistry of the thermal springs of Mount Taftan, southeastern Iran. J Volcanol Geotherm Res 178:829–836
Shakeri A, Ghoreyshinia S, Mehrabi B, Delavari M (2015) Rare earth elements geochemistry in springs from Taftan geothermal region SE Iran. J Volcanol Geoth Res 304:49–61. https://doi.org/10.1016/j.jvolgeores.2015.07.023
Sheikholeslami MR (2005). Mahallat Quadrangle Map 1:100,000. Geological Survey of Iran
USEPA United States Environmental Protection Agency (2009) National primary drinking water regulations. [Online] 2009. http://www.epa.gov/safewater/contaminants/index.html. Accessed 20 Apr 2019
Yazdi M, Taheri M, Navi P (2014) Environmental geochemistry and sources of natural arsenic in the Kharaqan hot springs, Qazvin, Iran. Environ Earth Sci 73(9):5395–5404. https://doi.org/10.1007/s12665-014-3794-4
Yazdi M, Navi P, Tahmasi O (2016) Hydrogeochemical characteristics of Mahallat hot springs, central Iran. J Tethys 4(2):169–179
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikpeyman, Y., Yazdi, M., Tahmasi, O. et al. The hydrogeochemical assessment of hot springs in Mahallat region, central Iran. Environ Earth Sci 78, 597 (2019). https://doi.org/10.1007/s12665-019-8612-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8612-6