Abstract
The Himalayan lakes are facing an acute problem of survival due to increased concentration of toxic elements in water and sediments. In the present study, the heavy metal contamination history of Renuka Lake during the calendar year (ca) 1839–2003 AD has been performed using 137Cs and 210Pb radionuclide-dated core sediment sample and Inductively-Coupled Plasma Mass Spectrometry (ICP-MS). The concentration of heavy metals (Mn, Cr, Cu, Zn, Ni, Pb and Co) during the said time period is found to have significant variations and suggested a contribution of both natural and anthropogenic factors like surrounding rocks, burning of fossil fuels, sewage and agro-chemicals. The highest concentration of Mn is observed during ca 1847, which is 4–5 times more than ca 2003. The Cr is found to be highest during ca 1962; whereas Cu, Zn, Ni and Pb are observed highest during 1883. Similarly, Co is determined highest during ca 1939. The contamination factor (Cf) indicated low to the considerable level of contamination, whereas the degree of contamination (Cd) revealed a low level of contamination in the lake. However, the geo-accumulation index (Igeo) marked the lake uncontaminated to moderately contaminated and on the other hand, the pollution load index (PLI) showed some signs of contamination in the Renuka Lake. The present study has been compared with other lakes and the background concentration of average shale, upper crust, crustal average and carbonate rocks. Although, keeping in view historical contamination, there is a need of stringent steps for the preservation of the Renuka Lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The millions of people all over the world are facing an acute shortage of fresh water for their domestic needs, due to the degradation of water quality (Pius et al. 2012; Gaury et al. 2018). Because, the rivers and lakes which store most of surface fresh water are degraded. Particularly, the contamination of lakes by toxic heavy metals has become a very serious global problem (Ali et al. 2016; Meena et al. 2017). Metals like Fe, Mn, Mo and Zn are essential for the human body, but when reaching more than a specific limit, they become toxic and cause serious health impairments (Mackay 2001; BIS 2012; Febriana et al. 2012; Bost et al. 2016). However, metals like Cd, Pb, As and Hg are non-essential for aquatic and non-aquatic lives, and even a small amount of these metals can be harmful to organisms (Kumar and Kumar 2019). Metals enter into the lakes through natural and anthropogenic point and non-point sources like surrounding rocks, soil, atmospheric deposition, industrial effluent, sewage and vehicular emission, through transporting agents like water, wind and animals (Rice et al. 2002; Xu et al. 2009; Nadaska et al. 2010; Koilakos 2017). They are adsorbed and enriched in sediments over time as the water of the lake escapes by evapotranspiration, but the metals remain in the system and thus increase in concentration. The toxic metals continuously release from sediment in the water column for a long time period under different environmental conditions and during seasonal mixing and mobility, and thereafter reach to the flora and fauna through direct uptake of drinking water and food chain (Salomons 1998; Khadka and Ramanathan 2013; Singh et al. 2005).
Therefore, it is essential to determine heavy metal loading in the lakes, rivers and other reservoirs to understand the level and the factors responsible for it. The Himalayan lakes have been monitored with different perspectives in past (Das and Kaur 2001; Das et al. 2008; Ding et al. 2016; Sarkar et al. 2016; Meena et al. 2017; Gaury et al. 2018; Kumar et al. 2019a), but very few studies reported contamination history of the lakes (Wang et al. 2010; Zeng et al. 2014; Meena et al. 2017). Since the recent past, Renuka Lake has been a center of anthropogenic activities due to its religious and cultural importance in the region. The waste materials left by tourists, a cultural fair, dumping of worshipping and fish feeding material in the lake, excess catchment erosion after civil development, etc., have led to a rapid change in the natural scenario of this lake. The anthropogenic activities have increased limiting nutrients that resulted in degradation of water quality and turned the Renuka Lake hyper-eutrophic in status (Kumar et al. 2019b). The rare earth elements in the lake are suggested to be the product of moderate weathering of surrounding rocks (Das et al. 2008). The seasonal change in the major ion chemistry of the lake is revealed by Kumar et al. (2019a). However, the mobilization and enrichment of the metals depend on the seasonal variations of lake water chemistry, organic matter and carbonate saturation in sediments. Heavy metal enrichment generally found to be higher during summer as compared to winter, due to higher surface water evaporation rate (Padma and Periakali 1999; Zhao et al. 2014; Smily and Sumithra 2017). The organic matter in the lake is derived from the terrestrial and planktonic source. The organic matter is mainly composed of humus, which is an oxygen-containing functional group and having a large surface area with a high cation exchange capacity that bound metal with it (Wu et al. 2009). Thus, metal partaking in complication reactions remains in solution or associated with plankton and settles to the lake floor. The main sources of calcium carbonate for the lakes are the calcite and aragonite minerals, and the sediments with higher CaCO3 content are alkaline in nature that associate the metal in the form of metal carbonates and metal oxides. However, the presence of the carbonic acid (H2CO3) dissolves the metal oxide and metal carbonate from the solution (Aziz et al. 2008).
The present study has three major objectives, the heavy metal contamination history of Renuka Lake during the calendar year ca 1839–2003 AD, to assess natural and anthropogenic loading of heavy metal and the effect of carbonate saturation on the metal fractionation using core sediment sample retrieved from the central part of the Renuka Lake and analyzed using 210Pb and 137Cs radionuclide dating technique. The present study will be helpful to know the heavy metal contamination history for preservation of the Renuka Lake (Wang et al. 2010; Oliveira et al. 2012).
Materials and methods
Study site and geological setting
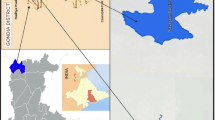
The Renuka Lake is a freshwater lake and located (620 m asl, 30°36′36″N, 77°27′30″E) in the Sirmaur District of Himachal Pradesh, India (Fig. 1). Physiographically, the lake is lying between the Lesser Himalayan sedimentary rocks comprising dolomitic limestone, slate, quartzite, calcareous, carbonate, siltstone, shale, sericite, etc. (Das et al. 2008). The catchment area of the lake is 254.3 ha, length 1706.7 m, width 204 m and depth 13.7 m with annual rainfall around 150–199.9 cm per annum (Das and Kaur 2001). The monsoon rain and underground water seepage are the main sources of lake water. This lake also feeds the Parshuram Tal located in the lower region. Owing to the tourist attraction and ecological significances, the Renuka Lake is declared as a Ramsar site since ca 2005.
Field work, laboratory procedure and data analysis
Sampling
To observe the contamination history of the Renuka Lake, a 2-m-long core from the central part of the lake was retrieved using a piston corer. The core was vertically bisected into U-shaped two channels and among them, one part was kept safe for future study and another part was again bisected for dating and heavy metal analysis. The bisected part of the core for heavy metal analysis was sliced into 20 sub-samples. These samples were kept dry at room temperature, and thereafter grinded using agate mortar, which was cleaned before grinding each sample with acetone to avoid contamination. A litholog has been prepared in order to understand the textural changes that occurred in the Renuka Lake sediments (Fig. 2).
ICP-MS analysis
The next step was the digestion of the homogenized sediment samples. A 100-mg sediment sample was taken in a Teflon crucible and digested with 10 ml of HF–HNO3 mixture (2:1). The content was kept for complete digestion on a hot plate, and thereafter treated with 2 ml of HClO4. Then, 10 ml of 20% HNO3 was added to the digested sample and total volume of the sample was made 100 ml by adding Milli Q water as proposed by Khanna et al. (2009) and Meena et al. (2017). The same procedure was followed for all the remaining samples. The samples were analyzed by PerkinElmer SCIEX quadrupole Inductively-Coupled Plasma Mass Spectrometry (ICP-MS) ELAN DRC in the central laboratory of Wadia Institute of Himalayan Geology (WIHG), Dehradun, India. The SO-1 USGS and MAG-1 standards were used for calibrating the instruments before the sample analysis (Meena et al. 2017).
210Pb and 137Cs radionuclide dating technique
The age of the core sediment sample was determined using 210Pb and 137Cs radionuclide dating technique at the Physical Research Laboratory, Navrangpura, Ahmedabad, India. The radionuclide 210Pb is having a half-life period of 22.26 years and was first inferred by Goldberg (1963). It is still most commonly used to estimate the age of the sediments up to a time period of 150–200 years on the basis of unsupported 210Pb. It is a natural decay product of Uranium series radionuclide present in the soil and considered as supported 210Pb. Instead, the 222Rn in the series decays to 210Pb in the atmosphere, which falls at the earth surface with rain, dust, etc., and known as unsupported 210Pb. That form of 210Pb on reaching the lake water settles down at the bottom and further decays with time. The lake sediments also contain 238U coming from weathering of the surrounding rocks; also decays to 210Pb. The level of 210Pb is estimated by subtracting activities of 226Ra and total 210Pb (Somayajulu et al. 1999). The 137Cs radionuclide is having a half-life period of 30 years and its maximum fallout was occurred during ca 1963 AD, owing to nuclear weapon testing (Robbins and Edgington 1975; Chillrud et al. 1999). 137Cs is an artificial radionuclide and its maximum value in the samples, thus, indicates the time period of ca 1963 AD; therefore, the depth of that sample since the time period of ca 1963 AD is divided into years, which, thus, is the age of the sediment deposition in the lake. The core sample retrieved from the Renuka Lake was sliced in different sub-samples. From each sample, 3–5 g was dried, and thereafter finely powdered and sealed in plastic vials. Those samples were then kept undisturbed for 20 days so as to establish 226Ra and 222Rn radioactive equilibrium (Sarkar et al. 2016). The samples were analyzed using Canberra HPGe well detector to know the gamma activity of 226Ra, 137Cs and 210Pb.
Statistical analysis
Contamination factor and degree of contamination
The contamination factor (Cf) and the degree of contamination (Cd) were calculated to understand the heavy metals contamination level of the Renuka Lake using the formula as given by Hakanson (1980):
where Mes represents the metal concentration in the sediments. Meav is the metal concentration of pure/uncontaminated sediments.
The average shale concentration proposed by Turekian and Wedepohl (1961) was used as a reference for the pure sediments. The contamination factor is divided into four classes (Nasr et al. 2006) as given in Table 1.
On the other hand, the degree of contamination was calculated from the contamination factor using below given formula:
The degree of contamination is divided into four classes (Leopold et al. 2012) as given in Table 2.
Geo-accumulation (I geo) and pollution load index (PLI)
The heavy metals contamination in the core sample of Renuka Lake was known using Geo-accumulation index (Igeo) as proposed by Muller (1969). This index compares the observed metal concentration with the pre-industrial level and was calculated using the formula (Turekian and Wedepohl (1961) as:
where Cn is the concentration of a particular observed metal. Bn is the background concentration of the metals.
The factor ‘1.5’ is used to avoid the disparity of the background data as a result of the lithological differences.
The Igeo is to be divided into different categories as shown in Table 3.
The pollution load index (PLI) for Renuka Lake has been calculated using the formula given by Tomlinson et al. (1980). This index is based on contamination factors equal to the observed metal concentration to the background level of contamination (Turekian and Wedepohl 1961). All of the contamination factors (Cf) as obtained from seven metals (Mn, Cr, Cu, Zn, Ni, Pb and Co) were multiplied with each other and then their seventh root was calculated to get PLI as:
where n is the number of metals and Cf is the contamination factor.
The value of PLI < 1 indicates perfection, PLI = 1 suggests a baseline level of contamination and PLI > 1 indicates the progressive deterioration in the lake (Manoj and Padhy 2014).
Results and discussion
Chronology
The chronology for the top 124-cm sediments from Renuka Lake is assigned on the basis of 210Pb and 137Cs radionuclides isotopes. On the basis of 210Pb excess values, the Renuka Lake showed two different sedimentation rates. The top part (0–19 cm) indicated 0.51 cm/year; followed by 0.78 cm/year in between 19- and 37-cm depth with average sedimentation rate of 0.64 cm/year. Based on 210Pb, the sedimentation rate of the top part from 0 to 19 cm revealed the time period from ca 2011 to 1974 AD and from 19 to 37 cm indicated as ca 1974–1950 AD.
The 210Pb activity was not detected below 37-cm depth. However, relatively well-resolved peaks of 137Cs activity in the profiles provided independent support for the present age-depth model. The 137Cs isotope showed the highest value at 37.5 cm, which corresponded to ca 1963 AD and correlated well with the maximum 137Cs fallout due to the nuclear weapon test in the year 1963 AD (Robbins and Edgington 1975). Based on the 137Cs activity, the calculated sedimentation rate is found to be 0.79 cm/year, which is well within the range of 210Pb-based calculated sedimentation rate (0.64 cm/year). The constant rate of supply (CRS) model as was used to determine the age of the samples at various depths is given in Table 4. The Fig. 2 shows the variation in the lithology of the Renuka Lake sediments and suggests that from 124 to 74 cm, the sediments are of very coarse silt sized; followed by the very fine sand up to 58-cm depth. Thereafter, from 58 to 44 cm, the grain size slightly decreased to very coarse silt. The top part of the core between 44 and 2 cm is characterized as coarse silt, whereas the topmost surface consisted of medium sized silt.
Temporal heavy metal loading
The concentration of heavy metals in the Renuka Lake core sediments during ca 1839–2003 AD is shown in Table 5. The group of metals (Pb, Cr, Co, Cu, Zn and Ni) except Mn shows a similar trend from ca 1839 to 2003 AD with different amplitude (Fig. 3).
Lead (Pb)
The Pb is a most abundant transition metal found in mineralised forms like galena and anglesite, and at trace amount in K-feldspar, plagioclase, mica, zircon and magnetite (Greenwood and Earnshaw 1984). The sedimentary enrichment of Pb is mainly controlled by the primary detrital minerals like feldspar, mica, sulfides and organic matter (Heinrichs et al. 1980). In the sediment core of the Renuka Lake, the highest concentration of Pb (33 µg/g) has been recorded during the year 1839, 1844 and 1939 AD that slightly decreased to 27 µg/g during ca 1883 AD and found to be lowest (25 µg/g) during ca 1862 AD. The detrital quaternary sediments (containing K-feldspar, plagioclase and carbonate minerals), shale and lake-based sulfide minerals are the potential source of natural Pb in the Renuka Lake. The man-made sources of Pb include surrounding vehicular emission, acid batteries, paints, sewage and leaded petrol. The Pb in the lake sediments is bounded with organic matter, CaCO3 and sulfide minerals, and therefore remains less mobile. The decreasing trend of the Pb in the Renuka Lake during post-1900 AD seems to be due to decreased natural and anthropogenic inputs to the lake after the construction of the first protection wall and other preventive actions. The higher concentration of the Pb (350 μg/l) in the Renuka Lake water (Singh and Sharma 2012) as compared to its permissible limit (10 μg/l) and crustal average 125 µg/g (Taylor and Mclennan 1985; Table 7) seems to be enriched from the sediments over the time. It is one of the most toxic metals that can cause health impairment among human beings if remains high in Renuka Lake sediments (Meena et al. 2017).
Chromium (Cr)
The Cr is a highly toxic metal that occurs in hexavalent (Cr6+) and trivalent (Cr3+) states in the water bodies, in which the hexavalent form of Cr is more toxic. The high stability and oxidizing capability make this metal highly toxic (Khan et al. 2013). The Renuka Lake sediments core exhibits the highest concentration of Cr during ca 1939 AD (62 µg/g), that decreased to 59 µg/g during ca 1883 AD and 42 µg/g between ca 1862 and 1890 AD. Overall, the Cr revealed an increasing trend in the last 164 years of contamination history. The quartzite and chlorite minerals are potential sources of Cr in Renuka Lake as these rocks are rich of Cr and found abundantly in the surroundings of the lake (Das et al. 2008; Randive et al. 2015). However, the anthropogenic sources of Cr may include stock farms, industries, agro–chemicals, etc. The Cr is a comparatively less mobile element in everglade lakes, because iron oxides minerals and humic substances bind it in the presence of organic matter and deposit at the bottom sediment (Guo et al. 1997). The concentration of the Cr in the Renuka Lake (23.63 μg/l) is in the range of permissible limit (50 μg/l). Therefore, it is suggested that the organic matter and iron-bearing minerals controlled the Cr concentration in Renuka Lake water. Higher concentration of the Cr during ca 1939 and ca 1883 AD may be due to higher input of the catchment wash or debris flow to the lake. The increasing trend of Cr since the last 164 years suggests anthropogenic input due to increased activities that slightly decreased during post-1950 due to preventive measures by the administration at the lake. The increased concentration of Cr may cause carcinogenic effect, respiratory problem and skin diseases and also reduce the abundance and diversity of benthic organism (EPA 2012; Sun et al. 2015).
Cobalt (Co)
The Co is found to be as an accessory element in several abundant minerals, i.e., olivine, pyroxene, mica and garnet (Ure and Berrow 1982). The highest concentration of Co (51 µg/g) was found to be during ca 1939 AD, followed by 45 µg/g in ca 1975AD, 38 µg/g in 1862 AD and 36 µg/g during 1890 AD. However, the Co indicated an increasing trend from beginning to the recent (ca 1839–2003 AD). This metal is comparatively immobile and shows less solubility in water (Das and Haake 2003). However, the acidic and reducing conditions can mobile Co at the surface environment. The low concentration of Co (20 µg/g) in Renuka Lake water as compared to the core sediments do suggest that the Co is bounding with the organic matter, because at decreasing Eh, cobalt prefers to associate with the organic matters and sulfide. Higher concentration of the Co during ca 1939 and ca 1883 seems to appear due to higher input of the catchment wash or debris flow to the lake. The increasing trend of Co in Renuka indicates increased input of Co through various anthropogenic activities around the lake since the last 164 years. The higher concentration of Co may cause heart diseases and decrease the population of lake-based fauna and flora.
Copper (Cu)
The Cu is an essential dietary element (0.05 mg/l), but its higher concentration (> 1.5 mg/l) may disturb human health through cardiovascular diseases, lung cancer, etc. Naturally, Cu comes from silicate, carbonate, as well as copper sulfide and other evaporate minerals. The trace level of Cu is widely present in the minerals like pyroxene, mica and amphibole. However, it is found to be more in mafic rocks as compared to felsic rocks. The Cu is enriched in fine grain clastic rocks, particularly in shale. The anthropogenic activities which may generate Cu are the coal burning, atmospheric deposition, vehicular emission and combustion of lubricants, diesel and gasoline (Meena et al. 2017). In Renuka lake, the Cu is found to be highest during ca 1883 (40 µg/g), that depleted in ca 1862 AD (35 µg/g), ca 1890 AD (31 µg/g), ca 1916 AD (30 µg/g) and ca 1939 AD (29 µg/g). The Cu mobiles under oxidizing acidic and high anoxic environment; howsoever, the copper under such conditions gets bound to sulfides and carbonate (Davison 1993). The high carbonate fraction with the highly anoxic condition of the Renuka Lake favors the enrichment of copper in the sediments. The Cu shows affinity with organic matter that adsorbs and bounds with sediments (Rashid 1974). The everglade Renuka Lake has the ideal condition to bind Cu in the sediments with abundantly available organic matter and sulfide minerals. Therefore, the concentration of Cu (10 µg/g) in the lake water appeared to be much lesser than the sediments; possibly due to high organic matter in the Renuka Lake that bounds the Cu with sediments. The overall trend of Cu is found to be decreasing over the last 164 years that may be due to the reduction of catchment input. However, Renuka Lake has a higher concentration of Cu than the permissible limit that may cause health problems for flora and fauna of the lake.
Zinc (Zn)
The Zn is an essential body nutrient and its deficiency (< 0.1 mg/l) may create neuropsychological disorder in human beings, but its higher concentration (> 0.5 mg/l) causes leucopenia and anemia (Chambers et al. 1999; Khan 2011). The Zn in Renuka Lake is increased during ca 1883 AD (127 µg/g) and decreased during ca 1890 AD (88 µg/g), followed by 1862 (85 µg/g), 1839 (81 µg/g) and 1867 AD (80 µg/g). The surrounding rocks and the anthropogenic activities (metal plating and related works) are the major source of Zn in the lake and its concentration not increased significantly during the last hundred years, probably due to less use of Zn in the region. The mobility of zinc depends on the availability of the binding material in the aquatic environment and with higher carbonate concentration the zinc gets bind with carbonate and precipitates as zinc carbonate and zinc hydroxide. The higher carbonate content in the Renuka Lake appears to be a main factor for the enrichment of Zn in sediment as zinc carbonate.
Nickel (Ni)
The Ni in the sediment profile of the Renuka Lake has shown higher concentration during ca 1883 (33 µg/g), 1862 (25 µg/g), 1890 (25 µg/g), 1939 (24 µg/g) and 1975 AD (23 µg/g), which is probably due to natural and anthropogenic activity. Nickel is found to be in the organic and residual fraction (Panda et al. 1995; Staelens et al. 2000) and its adsorption takes place in the ligands formation process and in the presence of high organic content (Jacobs et al. 1985). The behavior of Ni in anoxic condition shows binding with oxides for reduction and release to the water column (Yu et al. 2001) in the presence of carbonate (Purushothaman and Chakrapani 2012). It is strongly enriched in ultramafic and mafic rocks as compared to the felsic igneous rocks. The higher concentration of Ni during 1883 AD appears to be due to excess catchment wash, whereas its higher value during 1975 AD seems to be a result of the metallic waste dumping in the lake.
Manganese (Mn)
The Mn indicated significant variations with higher values from ca 1839–1875 AD, after that, it decreased and stabilized from ca 1875–2003 AD. The highest concentration of Mn noted during ca 1847 AD (3562.5 µg/g) is 4–5 times more than ca 2003 AD (774.46 µg/g). The Mn is a transition metal that abundantly occurs in the earth crust (Tobiason et al. 2016). However, it does not occur in the free state and found to be associated with minerals like silicates under oxic conditions and with sulfides and carbonates under highly anoxic condition (Davison 1993). The shale and dolomite minerals contain a higher concentration of Mn (Wedepohl 1978); thus, dominance of these in the surroundings of the Renuka Lake is a potential source of this metal. The concentration of Mn is significantly influenced by redox condition and easily mobilized under the anoxic condition and participated in the redox reaction (Hylander et al. 2000). The concentration of Mn in Renuka lake water (870 µg/l; Singh and Sharma 2012) is higher than the permissible limit (300 µg/l; BIS 2012). The anthropogenic sources of Mn may include burning of fossil fuels, sewage, mining, etc. The low concentration of Mn (100 µg/l) is essential for the proper life functioning of a human being, but beyond a specific limit (300 µg/l), it is harmful (BIS 2012). It can cause nervous system damage, bronchitis and lungs problems. Usually, the presence of Mn in the water body persists for a long time and if its concentration goes beyond the permissible limit, it may disturb aquatic organisms, hinder the growth of the microorganism and accumulate in fishes and disturb the proper function of their life cycle (Chen et al. 2015).
Contamination factor (C f), the degree of contamination (C d) and the geo-accumulation index (I geo)
The geochemical analysis suggests contamination factor (Cf) from low, moderate and considerable contamination during ca 1839–2003 AD (Fig. 4). Amongst all metals, the considerable contamination is shown by Mn during 1839 (3.28) and 1847 AD (4.19), whereas moderate contamination by Mn, Pb and Co is revealed during ca 1844 (2.55), 1854–1929 (1.0–2.28) and 1991 AD (1.09); ca 1839–2003 AD (0.45–1.34); and ca 1844 (1.16), 1862 (2.0), 1883 (1.21), 1890 (1.89), 1939 (2.68) and 1975–2003 AD (2.37–1.58), respectively. The moderate contamination by Zn is shown during ca 1883 AD (1.34). However, Co and Zn during the rest of the time periods exhibit low contamination in the range of 0.37–0.95 and 0.45–0.93, respectively. Besides, Cu, Ni and Zn are found to indicate low contamination (i.e., 0–1) during ca 1839–2003 AD. On the other hand, the degree of contamination (Cd) for all the metals is noticed below 7, which indicates low level of contamination in the Renuka Lake during ca 1839–2003 AD. The geo-accumulation index (Igeo) during ca 1839–2003 AD for different metals like Mn, Cr, Cu, Zn, Ni, Pb and Co is found to be in the range of 0–1. This indicates that the lake has been uncontaminated to moderately contaminated during the said time period.
Pollution load index (PLI) and the person’s correlation coefficient
The PLI in Renuka Lake is noticed in the range of 0.36–0.95, which is highest during ca 1883 (0.95); followed by 1890 (0.87), 1939 (0.843) and 1862 AD (0.842), whereas the lowest value of PLI is found to be during 1906 AD (0.36). Therefore, the PLI value < 1 indicates perfection in the lake during ca 1839–2003 AD. According to Pearson’s correlation coefficient, the strong positive correlation at the Renuka Lake is found to be among Cr–Cu, Ni, Pb; Cu–Zn, Ni, Pb; Zn–Ni, Pb and Ni–Pb, while the moderate positive correlation is observed between Cr–Zn and Co–Cr, Cu, Ni, Pb (Table 6). The concentration of heavy metals is also compared with the background level of the upper crust and crustal rocks as suggested by Taylor and Mclennan (1985) and Turekian and Wedepohl (1961). The average concentration of Mn during ca 1839–2003 AD (619.57–3562.52 µg/g (avg. 1297.22 ± 767.86 µg/g)) at Renuka Lake is found to be higher than average shale (850 µg/g) and carbonate rocks (1100 µg/g).
The average content of Cr [5–62 µg/g (avg. 24.45 ± 17.12 µg/g)], Cu [11–40 µg/g (avg. 20.9 ± 8.64 µg/g)] and Ni [11–33 µg/g (avg. 18.8 ± 5.75 µg/g)] during the said time period is found to be more than the rocks of the upper crust and carbonate rocks. The Pb [21–27 µg/g (avg. 22.8 ± 1.75 µg/g)] in the sediment profile of the Renuka Lake is observed more as compared to the background concentration of the average shale, carbonate rocks and upper crust. On the other hand, the concentration of Co [7–51 µg/g (avg. 20.25 ± 13.14 µg/g)] and Zn [43–127 µg/g (avg. 66.25 ± 20.44 µg/g)] is determined higher than the background concentration of these metals found to be in the carbonate rocks, average shale, upper crust and crustal average. However, due to excess of carbonate weathering in the surrounding of the Renuka Lake, the average metal concentration in the core sediment is noticed much higher than the background values of carbonate rocks.
Comparison of the Renuka with other lakes
The metals concentration of the Renuka Lake has also been compared with other Himalayan lakes and the lakes situated at the lower regions of India. The Mn content in the Renuka Lake is observed more than the other lakes, whereas the concentration of Cr is found to be more than the Vartur, Rewalsar, Nainital and Naukuchiatal Lake (Jumbe and Nandini 2009; Meena et al. 2017, etc.). The Zn in the lake is noticed to be more than the Nainital, Bhimtal, Jannapura and Rewalsar Lake, but having less concentration as compared to the rest of lakes. The concentration of Ni in Renuka Lake is found to be more than Kolleru Lake, but less than Chilka, Rewalsar, Nainital Lake, etc.; whereas Pb in the Renuka Lake is noticed more than the Rewalsar, Nainital, Kolleru and Jannapura, but less than Chilka, Bhimtal, Vartur, Naukuchiatal and Akkulam-Veli Lake. On the other hand, the concentration of Co in the Renuka Lake is observed less than Vartur and Naukuchiatal Lake, but more than other lakes (Table 7).
Conclusions
The present study provides the heavy metals (Pb, Cr, Co, Cu, Zn, Ni, Mn) contamination history of the Renuka Lake for the last 164 years (ca 1839–2003 AD), based on 210Pb and 137Cs radionuclide-dated sediments core. The metals enrichment in the lake is associated with the dominance of natural factor, but the significant contribution is also made by anthropogenic input in the recent past. The enrichment of metals is controlled by detrital quaternary sediments, carbonate lithology, lake-based sulfide minerals and organic matter. The detrital quaternary sediments (containing K-feldspar, plagioclase and carbonate minerals), shale and lake-based sulfide minerals are the potential source of Pb in the Renuka Lake. The man-made sources of Pb include surrounding vehicular emission, acid batteries, paints, sewage and leaded petrol. The Pb in the lake sediments bounds with organic matter, CaCO3, sulfide minerals, and therefore remains less mobile. The decreasing trend of the Pb in Renuka Lake during the post-1900 AD might be due to decreased natural and anthropogenic input to the lake after the preventive actions. Overall, the Cr shows an increasing trend during the last 164 years of contamination history. The quartzite and chlorite minerals are a potential source of Cr in Renuka Lake as these minerals are rich of Cr and found abundantly in the surroundings of the Renuka Lake. The organic matter and iron-bearing minerals bonded the Cr in the Renuka Lake. Higher concentration of the Cr during ca 1939 and ca 1883 appears to be due to higher input of the catchment wash or debris flow to the lake. The increasing trend of the Cr since the last 164 years suggests increased anthropogenic input that slightly decreased during the post-1950 due to preventive measures. The low concentration of Co (20 µg/g) in Renuka Lake water, rather than its core sediments suggests that the Co is bounding with the organic matter, because at decreasing Eh, cobalt prefers to associate with the organic matters and sulfide. Higher concentration of the Co during 1939 and 1883 AD seems to appear due to higher input of the catchment wash or debris flow to the lake and the anthropogenic activities also increased the Co concentration during the last 164 years. The concentration of Cu (10 µg/g) in the lake water appeared to be much lesser than the sediments; possibly due to high organic matter in everglade nature of the Renuka Lake that bounds Cu with sediments. The overall trend of Cu is found to be decreasing over the last 164 years that may be due to decreased catchment input. However, the Renuka Lake possesses higher Cu concentration than the permissible limit that may cause health problem for flora and fauna of the lake. The surrounding rocks and the anthropogenic activities (metal plating and related works) are the primary source of Zn in the lake and its concentration not increased significantly during the last hundred years, probably due to less use of Zn in the region. The higher carbonate content in the Renuka Lake seems to be a major factor of Zn enrichment in sediments in the form of zinc carbonate. The higher concentration of Ni during 1883 AD appears to be a result of excess catchment wash; whereas, higher values during 1975 AD seem to be the result of metallic waste dumping in the lake. The shale and dolomite contain a higher concentration of the Mn and the dominance of these in the surroundings of the Renuka Lake is a potential source of this metal. The concentration of Mn is significantly influenced by redox condition and easily mobilized under the anoxic condition and instead participated in the redox reaction. The anthropogenic sources of Mn may include burning of fossil fuels and sewage.
The contamination factor (Cf) has indicated low to the considerable level of contamination, whereas the degree of contamination (Cd) revealed a low level of contamination in the Renuka Lake. However, the geo-accumulation index (Igeo) marked the lake uncontaminated to moderately contaminated and on the other hand, the pollution load index (PLI) has shown some signs of contamination in the Renuka Lake.
Most of the metals are having strong and moderate positive correlations, which suggest their similar sources, probably due to weathering of the surrounding rocks and anthropogenic activities around the Renuka Lake. The heavy metal contents of Renuka Lake also varied from other Himalayan lakes and those situated in the lower regions of this country. The Mn and Cr show higher concentration as compared to average shale, crustal average and other lakes. The local lithology and lake-based anthropogenic activities dominate the metal loading in the Renuka Lake. However, the lake requires further detail study to understand the degradation by various sources.
References
Ali MM, Ali ML, Islamc MS, Rahmanda MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli river, Bangladesh. Environ Nanotechnol Monit Manag 5:27–35
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr (III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99(6):1578–1583
BIS (2012) Drinking water specification, 2nd edn. Bureau of Indian Standards, New Delhi
Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I (2016) Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol 35:107–115
Chambers BJ, Lord EI, Nicholson FA, Smith KA (1999) Predicting nitrogen availability and losses following application of organic manures to arable land: MANNER. Soil Use Manag 15(3):137–143
Chen Y, Xiao F, Liu Y, Wang D, Yang M, Bai H, Zhang J (2015) Occurrence and control of manganese in a large scale water treatment plant. Front Environ Sci Eng 9(1):66–72
Chillrud SN, Bopp RF, Simpson HJ, Ross JM, Shuster EL, Chaky DA, Yarme A (1999) Twentieth century atmospheric metal fluxes into central park lake, New York City. Environ Sci Technol 33(5):657–662
Das BK, Haake BG (2003) Geochemistry of Rewalsar Lake sediment, Lesser Himalaya, India: implications for source-area weathering, provenance and tectonic setting. Geosci J 7(4):299–312
Das BK, Kaur P (2001) Major ion chemistry of Renuka Lake and weathering processes, Sirmaur District, Himachal Pradesh, India. Environ Geol 40:908–917
Das BK, Gaye B, Kaur P (2008) Geochemistry of Renuka lake and wetland sediments, lesser Himalaya (India): implication for source-area weathering, provenance and tectonic setting. Environ Geol 54:147–163
Davison W (1993) Iron and manganese in lakes. Earth Sci Rev 34(2):119–163
Ding Y, Qin B, Xu H, Wang X (2016) Effects of sediment and turbulence on alkaline phosphatase activity and photosynthetic activity of phytoplankton in the shallow hyper-eutrophic Lake Taihu, China. Environ Sci Pollut Res 23(16):16183–16193
EPA (2012) Chromium in drinking water. Environmental Protection Agency, Washington
Febriana SA, Jungbauer F, Soebono H, Coenraads PJ (2012) Inventory of the chemicals and the exposure of the workers’ skin to these at two leather factories in Indonesia. Int Arch Occup Environ Health 85(5):517–526
Gaury PK, Meena NK, Mahajan AK (2018) Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environ Monit Assess 190(2):84
Goldberg ED (1963) Geochronology with 210Pb radioactive dating. In: International atomic energy agency symposium proceedings, Vienna 1962 (1963):121–131
Greenwood NN, Earnshaw A (1984) Germanium, tin and lead. Chemistry of the elements. Pergamon Press, Oxford, pp 427–465
Guo T, DeLaune RD, Patrick WH (1997) The influence of sediment redox chemistry on chemically active forms of arsenic, cadmium, chromium, and zinc in estuarine sediment. Environ Int 23:305–316
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Resour 110:49–55
Heinrichs H, Schulz-Dobrick B, Wedepohl KH (1980) Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim Cosmochim Acta 44(10):1519–1533
Hylander LD, Meili M, Oliveira LJ, Silva EDC, Guimarães JR, Araujo DM, Neves RP, Stachiw R, Barros AJ, Silva GD (2000) Relationship of mercury with aluminum, iron and manganese oxy-hydroxides in sediments from the Alto Pantanal, Brazil. Sci Total Environ 260(1–3):97–107
Jacobs L, Emerson S, Skei J (1985) Partitioning and transport of metals across the O2H2S interface in a permanently anoxic basin: Framvaren Fjord, Norway. Geochim Cosmochim Acta 49(6):1433–1444
Jumbe AS, Nandini N (2009) Impact assessment of heavy metals pollution of Vartur Lake, Bangalore. J Appl Nat Sci 1(1):53–61
Khadka UR, Ramanathan AL (2013) Major ion composition and seasonal variation in the Lesser Himalayan lake: Case of Begnas Lake of the Pokhara Valley, Nepal. Arabian J Geosci 6(11):4191–4206
Khan TA (2011) Trace elements in the drinking water and their possible health effects in Aligarh City, India. J Water Resour Prot 3:522–530
Khan DA, Mushtaq S, Khan FA, Khan MQ (2013) Toxic effects of chromium on tannery workers at Sialkot (Pakistan). Toxicol Ind Health 29(2):209–215
Khanna PP, Saini NK, Mukherjee PK, Purohit KK (2009) An appraisal of ICP-MS technique for determination of REEs: long term QC assessment of silicate rock analysis. Himal Geol 30(1):95–99
Koilakos DI (2017) Aspects of hexavalent chromium pollution of Thebes plain aquifer, Boeotia, Greece. Water 9(8):611
Kumar P, Kumar P (2019) Removal of cadmium (Cd-II) from aqueous solution using gas industry-based adsorbent. SN Appl Sci 1(4):365
Kumar P, Meena NK, Mahajan AK (2019a) Major ion chemistry, catchment weathering and water quality of Renuka Lake, north-west Himalaya, India. Environ Earth Sci 78(10):319
Kumar P, Mahajan AK, Meena NK (2019b) Evaluation of trophic status and its limiting factors in the Renuka Lake of Lesser Himalaya, India. Environ Monit Assess 191(2):105
Leopold EN, Baussand P, Emmanuel EG (2012) Heavy metals accumulation in sediment cores of the Municipal Lake of Yaounde, Cameroon. Glob J Environ Res 6(3):100–110
Mackay D (2001) Multimedia environmental models: the fugacity approach, 2nd edn. CRC Press, Boca Raton, pp 39–44
Manoj K, Padhy PK (2014) Distribution, enrichment and ecological risk assessment of six elements in bed sediments of a tropical river, Chottanagpur Plateau: a spatial and temporal appraisal. J Environ Prot 5:1419–1434
Meena NK, Prakasam M, Bhushan R, Sarkar S, Diwate P, Banerji U (2017) Last-five-decade heavy metal pollution records from the Rewalsar Lake, Himachal Pradesh, India. Environ Earth Sci 76:39
Muller G (1969) Index of geo-accumulation in the sediments of the Rhine river. GeoJournal 2:108–118
Nadaska G, Lesny J, Michalik I (2010) Environmental aspect of manganese chemistry. Hungarian Elect J Sci 100702:1–6
Nasr SM, Okbah MA, Kasem SM (2006) Environmental assessment of heavy metal pollution in bottom sediments of Aden Port, Yemen. Int J Oceans Oceanogr 1(1):99–109
Oliveira SMBD, Pessenda LCR, Favaro DIT, Babinski M (2012) A 2400-year record of trace metal loading in lake sediments of Lagoa Vermelha, southeastern Brazil. J S Am Earth Sci 33(1):1–7
Padma S, Periakali P (1999) Physico-chemical and geochemical studies in Pulicat Lake, east coast of India. Indian J Mar Sci 28:434–437
Panda D, Subramanian V, Panigrahy RC (1995) Geochemical fractionation of heavy metals in Chilka Lake (east coast of India)—a tropical coastal lagoon. Environ Geol 26(4):199–210
Pius A, Jerome C, Sharma N (2012) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184(7):4067–4077
Purushothaman P, Chakrapani GJ (2012) Trace metals biogeochemistry of Kumaun Himalayan Lakes, Uttarakhand, India. Environ Monit Assess 184(5):2947–2965
Puttaiah ET, Kiran BR (2007) Heavy metal transport in a sewage fed lake of Karnataka, India. In: Proceeding of Taal: The 12th world lake conference pp 347–354
Randive KR, Korakoppa MM, Muley SV, Varade AM, Khandare HW, Lanjewar SG, Tiwari RR, Aradhi KK (2015) Paragenesis of Cr-rich muscovite and chlorite in green-mica quartzites of Saigaon-Palasgaon area, Western Bastar Craton, India. J Earth Syst Sci 124(1):213–225
Rashid MA (1974) Absorption of metals on sedimentary and peat humic acids. Chem Geol 13(2):115–123
Rice KC, Conko KM, Hornberger GM (2002) Anthropogenic sources of arsenic and copper to sediments in a suburban lake, Northern Virginia. Environ Sci Technol 36(23):4962–4967
Robbins JA, Edgington DN (1975) Determination of recent sedimentation rates in Lake Michigan using Pb-210 and Cs-137. Geochimica et Cosmochimica Acta 39(3):285–304
Salomons W (1998) Biogeodynamics of contaminated sediments and soils: perspectives for future research. J Geochem Explor 62:37–40
Sarkar S, Prakasam M, Banerji US, Bhushan R, Gaury PK, Meena NK (2016) Rapid sedimentation history of Rewalsar Lake, Lesser Himalaya, India during the last fifty years—estimated using 137Cs and 210Pb dating techniques: a comparative study with other North-Western Himalayan Lakes. Himal Geol 37(1):1–7
Sekhar KC, Chary NS, Kamala CT, Raj DSS, Rao AS (2004) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru Lake by edible fish. Environ Int 29:1001–1008
Sheela AM, Letha J, Joseph S, Thomas J (2012) Assessment of heavy metal contamination in coastal lake sediments associated with urbanization: Southern Kerala, India. Lakes Reserv Res Manag 17:97–112
Singh O, Sharma MK (2012) Water quality and eutrophication status of the Renuka Lake, District Sirmaur (HP). J Indian Water Resour Soc 32(3–4):1–7
Singh KP, Malik A, Sinha S, Singh VK, Murthy RC (2005) Estimation of source of heavy metal contamination in sediments of Gomti River (India) using principal component analysis. Water Air Soil Pollut 166(1–4):321–341
Smily JRMB, Sumithra PA (2017) Optimization of chromium biosorption by fungal adsorbent, Trichoderma sp. BSCR1 and its desorption studies. HAYATI J Biosci 24(2):65–67
Somayajulu BLK, Bhushan R, Sarkar A, Burr GS, Jull AJT (1999) Sediment deposition rates on the continental margins of the eastern Arabian Sea using 210Pb, 137Cs and 14C. Sci Total Environ 237:429–439
Staelens N, Parkpian P, Polprasert C (2000) Assessment of metal speciation evolution in sewage sludge dewatered in vertical flow reed beds using a sequential extraction scheme. Chem Speciat Bioavailab 12(3):97–107
Sun Z, Mou X, Tong C, Wang C, Xie Z, Song H, Sun W, Lv Y (2015) Spatial variations and bioaccumulation of heavy metals in intertidal zone of the Yellow River estuary, China. CATENA 126:43–52
Taylor SR, Mclennan SM (1985) The continental crust: its composition and evolution. Blackwell, Oxford, p 312
Tobiason JE, Bazilio A, Goodwill J, Mai X, Nguyen C (2016) Manganese removal from drinking water sources. Curr Pollut Rep 2:168–177
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problem in the assessment of heavy metals level in estuaries and the formation of a pollution index. Helgolander Meeresunterssuchungen 33:566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72(2):175–192
Ure AM, Berrow ML (1982) Environmental chemistry. Spec Period Rep 2:94–204
Wang X, Yang H, Gong P, Zhao X, Wu G, Turner S, Yao T (2010) One century sedimentary records of polycyclic aromatic hydrocarbons, mercury and trace elements in the Qinghai Lake, Tibetan Plateau. Environ Pollut 158:3065–3070
Wedepohl KK (1978) Handbook of geochemistry, vol. II/5. Heidelberg–New York, Springer–Verlag Berlin, p 1546
Wu JC, Zhang JF, Sun YY, Wang XR (2009) Water environmental chemistry. China Forestry Publishing, Beijing
Xu B, Yang X, Gu Z, Zhang Y, Chen Y, Lv Y (2009) The trend and extent of heavy metal accumulation over last one hundred years in the Liaodong Bay, China. Chemosphere 75(4):442–446
Yu KC, Tsai LJ, Chen SH, Ho ST (2001) Chemical binding of heavy metals in anoxic river sediments. Water Res 35:4086–4094
Zeng H, Wu J, Liu W (2014) Two-century sedimentary record of heavy metal pollution from Lake Sayram: a deep mountain lake in central Tianshan, China. Quatern Int 321:125–131
Zhao S, Shi X, Li C, Zhang H, Wu Y (2014) Seasonal variation of heavy metals in sediment of Lake Ulansuhai, China. Chem Ecol 30(1):1–14
Acknowledgements
The authors thank the Hon’ble Vice Chancellor, Central University of Himachal Pradesh, India, for their logistic support. The authors also thank the Director, Wadia Institute of Himalayan Geology (WIHG), Dehradun. Ravi Bhushan acknowledges the Director Physical Research Laboratory (PRL), Ahmedabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, P., Meena, N.K., Diwate, P. et al. The heavy metal contamination history during ca 1839–2003 AD from Renuka Lake of Lesser Himalaya, Himachal Pradesh, India. Environ Earth Sci 78, 549 (2019). https://doi.org/10.1007/s12665-019-8519-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8519-2