Abstract
The sources impacting the water chemistry of the Nakdong River (NR) in South Korea were investigated in order to examine the pollution mechanism including the fate and transport of the contaminants and how much such sources may affect its main channel of water resource. Water samples were collected between 2007 and 2008 from 8 sites along a 510 km downstream transect of the NR, and chemical and isotopic compositions of the water samples were evaluated to identify natural and anthropogenic sources contributing to the water chemistry of the NR. The results showed that the major ion concentrations were mainly controlled by chemical weathering that occurred in the watershed, in which a silicate weathering is more dominant than a carbonate weathering. The 87Sr/86Sr ratios of the water samples were in a range from 0.71043 to 0.71520 within those of the Mesozoic volcanogenic sedimentary rocks long developed in the watershed, thereby supporting the fact that the water chemistry is governed by a chemical weathering. The δ34S values varied in a narrow range of 1.8–3.1‰, regardless of spatial and seasonal variations. Mass balance calculations indicated that the contributions of chemical weathering and anthropogenic sources during summer time increased along a downstream transect, from 67.3 ± 1.3 to 73.6 ± 0.5% and from 6.0 to 15.7%, respectively. In contrast, the contribution of chemical weathering during winter time decreased from 82.7 ± 0.8 to 72.5 ± 0.3%, while anthropogenic contribution increased up to 22.2%. These results indicated that the water chemistry of the NR was mainly caused by a chemical weathering, followed by anthropogenic inputs and rainwater. This study will provide baseline information for comparing the water quality issue before and after the implementation of the Four Rivers Restoration Project of South Korea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been a big issue in numerous studies to quantify mineralogical sources of Ca and Mg in rivers because the release of Ca and Mg from minerals during chemical weathering is closely related to biogeochemical processes and global carbon cycle (e.g., Berner et al. 1983; Likens et al. 1996; White et al. 2005). However, this is quite difficult to explain how the chemical weathering is related to various type of rocks that contain multiple Ca- and Mg-bearing minerals, and therefore, most studies have indirectly constrained source apportionment using major ion balances or strontium isotope ratios (87Sr/86Sr) (Gaillardet et al. 1999; Miller et al. 1993; Négrel et al. 1993; Spence and Telmer 2005; Wu et al. 2008). Elemental and isotope tracers are useful because they reveal reaction pathways, transport processes, and source apportionments that may otherwise be difficult to discern with normative mineral calculations and other bulk elemental accounting methods.
The sulfate (SO4) in rivers is generally derived from soil sulfur (Balík et al. 2007; Mayer et al. 1995; Stevenson and Cole 1999; Yu and Park 2004); total S ≈ 10–2500 mg/L, atmospheric inputs, [SO4] ≈ 0.3–16 mg/L, sulfide oxidation, and anthropogenic sources from chemical fertilizers, domestic wastewater, and detergents, [SO4] ≈ 1.0–130 mg/L. Their relative proportions can determine the riverine sulfur isotopic composition (δ34S) (Krouse and Mayer 2000; Mayer et al. 1995; Yu and Park 2004); soil has δ34S values of −1.0 to 8.0‰, precipitation of −3.9 to 8.2‰ (n = 23), sulfide oxidation of −35 to 8.0‰, detergents of 18.0 to 31.3‰ (n = 5), domestic wastewater of 15.1 to 24.2‰ (n = 2), and fertilizers of −7.6 to −2.6‰ (n = 5), respectively. Thus, δ34S value combined with SO4 concentration can be a powerful tracer to identify the riverine SO4 sources (Grasby et al. 1997; Mayer 2005).
Likewise, boron (B), which is abundant in coal, [B] ≈ 5–400 mg/L (Swaine 1994; Swaine and Goodarzi 1995) and man-made materials such as detergents and chemical fertilizers, 0.5–100% as a form of various boron compounds (Ghavanloughajar 2015), can be used to apportion their sources in river waters because it is not affected by adsorption or microbial activity in rivers, and is not even removed by traditional wastewater treatment process (Chetelat and Gaillardet 2005; Petelet-Giraud et al. 2009; Sakata et al. 2010). Although numerous studies have suggested an excess of B in surface/subsurface waters due to anthropogenic inputs including sewage, urban irrigation, and industrial effluent (Chetelat and Gaillardet 2005; Hasenmueller and Criss 2013; Lee et al. 2005), chemical weathering of basalts and carbonate rocks was a major contributor (Stefánsson and Gíslason 2001; You et al. 1993).
The Nakdong River (NR) is the longest river in South Korea and flows through various land areas such as national industrial districts, a great number of wastewater treatment plants, agricultural areas, and metropolitan areas, and eventually reaches to the South Sea, Korea. In spite of the socioeconomic importance of the NR as a major source of drinking water supply to over 6.5 million populations in the South-East Korean Peninsula, however, no up-to-date watershed-scale information is available on the chemical and isotopic characteristics of the rivers. Only a few studies investigated regional water contaminations focusing on tributaries draining industrial districts and agricultural areas (Chung et al. 1997, 1998; Lee et al. 2005; Seo et al. 2003). In a recent event, the Four Rivers Restoration Project, a part of the “Green New Deal” policy that launched in 2009, was commenced and finished at the Korean major rivers including the NR until 2013. During this mega civil project, many weirs were constructed in a number of locations of the four rivers, and many tributaries were subject to restoration.
The study was conducted on a watershed scale, quantifying the seasonal and spatial variations of chemical and isotopic compositions of the NR waters, and identifying various sources affecting the river water chemistry. This study will provide baseline information on the overall characteristics of the NR water chemistry and further comparison with the water quality in NR after the implementation of the ongoing controversial Four Rivers Restoration Project. Furthermore, it will provide a basis for future biogeochemical watershed-scale investigations in the NR.
Materials and methods
Description of the study area
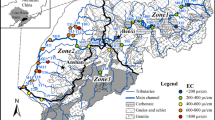
The NR, with a length of 510 km, is the longest river in South Korea and has the second-largest drainage area of 23,384 km2 (Nakdong River Flood Control Office 2016). The discharge rates during the sampling activities between August 2007 and February 2008 were 1248 and 611 m3/s, respectively (Water Resources Management Information System 2016). The NR flows from the northeastern to southeastern area of South Korea. There are two dam structures such as Andong and Imha in the upstream reaches and three dams such as Hapcheon, Namgang, and Milyang at the main tributaries in the upper to lower reaches (Fig. 1). The NR land cover was estimated using ArcGIS 10 (Esri, Redlands, CA, USA), which mainly consists of areas including forest (69%), agricultural (23%), river water (5%), and residential/business and industrial (4%) zones (Fig. 1a). The proportion of agricultural area increases from 14% in the upper reach to 23% in the lower reach, whereas that of forest decreases from 81 to 69%. Two national industrial complex towns (Gumi and Daegu) are located near the main channel in the middle reaches, and scores of small industrial and agricultural areas are included near the Nakdong watershed (Korea Industrial Complex Corp. 2016) (Fig. 1a).
The NR basin is mainly composed of Precambrian metamorphic rocks, Mesozoic granitic rocks, and Mesozoic clastic sedimentary and volcanic rocks (e.g., conglomerate, sandstone, mudstone, andesite, rhyolite, tuff, tuffaceous sandstone/conglomerate) (Fig. 1b) (The Geological Society of Korea 1999). The proportion of Mesozoic sedimentary and volcanic rocks is over 60% of the outcrop area and greatly increases in the mid- and lower reaches. Paleozoic carbonate rock is barely distributed in the upper reaches of the NR watershed (Fig. 1b).
South Korea has a temperate climate with four seasons, and approximately 70% of the annual precipitation is concentrated from July through September during the summer season (Korea Meteorological Administration 2015). The winter season is typically from late November through early March.
Sampling and analysis
Water samples were collected at the eight sites along a downstream transect during sampling activities in Fig. 1. The sampling periods, characterized with high- and low-water stages, were likely to show water chemistry indicative of the river. All sampling sites were carefully selected to reflect impact from the dominant land use in order to avoid anthropogenic contamination and direct influences from small tributaries. Water temperature, pH, and electrical conductivity were measured in situ, using an Orion 5-star meter equipped with an Orion combination epoxy pH electrode and DuraProbe 4-electrode conductivity cells; uncertainties were ±0.1 °C, ±0.02 pH, and ±0.01 μS/cm, respectively. Water samples were introduced through 0.45-μm mixed cellulose ester membrane filters and collected in acid-cleaned Nalgene LDPE bottles. When analyzing cations, the 87Sr/86Sr were acidified to pH < 2 in the field, using ultrapure HNO3 solution. To analyze the δ34S of SO4, the samples were filtered with 0.45-μm membrane filters and transferred to a 4-L sterilized container. All of the water samples were stored at 4 °C until the analysis was finished up to a week.
Alkalinity was determined in a laboratory with 0.01 N HCl using the Gran titration method (Gran 1952). Anions and cations were analyzed with ion chromatography (IC) system (Dionex ICS-1500 IC, Sunnyvale, CA, USA) at the Korea Institute of Geoscience and Mineral Resources (KIGAM) and with inductively coupled plasma–optical spectrometer (ICP-OES) (PerkinElmer Optima 4300DU ICP-OES, Akron, OH, USA) at the Korea Basic Science Institute (KBSI). Repeat analyses of two international standards (SLRS-4 and TMDW-A) yielded external reproducibility values <±5%. Most of the water samples showed a percent charge balance error [CBE (%) = (TZ+ − TZ−)/(TZ+ + TZ−) × 100] within the range of ±8%, where TZ+ = Na+ + K+ + 2Ca2+ + 2Mg2+ and TZ− = Cl− + 2SO4 2− + NO3 − + HCO3 −. The ratio of 87Sr/86Sr was determined using multicollector inductively coupled plasma–mass spectrometry (VG Elemental Axiom MC-ICP-MS, Winsford, UK) at the KBSI. The analytical reproducibility of the ratios of 87Sr/86Sr by multiple repeating analyses of NBS987 was 0.710247 ± 0.000020 (2σ, n = 24), and the total blank of the Sr was negligible. To analyze the δ34S of SO4, the content of SO4 in the water samples was precipitated as a form of BaSO4 using 10% BaCl2 solution. The precipitate was recovered by passing the treated samples through a 0.45-μm membrane filter, being washed with hot distilled water, and thoroughly dried at room temperature. Following the method reported by Yanagisawa and Sakai (1983), SO2 gas was extracted by the thermal decomposition of the precipitate. The δ34S was determined, using an isotope ratio mass spectrometer (IRMS) (Micromass Optima, Ringoes, NJ, USA) at the KBSI, and is expressed in delta (δ) notation relative to Canyon Diablo Troilite (CDT), where δ34S (‰) = [(34S/32S)sample/(34S/32S)standard − 1] × 1000. The analytical reproducibility for the δ34S was ±0.2‰.
Correction of atmospheric inputs
The major ions in rivers stem from atmospheric inputs, chemical weathering, and anthropogenic inputs, and numerous studies have quantitatively calculated the contributions of such three sources to river water chemistry (Fan et al. 2014; Gaillardet et al. 1999; Shin et al. 2011). The previous studies have used Mg/Na and Ca/Na ratios for carbonate, silicate, and evaporite mineral end members, as suggested by Gaillardet et al. (1999), and suggested that the water chemistry was mainly characterized by the representative rock types in their respective drainage areas; anthropogenic input was ignored or was not a main source. The contribution of chemical weathering can be estimated after extracting the contribution of atmospheric input.
Assuming that the lowest Cl concentration measured in this study was entirely derived from precipitation, the contribution of an element derived from atmospheric input to river water (R) could be determined as follows:
where E represents elemental concentration measured in the precipitation. The lowest Clriver was 55 μM, and the annual average Clprecipitation value used was 43.5 μM for 1999 through 2006 in South Korea [Ministry of Environment (MOE) 2006]. The lowest Clriver concentration in this study was much lower than that of water samples collected from six major rivers in South Korea during the similar sampling period, which was 88 μM (Shin et al. 2011). Therefore, the lowest Clriver concentration in this study could be regarded as representative of precipitation, which was derived Cl in the NR, and the ratio of each element to Cl in precipitation is as follows: Ca/Cl = 0.57, K/Cl = 0.26, Mg/Cl = 0.22, Na/Cl = 0.69, SO4/Cl = 1.07. Corrected concentrations are shown in Table 1 and are denoted using an asterisk (e.g., Ca*, Mg*, Na*, Cl*, and \({\text{SO}}_{4}^{*}\)).
Results and discussion
The chemical compositions and isotopic ratios (δ34S and 87Sr/86Sr) of the water samples and the variables measured in the field are represented in Table 1. On average, pH was somewhat lower in the summer time (average of 6.4 ± 0.1) than in the winter time (average of 7.0 ± 0.7), showing little notable variation. Its range was from 6.3 to 6.5 in the summer time. In the winter time, the pH gradually increased from 6.4 up to 8.4 in accordance with the distance of downstream. The total dissolved solids (TDS = Na+ + K+ + Ca2+ + Mg2+ + Cl− + SO4 2− + NO3 − + HCO3 −) for the water samples ranged from 28.4 to 60.7 mg/L (average of 44.3 ± 9.0 mg/L) in the summer time and from 53.7 to 76.7 mg/L (average of 70.6 ± 7.6 mg/L) in the winter time.
Figure 2 shows the spatial and seasonal variations in major ion concentrations with the distance from the upper reaches. The confluence of water flowing from the two dams in the most upper reaches is hereafter considered pristine water, and the distance shown represents the distance from the confluence. Most of the water samples showed higher ion concentrations in winter than in summer. Overall, the ion concentrations tended to increase along a downstream, except for the NO3 concentration in summer. It was found that a water sample collected in winter at the middle reaches (NR-4) included markedly high ion concentrations compared to those of other samples in the same season. Water types were mainly Ca–HCO3–(Cl, SO4) in summer, regardless of spatial variations, but those in winter changed from Ca–HCO3 at the most upper reaches to Na–(Cl, SO4) at the lower reaches (not shown).
The δ34S of the water samples ranged from 0.9 to 4.0‰, with an average of 2.5 ± 0.7‰. On average, the δ34S was slightly higher in summer with 2.9 ± 0.5‰ than in winter with 2.2 ± 0.6‰. In summer, the δ34S tended to be lowered in accordance with the distance of downstream, while in winter, the trend oppositely occurred (Fig. 2g). The δ34S values of both seasons were similar at the middle and the lower reaches of the river. The 87Sr/86Sr ratio, which was only determined for water samples collected in summer, ranged from 0.71043 to 0.71520 (0.71307 ± 0.00177 in average). The ratio exhibited obvious spatial variation. Specifically, the ratio was the lowest at the most upper reaches, with the highest value occurring at ca. 100 km from pristine water, and then gradually decreased toward the lower reaches. Nonetheless, the 87Sr/86Sr ratios were within the range of those of Cenozoic volcanic-derived sedimentary and volcanic rocks and Mesozoic silicate rock reported in South Korea, 0.70402–0.75594 (Kim et al. 2013).
Chemical weathering as a main source of dissolved ions
Correlation between major ions
After the correction on the atmospheric input, Ca*/Na* ranged from 0.74 to 1.74 in summer and from 0.36 to 1.29 in winter, and Mg*/Na* ranged from 0.28 to 0.62 in summer and from 0.14 to 0.40 in winter. On a plot of Mg*/Na* and Ca*/Na*, the water samples fell between the silicate and carbonate end members supported by Gaillardet et al. (1999), and most samples were plotted toward silicates (Fig. 3a). This occurrence was observed more evidently in winter than in summer when rainwater contributed more substantially to water chemistry. The Ca*/Na* and Mg*/Na* ratios of the water samples are between 0.29 and 1.42, and between 0.13 and 0.48, respectively, which are consistent with the previous study (Shin et al. 2011). It indicates that water chemistry is mainly controlled by both the weathering of silicates and carbonates and that silicate weathering is more dominant in the NR.
Plots of Mg*/Na* versus Ca*/Na* (a) and 2(Ca + Mg) versus HCO3 + 2SO4 (b) for the water samples [the three end members in a are from Gaillardet et al. (1999). Each sample name was simplified as the numbers near the symbol in b without prefix (NR-)]
Theoretically, carbonic acid in the soil zone reacts with carbonate minerals in the watershed, resulting in 2 mol of HCO3 for each mole of Ca and Mg (Appelo and Postma 2005) as follows: H2O + CO2 + CaMg(CO3)2 → Ca2+ + Mg2+ + 4HCO3 −. Furthermore, the oxidation of sulfide minerals in the presence of oxygen and water generates sulfuric acid, which participates in chemical weathering (Calmels et al. 2007). In this study, the water samples had 1.75 of (Ca + Mg)/HCO3 ratio. Overall, the water samples were near, but plotted below, the binary line of 2(Ca + Mg) and HCO3 + 2SO4 (Fig. 3b). Thus, it appeared that the water chemistry was mainly determined by chemical weathering arising from a combination of carbonic and sulfuric acid generated in the watershed, attributing to an additional source (e.g., anthropogenic source).
Variation in 87Sr/86Sr ratio with the distance of downstream
The 87Sr/86Sr ratios for water samples in the river ranged from 0.7104 to 0.7152, which are similar to those for stream and groundwater within this watershed (0.70690–0.71332; Song et al. 2014). Although the Nakdong River drains Precambrian metamorphic rocks, Mesozoic granitic rocks, and Mesozoic clastic sedimentary and volcanic rocks, most of the 87Sr/86Sr ratios for water samples in this study are within those of the Cenozoic and Mesozoic granites (0.70514–0.73359; Cheong and Chang 1996; Kim and Kim 1997; Na et al. 1997) but higher than those of the Cenozoic volcanic-derived sedimentary and volcanic rocks (0.70402–0.70623; Kim et al. 1999). Because precipitation has a significantly low Sr concentration (<0.88 μg/L; Miller et al. 1993) and the lowest Sr concentration in the NR-1 sample is approximately 90 times higher than that of precipitation, riverine Sr concentrations from the upper to the lower reaches, even prior to NR-1, are attributed to chemical weathering and/or anthropogenic inputs. That is, 87Sr/86Sr ratios of the water samples can be explained by weathering of the Mesozoic volcanogenic sedimentary rocks and the granitic rocks. Given that potential anthropogenic sources in South Korea have 87Sr/86Sr ratios as follows: 0.71111–0.71260 for detergent (n = 5), 0.71004–0.71455 for chemical fertilizer (n = 5), and 0.71453–0.71629 for sewage (e.g., Lee et al. 2014), the 87Sr/86Sr ratios of water samples indicates that the Nakdong River would be also affected by those anthropogenic sources.
Effects of anthropogenic inputs on water chemistry
Increase of anion in accordance with the distance of downstream
Given that the water chemistry of surface water is entirely influenced by rainwater input, the elemental ratios in surface water should be the same as those of seawater (Sarin et al. 1989; Stallard and Edmond 1983). The Na/Cl ratio of seawater reported by Zhang et al. (1995) was 0.85, and the precipitation monitored in South Korea from 1999 to 2006 was Cl and Na of 43.5 and 30.2 μM in average, respectively (MOE 2006), which yielded a correlation of Na = (0.60 × Cl) + 4.09. In this study, the water samples could be expressed by the equation; Na = (1.75 × Cl) + 33.0 (R 2 = 0.96). With the fact that relatively high Na/Cl ratio of the water samples compared to both seawater and local precipitation, and high Na and Cl concentrations in the water samples with 439 and 799 μM, respectively, it seemed like that the water chemistry was influenced by some anthropogenic factors in the watershed because there were no evaporites in South Korea (Chough et al. 2000).
In winter, concentrations of Cl, NO3, and SO4 increased rapidly from the upper reaches through the middle (NR-1 to NR-4) and then increased slightly or decreased to the lower (NR-4 to NR-8). However, for water samples collected in summer having relatively lower ion concentrations, this trend was not obvious, probably due to the dilution by heavy rain. The overall NO3 concentration became lower with passage through the watershed, except for NR-5. Because atmospheric inputs and chemical weathering were not accountable for spatial and seasonal variations in ion concentrations, particularly elevated Cl concentration toward the downstream region, it could be induced that anthropogenic inputs related to land-use patterns might impact the river water chemistry. As earlier discussed, the relationship between land-use patterns and the water chemistry would be of help in precisely identifying the sources contributing to the water chemistry, and its spatial and seasonal variations.
Contribution of land covers to each sampling site
The proportions of agricultural area, forest, industrial district, and residential/business areas drained into each sampling site were estimated using ArcGIS software (RockWare, Ver. 2015). The NR-1 site was occupied with 13.8% of agricultural area and 80.9% of forest, whereas the NR-2 was with 22.0% of agricultural area and 71.9% of forest. The percentages of agricultural area and forest of the NR-2 and the NR-8 were similar. At the downstream, the percentages of industrial district and residential/business areas were interestingly small, compared to agricultural area and forest.
On the plot of NO3/HCO3 versus NO3/Na (Fig. 4), the summer water samples were positively correlated and the NR-1 was characterized by the highest NO3/HCO3 and NO3/Na ratios. In contrast, such ratios of the winter samples were drawn scattered. The NR-1 was completely opposite to the summer samples. A study showed that the NO3 concentration in river water was negatively correlated with a percentage of forest cover in watershed (Herlihy et al. 1998). Considering the results and land-use pattern in the NR, it can be inferred that the highest ratios of NO3/HCO3 and NO3/Na of the NR-1 during summer were due to agricultural activities at the upper reaches. However, the ratios of NO3/HCO3 and NO3/Na became lower even with the increased activity from agricultural areas because of the enhanced chemical weathering. In addition, the ratios of NO3/Na of most winter samples were lower than those of the summer samples. The results imply that land-use pattern greatly affects the water chemistry at the upstream, but its effect on the water chemistry at the downstream becomes minimal because of the enhanced weathering inputs.
Sulfur isotopic composition
Typically, the transformation processes during the sulfur cycle, including sulfide oxidation, mineralization, leaching, and assimilation, result in little or no sulfur isotope fractionation (Krouse 1980; Tuttle et al. 2009; Van Stempvoort and Krouse 1994). Although large sulfur isotope fractionation often occurs during the reduction by bacterial dissimilatory sulfate reduction (BDSR) processes, it is not the case in riverine environment. Therefore, sulfur isotopic composition in river water can be an effective tracer to apportion various sources in associated with the water chemistry in river. In the study, the SO4 concentration of water samples ranged from 92.1 to 234 μM in summer and from 130 to 535 μM in winter (Table 1). The lower SO4 concentration of 156 μM in average in summer than 397 μM in average in winter was typically due to dilution from the intensified rainfall that occurred in the summer season. Although water samples from the middle and lower reaches generally had relatively higher SO4 concentrations because of anthropogenic inputs derived from agricultural areas and industrial districts, the δ34S values of the water samples varied within a substantially narrow range (1.8–3.1‰) without seasonal variations. This result indicates that both sulfide oxidation and soil SO4 had an impact with negligible effect of anthropogenic input on δ34S of the NR (Fig. 5).
For the NR-1, the higher δ34S value in summer (4.0‰) than in winter (0.9‰) might be attributed to SO4 contents flushed from the soil zone by intensified rain for a certain short time during summer. However, this phenomenon was gradually masked up as river flows. The small δ34S variation with big variation of the SO4 concentration indicated that SO4 was possibly derived from sources that do not depend on seasonal change. The δ34S values of anthropogenic inputs, ranging from −7.1 to 31.3‰, were much bigger than those of the water samples. If anthropogenic inputs were not dominant in river water, chemical weathering would control a riverine δ34S value (Yang et al. 1996). Based on the fact that the soil water in the vicinity of a refinery located at the most upper reaches of the NR watershed had the δ34S values of 3–5‰, thus the sulfur content in the soil water was not derived directly from the refinery (National Institute of Environmental Research; NIER 2013), and the main source of SO4 was likely due to sulfide oxidation rather than anthropogenic inputs.
Use of boron (B) for identifying sources
Potential sources of boron
The boron (B) concentration of samples collected during summer exhibited an increasing trend with the distance from 1.52 to 3.25 μM with relatively high amplitude in the NR-3 (Table 1). In comparison, that of winter samples increased from 1.37 μM at NR-1 to 8.80 μM at NR-3 and then tended to gradually decrease with the distance. It was figured that a tributary affected by effluent from an industrial district had a high B concentration of 12.2 μM (Lee et al. 2005). Therefore, the national industrial districts were located in the near upper part of NR-3, and the tributary between NR-3 and NR-4 played a role in being a point source of B in river water. If consistent with the assumptions above, the NR-3 displayed the highest B concentration. Nonetheless, anthropogenic inputs should be identified with a caution because groundwater developed within Mesozoic volcanogenic sedimentary rocks displayed extremely high B concentrations of 37.9 up to 427 μM (Choo et al. 2009). Also, it was reported that B-bearing minerals (e.g., tourmaline, dumortierite) existed within the NR watershed (Koh et al. 2000; Woo et al. 2014). For example, tourmaline obtained from the watershed had a B content of 269–309 μmol (9.38–10.8 wt% as B2O3). Thus, further studies using B isotopes can be useful to identify the B source.
Main source of boron (B) affecting the water chemistry
The B concentrations at the upper reaches are between 1.37 and 1.69 μM, regardless of season, which are within the range reported in rainwater (Chetelat et al. 2005, 2009; Hasenmueller and Criss 2013; Park and Schlesinger 2002; Rose et al. 2000). Therefore, the B concentrations of the upper reaches can be a representative of rainwater. Figure 6 shows the relationship between major elements such as Cl and SO4 and B for the water samples. To efficiently identify B sources from the watershed, the ions were normalized by Na to minimize the effects of factors such as dilution and evaporation on the water chemistry. The ratios of precipitation in South Korea, and surface water affected mainly by wastewater, and groundwater associated with volcanic rock (e.g., volcanic breccia, tuffaceous conglomerate, and basaltic rocks) developed in the watershed were illustrated together with the water samples on a plot of 1000 × (B/Na) versus Cl/Na and versus SO4/Na (Fig. 6a, b). Because there were few data on available B concentration in the precipitation in South Korea, the ratio of 1000 × (B/Na) was calculated using data from the studies (Chetelat et al. 2005, 2009; Rose et al. 2000); the ratios corresponding from the 25th through 75th quartiles were 5.33–27.2.
The molar ratios of 1000 × (B/Na) of summer samples were from 5.50 to 8.79, plotted within precipitation region, while the ratios of winter samples were from 3.25 to 4.09 (except for NR-3), plotted within tributary region affected by wastewater with a ratio of 0.86–3.48. Interestingly, the Cl/Na and SO4/Na molar ratios of the samples did not stay in a remarkable spatial variation, and most were lower than those of precipitation and/or within the range of groundwater related to the volcanogenic source. Although more than 50% of winter samples were plotted within the ranges of the tributary mainly affected by wastewater, it was not entirely dependent on wastewater input. Firstly, the sample NR-3 expecting the largest contribution of wastewater was far from the cluster of tributaries contaminated by wastewater (Fig. 6). Secondly, the low 1000 × (B/Na) ratio in winter could be due to groundwater input experiencing the enhanced chemical weathering of sodium-rich rock (e.g., granite) in the watershed, because granitic rocks (e.g., leucogranite, diorite, granodiorite, granite) had 1000 × (B/Na) values of 0.23–1.38 (n = 8) (Hu and Gao 2008). Furthermore, the contribution of agricultural activities to the water chemistry could be ignored in this study because intensive agricultural practice caused high molar ratios of NO3/Na (more than 6) and Cl/Na (more than 4) along with relatively high B concentrations (Chetelat et al. 2005). In this study, the NO3/Na molar ratios in summer decreased overall from ~0.90 to ~0.20 with the distance (except for NR-5), and those in winter were all below 0.3. Therefore, the results suggest that rainfall in summer was a potential main source of B at the upper reaches, but its effect decreased gradually with the distance as chemical weathering was dominant. Still, chemical weathering was likely to be the main source of B concentration in winter.
Contributions of natural and anthropogenic sources to the water chemistry
Considering no evaporites in South Korea, rain-corrected Cl* in river water should be entirely derived from anthropogenic inputs. Assuming that SO4 in river water is mainly derived from the oxidation of sulfide minerals, \({\text{Cl}}_{\text{river}}^{*}\) and \({\text{SO}}_{{4\;{\text{river}}}}^{*}\) can be expressed as follows:
It can be possible to estimate the relative contributions of rain, chemical weathering, and anthropogenic sources to the water chemistry. Variation in \(\left[ {{\text{SO}}_{4} } \right]_{\text{river}}^{*}\) concentration corresponds to the proportions of chemical weathering and anthropogenic sources (Fig. 7a, b); here, sulfate derived from chemical weathering was entirely triggered by the hydrolysis of sulfide minerals because there is no evaporite in this study area. In summer, the contributions of chemical weathering and anthropogenic sources increased as river flows downstream, from 67.2 to 73.5%, and from 6.0 to 15.7%, respectively. In contrast, the contribution of chemical weathering in winter decreased from 82.6% in the upstream to 72.5% in the downstream, while that of anthropogenic inputs increased by up to 22.2% toward the middle and lower reaches. The fact that summer \(\left[ {{\text{SO}}_{4} } \right]_{\text{river}}^{*}\) was more correlated with the contribution of chemical weathering (R 2 = 0.66) than that of anthropogenic sources (R 2 = 0.59), but winter had the opposite (R 2 = 0.87) which indicates that high discharge in summer makes the effect of anthropogenic inputs diluted. Therefore, the use of \(\left[ {{\text{SO}}_{4} } \right]_{\text{river}}^{*}\) should be carefully considered when for estimating chemical weathering.
Chemical weathering rates and CO2 consumption rates
Based on the results and assumptions above, chemical weathering rates and associated CO2 consumption rates can be estimated. Using hydrological information and rain-corrected chemical compositions of river water, the chemical weathering rate of silicates and carbonates in the NR can be calculated as follows:
where SSW, CSW, and SW represent silicate weathering by sulfuric acid, by carbonic acid, and by both acids, respectively. SSWR, CSWR, and TSWR represent silicate weathering rate corresponding to each of them, and Φ indicates yield (t/km2/year). Similarly, carbonate weathering rate can be calculated as follows:
where SCW, CCW, and CW represent carbonate weathering by sulfuric acid, by carbonic acid, and by both acids, respectively. SCWR, CCWR, and TCWR represent carbonate weathering rate corresponding to each of them. The calculated chemical weathering rates are given in Table 2. In short, chemical weathering rates were higher in summer than those in winter, specifying that chemical weathering rates were much more related to discharge rates than elemental concentrations.
Assuming that bicarbonate and cations derived from chemical weathering are in charge-balanced state (Roy et al. 1999), related CO2 consumption rates can be calculated as follows:
where SW and CW represent silicate and carbonate weathering by only carbonic acid, respectively. The calculated CO2 consumption rates by SW and CW was 2062 × 103 and 1282 × 103 mol/km2/year in summer, and 1930 × 103 and 559 × 103 mol/km2/year in winter, respectively. The findings were that the CO2 consumption rate by SW was relatively consistent regardless of a season, while the rate by CW was higher in summer than in winter. Such difference may explain that the CO2 consumption rates were also discharge dependent, so elemental concentrations in winter were likely influenced by groundwater input developed in a silicate-dominant basin.
Conclusions
Water samples were collected from sampling activities along a downstream transect in the NR in order to identify and estimate the sources impacting the water chemistry with the help of elemental and isotope geochemistry. The concentrations of most dissolved ions were lower in summer than in winter because of the dilution effect arising from intensified rainfall in summer. The δ34S values of water samples were plotted within the ranges of sulfide oxidation and soil SO4, and there was no distinct spatio-seasonal variation for the δ34S values with the exception of the most upper samples. The 87Sr/86Sr ratios varied from 0.71043 to 0.71520, being consistent with the range of the Mesozoic volcanogenic sedimentary rocks in the catchment. These results indicated that chemical weathering was a major controlling factor over the water chemistry in the NR, although the effect of anthropogenic inputs existed. As for combined boron with major elements, the findings were that rainfall in summer was a main source of B concentration at the upper reaches, but its effect decreased gradually with the distance as chemical weathering became dominant. Still, chemical weathering was likely to be linked with B concentrations in winter. The calculated chemical weathering rates and CO2 consumption rates were more discharge dependent, and elemental concentrations in winter were likely influenced by groundwater input developed in a silicate-dominant basin. One another finding was that the influence of many potential anthropogenic inputs in the watershed on the water chemistry could be masked up, which resulted from the large buffering capacity of the NR, and will eventually provide baseline data and actual information when comparing the water quality of before and after implementation of the a mega-size civil and environmental infrastructure project such as the Four Rivers Restoration Project of South Korea.
References
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A.A. Balkema Publishers, Boca Raton
Balík J, Pavlíková D, Tlustoš P, Černý J, Jakl M (2007) The fluctuation of copper content in oilseed rape plants (Brassica napus L.) after the application of nitrogen and sulphur fertilizers. Plant Soil Environ 53:143–148
Berner RA, Lasaga AC, Garrels RM (1983) The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am J Sci 283:641–683
Calmels D, Gaillardet J, Brenot A, France-Lanord C (2007) Sustained sulphide oxidation by physical erosion processes in the Mackenzie River basin: climatic perspectives. Geology 35:1003–1006
Cheong CS, Chang HW (1996) Tectono-magmatism, -metamorphism, and -mineralization of the central Ogcheon belt, Korea (I): Sr, Nd and Pb isotopic systematic and geochemistry of granitic rocks in the Boeun area. J Geol Soc Korea 32:91–116
Chetelat B, Gaillardet J (2005) Boron isotopes in the Seine River, France: a probe of anthropogenic contamination. Environ Sci Technol 39:2486–2493
Chetelat B, Gaillardet J, Freydier R, Négrel P (2005) Boron isotopes in precipitation: experimental constraints and field evidence from French Guiana. Earth Planet Sci Lett 235:16–30
Chetelat B, Gaillardet J, Freydier R (2009) Use of B isotopes as a tracer of anthropogenic emissions in the atmosphere of Paris, France. Appl Geochem 24:810–820
Choo CO, Lee JK, Lee CJ, Park KH, Jeong GC (2009) Origin of B, Br and Sr in groundwater from Bukahn-myeon, Yeongcheon, Gyeongbuk Province, with emphasis on hydrochemistry. J Eng Geol 19:235–250
Chough SK, Kwon ST, Ree JH, Choi DK (2000) Tectonic and sedimentary evolution of the Korean peninsula: a review and new review. Earth Sci Rev 52:175–235
Chung JB, Kim BJ, Kim JK (1997) Water pollution in some agricultural area along Nakdong River. Korean J Environ Agric 16:187–192
Chung JB, Kim BJ, Kim JK, Kim MK (1998) Water quality of streams in some agricultural areas of different agricultural practices along Nakdong River basin. Korean J Environ Agric 17:140–144
Fan BL, Zhao ZQ, Tao FX, Liu BJ, Tao ZH, Gao S, Zhang LH (2014) Characteristics of carbonate, evaporate and silicate weathering in Huanghe River basin: a comparison among the upstream, midstream and downstream. J Asian Earth Sci 96:17–26
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Ghavanloughajar M (2015) Use of boron in detergents and its impact on reclamation. http://eprints.cdlib.org/uc/item/2rw7k2r7. Accessed 20 Apr 2016
Gran G (1952) Determination of the equivalence point in the potentiometric titrations. Part II. Analyst 77:661–671
Grasby SE, Hutcheon I, Krouse HR (1997) Application of the stable isotope composition of SO4 to tracing anomalous TDS in Nose Creek, southern Alberta, Canada. Appl Geochem 12:567–575
Hasenmueller EA, Criss RE (2013) Multiple sources of boron in urban surface waters and groundwaters. Sci Total Environ 447:235–247
Herlihy AT, Stoddard JL, Johnson CB (1998) The relationship between stream chemistry and watershed land cover data in the Mid-Atlantic region, U.S. Water Air Soil Pollut 105:377–386
Hu Z, Gao S (2008) Upper crustal abundances of trace elements: a revision and update. Chem Geol 253:205–221
Kim CS, Kim GS (1997) Petrogenesis of the early Tertiary A-type Namsan alkali granite in the Kyongsang Basin, Korea. Geosci J 1:99–107
Kim KH, Tanaka T, Nagao K, Jang SK (1999) Nd and Sr isotopes and K-Ar ages of the Ulreungdo alkali volcanic rocks in the East Sea, South Korea. Geochem J 33:317–341
Kim GE, Shin WJ, Ryu JS, Choi MS, Lee KS (2013) Identification of the origin and water types of various Korean bottled waters using strontium isotopes. J Geochem Explor 132:1–5
Koh SM, Takagi T, Kim MY, Naito K, Hong SS, Sudo S (2000) Geological and geochemical characteristics of the hydrothermal clay alteration in South Korea. Resour Geol 50:229–242
Korea Industrial Complex Corporation. http://www.e-cluster.net/new_app/main/Login/index.jsp. Accessed 28 May 2016
Korea Meteorological Administration. http://www.kma.go.kr/weather/climate/data_sfc_ann_mon.jsp. Accessed 6 Oct 2015
Krouse HR (1980) Sulphur isotopes in our environment. In: Fritz P, Fontes JCh (eds) Handbook of environmental isotope geochemistry. Elsevier, Amsterdam, pp 435–545
Krouse HR, Mayer B (2000) Sulphur and oxygen isotopes in sulphate. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer, Boston, pp 195–231
Lee JM, Park CK, Kim C (2005) A study for source and distribution of boron in Nakdong River. J Korean Soc Water Qual 21:236–241 (in Korean with English abstract)
Lee SW, Ryu JS, Lee KS (2014) Magnesium isotope geochemistry in the Han River, South Korea. Chem Geol 364:9–19
Likens GE, Driscoll CT, Buso DC (1996) Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272:244–246
Mayer B (2005) Assessing sources and transformations of sulphate and nitrate in the hydrosphere using isotope techniques. In: Aggarwal PK, Gat JR, Froehlich KFO (eds) Isotopes in the water cycle: past, present and future of a developing science. Springer, Dordrecht, pp 67–89
Mayer B, Fritz P, Prietzel J, Krouse HR (1995) The use of stable sulfur and oxygen isotope ratios for interpreting the mobility of sulfate in aerobic forest soils. Appl Geochem 10:161–173
Miller EK, Blum JD, Friedland AJ (1993) Determination of soil exchangeable-cation loss and weathering rates using Sr isotopes. Nature 362:438–441
MOE (2006) Annual report of ambient air quality in Korea. Ministry of Environment
Na CK, Lee IS, Chung JI (1997) Petrogenetic study on the foliated granitoids in the Chonju and the Sunchang area (II): in the light of Sr and Nd isotopic properties. Econ Environ Geol 30:249–262 (in Korean with English abstract)
Nakdong River Flood Control Office. http://www.nakdongriver.go.kr/html/about/district_12.jsp. Accessed 25 May 2016
Négrel P, Allègre CJ, Dupré B, Lewin E (1993) Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water: the Congo Basin case. Earth Planet Sci Lett 120:59–76
NIER (2013) Method to trace source of nitrogen and sulfur oxides in soil and stream water. National Institute of Environmental Research, Korea
Park H, Schlesinger WH (2002) Global biogeochemical cycle of boron. Glob Biogeochem Cycles 16:1072. doi:10.1029/2001GB001766
Petelet-Giraud E, Klaver G, Negrel P (2009) Natural versus anthropogenic sources in the surface- and groundwater dissolved load of the Dommel river (Meuse basin): constraints by boron and strontium isotopes and gadolinium anomaly. J Hydrol 369:336–349
Rose EF, Carignan J, Chaussidon M (2000) Transfer of atmospheric boron from the oceans to the continents: an investigation using precipitation waters and epiphytic lichens. Geochem Geophys Geosyst 1:2000GC000077
Roy S, Gaillardet J, Allègre CJ (1999) Geochemistry of dissolved and suspended loads of the Seine River, France: anthropogenic impact, carbonate and silicate weathering. Geochim Cosmochim Acta 63:1277–1292
Sakata M, Natsumi M, Tani Y (2010) Isotopic evidence of boron in precipitation originating from coal burning in Asian continent. Geochem J 44:113–123
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Seo JK, Lee JJ, Yang SY, Chung IK (2003) Effect of the sewage and wastewater plant effluent on the algal growth potential in the Nakdong River basin. Algae 18:157–167
Shin WJ, Ryu JS, Park Y, Lee KS (2011) Chemical weathering and associated CO2 consumption in six major river basins, South Korea. Geomorphology 129:334–341
Song BY, Ryu JS, Shin HS, Lee KS (2014) Determination of the source of bioavailable Sr using 87Sr/86Sr tracers: a case study of hot pepper and rice. J Agric Food Chem 62:9232–9238
Spence J, Telmer K (2005) The role of sulfur in chemical weathering and atmospheric CO2 fluxes: evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera. Geochim Cosmochim Acta 69:5441–5458
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Stefánsson A, Gíslason SR (2001) Chemical weathering of basalts, Southwest Iceland: effect of rock crystallinity and secondary minerals on chemical fluxes to the ocean. Am J Sci 301:513–556
Stevenson FJ, Cole MA (1999) Cycles of soil—carbon, nitrogen, phosphorus, sulfur, micronutrients. Wiley, Hoboken
Swaine DJ (1994) Trace elements in coal and their dispersal during combustion. Fuel Process Technol 39:121–137
Swaine DJ, Goodarzi F (1995) Environmental aspects of trace elements in coal. Kluwer, Dordrecht
The Geological Society of Korea (1999) Geology of Korea. Sigma Press, Seoul
Tuttle MLW, Breit GN, Cozzarelli IM (2009) Processes affecting δ34S and δ18O values of dissolved sulfate in alluvium along the Canadian River, central Oklahoma, USA. Chem Geol 265:455–467
Van Stempvoort DR, Krouse HR (1994) Controls of δ18O in sulfate: review of experimental data and application to specific environments. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. ACS symposium series, vol 500. American Chemical Society, Washington, pp 446–480
Water Resources Management Information System. http://www.wamis.go.kr/. Accessed 28 Mar 2016
White AF, Schulz MS, Vivit DV, Blum AE, Stonestrom DA, Harden J (2005) Chemical weathering rates of a soil chronosequence on granitic alluvium: part III. Hydrochemical evolution and contemporary solute fluxes and rates. Geochim Cosmochim Acta 69:1975–1996
Woo HD, Park SE, Jang YD, Kim JJ (2014) Mineralogical and geochemical studies on tourmaline in felsites from the Daeduk Mountain, Daegu, South Korea. J Miner Soc Korea 27:85–95
Wu W, Xu S, Yang J, Yin H (2008) Silicate weathering and CO2 consumption deduced from the seven Chinese rivers originating in the Qinghai-Tibet Plateau. Chem Geol 249:307–320
Yanagisawa F, Sakai H (1983) Thermal decomposition of barium sulfate–vanadium pentoxide–silica glass mixtures for preparation of sulfur dioxide in sulfur isotope ratio measurements. Anal Chem 55:985–987
Yang C, Telmer K, Veizer J (1996) Chemical dynamics of the “St. Lawrence” riverine system: δDH2O, δ18OH2O, δ13CDIC, δ34Ssulfate and dissolved 87Sr/86Sr. Geochim Cosmochim Acta 60:851–866
You CF, Spivack AJ, Smith JH, Gieskes JM (1993) Mobilization of boron in convergent margins: implications for the boron geochemical cycle. Geology 21:207–210
Yu JY, Park Y (2004) Sulfur isotope and chemical compositions of the natural waters in the Chuncheon area, Korea. Appl Geochem 19:843–853
Zhang J, Huang WW, Létolle R, Jusserand C (1995) Major element chemistry of the Huanghe (Yellow River), China—weathering processes and chemical fluxes. J Hydrol 168:173–203
Acknowledgements
We thank Dr. H.S. Shin and Ms. S.-J. Oh at the KBSI and Dr. D.C. Koh at the KIGAM for the equipment support and analyses. This work was supported by the Korea Ministry of Environment (MOE) as K-COSEM Program, along with funds from the Bio and Medical Technology Development Program of the National Research Foundation (NRF) by the Ministry of Science, ICT and Future Planning (NRF-2014M3A9E1070336) and the KBSI Grant (C37701).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, WJ., Ryu, JS., Min, JS. et al. Identification of sources affecting water chemistry in the Nakdong River, South Korea. Environ Earth Sci 76, 376 (2017). https://doi.org/10.1007/s12665-017-6690-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6690-x