Abstract
Managed aquifer recharge is one of the most popular methods for dealing with local water shortage issues, and the bacterial community could be a vital factor influencing groundwater quality during this process. In this study, analysis of variations in groundwater components during artificial recharge revealed three stages at a text site in China. During stage I, total iron and dissolved organic carbon levels are stable basically, dissolved oxygen and SO4 2− levels have rising trends, NO3 − curve varied not obviously. Variation curves show increases in dissolved oxygen, NO3 −, SO4 2− and stabilization in dissolved organic carbon and total iron at stage II. During stage III, dissolved oxygen and NO3 − have rising trends, dissolved organic carbon, total iron, and SO4 2− keep stable. At 25 and 70 days the Simpson and Shannon–Wiener indices show that microbial community richness and population diversity underwent a gradual dynamic change after recharge water arrived. Correlation analysis shows that the Simpson index was mainly affected by dissolved oxygen and NO3 −. PCR-DGGE confirmed these findings. Overall, the results revealed that the main bacterial communities reduce total nitrogen, total phosphorous, and chemical oxygen demand, which corresponded to the calculated correlation index.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over-extraction of groundwater resources has led to a range of geo-environmental problems, including cone of groundwater level depression (Qian et al. 2006; Du et al. 2013; Campos-Gaytan et al. 2014), groundwater quality deterioration (Kruawal et al. 2005; Zhai et al. 2013; Pophare et al. 2014), seawater intrusion (Vandenbohede et al. 2009; Werner et al. 2013) and land subsidence and surface fissures (Galloway and Burbey 2011; Zhang et al. 2014). Artificial recharge has become a commonly used and apparently effective method to alleviate and control groundwater-related geo-environmental problems (Du et al. 2013; Xu and Du 2014; Zhang et al. 2015). Artificial recharge also addresses the issue of water supply shortages while increasing the amount of available groundwater (Barnett et al. 2000; Bouwer 2002; Dillon 2005). However, the groundwater recharge process often results in disturbances in the hydrodynamic and hydrochemical status of groundwater, and inevitably affects microbial habitats in the recharged aquifer (Worrall and Kolpin 2004; Du 2012; Su et al. 2014a, b).
Previous studies have shown that microorganisms inhabiting aquifers are actively involved in various inorganic and organic reactions during MAR (Li et al. 2013; Alidina et al. 2014; Valhondo et al. 2015). Including the spatial and temporal distribution of microl community and their responses to environmental factor changes (Crump et al. 2003; Lu and Yan 2011; Xu and Huang 2011). These results demonstrate that the dominant microbial populations are more responsive to the environment they inhabit under different temperature and environmental conditions (An et al. 2012). Moreover, changes in groundwater temperature, dissolved oxygen and redox potential (Eh) strongly affect trace element water–rock interactions in the recharged aquifer during the deep groundwater recharge process (McNab et al. 2009; Shi et al. 2013; Stuyfzand 2015).
In this study, the correlations between groundwater components and microbial communities during a field scale MAR have been investigated, and polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE) was used to examine microbial diversity and associated changes during artificial recharge of groundwater in the deep aquifer in. Owing to differences in the concentrations of components between recharged water and original groundwater, artificial recharge will inevitably introduce variation into the underground environment, resulting in major changes in groundwater temperature, redox conditions and water components. This will in turn influence microbial community structure and groundwater quality in the recharged aquifer.
Field conditions
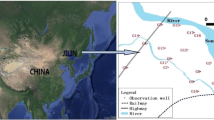
The study area was at an artificial recharge test site in China that contained ten observation wells (J1–J10) and one recharge well (R) (Fig. 1).
The recharged aquifer, which is located in the fourth confined aquifer (Fig. 2), has weak hydraulic connections to the overlying and underlying aquifers. The aquifer is 40–50 m thick, and groundwater recharge and discharge mainly occur through lateral runoff.
In the recharged aquifer, groundwater quality reflects freshwater (salinity < 1 g/L). The recharge water is also of good quality according to the hygienic standard for drinking water, China (GB5749-2006). Because of their different environmental origins, the water quality of the recharge water and the original groundwater differs significantly, particularly their temperature, dissolved oxygen and pH. Based on the related data, the aquifer has a large burial depth, with groundwater temperature and electrical conductivity (Eh) that ranged from 20 to 23 °C and 0–110 mV, respectively, for many years. The temperature of the recharge water generally varies with atmospheric temperature, ranging from 0 to 30 °C, while the Eh ranging from 560 to 640 mV.
Materials and methods
Groundwater sample collection
Nine groundwater samples were collected and prepared for microbial analysis, including seven groundwater samples collected during the artificial recharge period, an original groundwater sample and a recharge water sample collected before artificial recharge commenced. The sampling points and collection times are shown in Table 1.
The levels of microbial biomass are low in groundwater and the detection limits of common molecular biological methods are minimal. Therefore, each groundwater sample (2.5 L) was filtered through a sterile filter membrane (pore size 0.22 µm) in the field for enrichment purposes. Filter membranes containing the enriched microbial cells were kept separately in labeled sterile centrifuge tubes or petri dishes and stored at 4 °C until analysis.
Total DNA extraction
Total genomic DNA was extracted from groundwater samples using a PowerSoil® DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions and previously described methods (Nimnoi et al. 2010). Total DNA extracts were collected and stored at −20 °C until analysis.
The success of the DNA extraction was confirmed by agarose gel electrophoresis at 108 V for 50 min (Leite et al. 2012). Gels were post-stained, visualized and photographed using an ultraviolet transilluminator (Scion Co. Francisco USA) equipped with a Gel Smart 7.3 system (Clara Vision, Les Ulis, France).
PCR-DGGE fingerprinting
PCR amplification was conducted using an Mx3000P real-time quantitative PCR machine (Stratagene Inc., La Jolla, CA USA) with the following universal bacterial primers: forward, GC-338F (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGCCTACGGGAGGCAGCAG-3′); reverse, 518R (5′-ATTACCGCGGCTGCTGG-3′). The PCR program was as follows: denaturation at 95 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, elongation at 72 °C for 1 min, and then final elongation at 72 °C for 2 min. The PCR products were checked by agarose gel electrophoresis and stored at −20 °C until DGGE analysis.
Finally, the PCR products were separated by DGGE. Gels were prepared with 8 % polyacrylamide (PAGE), and the linear concentration of denaturant was 30–60 %. After complete polymerization of the PAGE gel (approx. 40 min), electrophoresis was started in a 1× Tris–acetate–EDTA buffer at a voltage of 120 V and a constant temperature of 60 °C. After 400 min of electrophoresis, DGGE fingerprints were obtained using a DCode system (BioRad Laboratories, Segrate, Italy).
Calculation of microbial diversity indices
The number and intensity of DNA bands in the DGGE fingerprint of each sample were analyzed using Quantity One (BioRad Laboratories, USA). In addition, two α biodiversity indices, the Shannon–Wiener diversity index (H′) and the Simpson dominance index (D), were calculated. The results were then used to evaluate the spatial and temporal diversity in the microbial community and associated changes in the deep confined aquifer during artificial recharge.
The H′ value reflects the richness of the structure and diversity of the microbial community structure. A larger H′ value indicates a richer microbial community structure and greater diversity. The D value reflects the function and status of the dominant population in the community. For the same species, a larger D value indicates higher function and status of the dominant population in the community.
The microbial diversity indices described above were calculated as follows (Shi et al. 2013):
where S is the total number of bacterial populations and P i is the proportion of the ith bacterial population in the community.
Results and discussion
Variation in concentrations of groundwater components during artificial recharge
Typical groundwater indicators (SO4 2−, NO3 −, total iron and TOC) and an environmental factor (dissolved oxygen in this study) are presented in Fig. 3 to directly reflect the variability in groundwater components during artificial recharge. The results of observation well J4 which is 10 m away from recharge well revealed that the concentration variation could be divided into three stages.
Stage I (0–25 days after artificial recharge starts): during this stage, total iron and dissolved organic carbon levels are basically stable (<0.05, 0.6 and 1.06, 0.8 mg/L in recharge water and original groundwater, respectively). Dissolved oxygen and SO4 2− levels (8.01, 50 and 1.12, 22 mg/L in recharge water and original groundwater, respectively) show increasing trends, with the same tendency as in the theoretical mixing curves. The NO3 − curve was expected to increase, but no obvious variations were actually observed (9.5 and 0.0 mg/L in recharge water and original groundwater, respectively). This is because time is needed for the groundwater environment and oxidation capacity of different hydrochemicals in groundwater to decrease. Overall, the investigated parameters occurred in the order O2 > NO3 − > Fe > SO4 2− (Kedziorek et al. 2008; Burke et al. 2014). When the recharge water arrived with sufficient O2, NO3 − was not involved in oxidation–reduction reactions. Because the original groundwater environment is a reducing environment, changes in NO3 − are mainly the result of mixing actions during artificial recharge.
Stage II (25–70 days after artificial recharge started): during this period, all selected indicators should remain stable when recharge water arrives at the observation wells. However, injection of recharge water causes increases in dissolved oxygen, NO3 − and SO4 2−, as well as stabilization of dissolved organic carbon and total iron. It is assumed that reactions related to dissolve organic carbon were not sufficient to induce changes in dissolved organic carbon, and that dissolved oxygen plays an active role in hydrochemical reactions, microbial reactions and microbial population changes. Research has shown that abundant dissolved oxygen actively contributes to reactions between aerobic microorganisms that consume dissolved oxygen in groundwater. Accordingly, high dissolved oxygen increases the possibility of survival of aerobic microorganisms (Terry et al. 1991). The conclusions suggest the appearance of an anaerobic microbial population. Because the dissolved oxygen level is much higher in recharge water than in groundwater (8.01 and 1.02 mg/L, respectively), dissolved oxygen tended to increase.
As explained in stage I, when there is sufficient O2 in the environment, NO3 − is rarely involved in oxidation–reduction reactions. Thus, changes in NO3 − are mainly the result of mixing actions during artificial recharge. Because many hydrochemical reactions and microbial reactions that involved total iron only changed its valence, the total amount of Fe did not change. Owing to the high content of SO4 2− in recharge water, injection of more recharge water led to increasing SO4 2− levels and therefore more SO4 2− consuming microorganisms.
Stage III (from approximately 70 days after the start of artificial recharge until the end of the present study): during this stage, dissolved organic carbon, total iron and SO4 2− should remain stable, and dissolved oxygen and NO3 − should show an increasing trend. At this stage, variation curves and theoretical curves are basically identical. However, the increasing trend of dissolved oxygen is higher in theoretical mixing curves than monitoring variation curves, and the increasing trend of NO3 − is higher in observed variation curves than theoretical mixing curves. This is because dissolved oxygen plays an active role in the reactions, resulting in its consumption. As a result, the actual increase in dissolved oxygen is lower than the theoretical increase. Except for mixing actions, some reactions may reduce more NO3 − than microorganisms can consume, resulting in the observed increase in NO3 − being higher than expected.
Variation in groundwater bacterial community diversity during artificial recharge
To directly reflect the variability in groundwater microorganisms during the recharge process, microbial diversity in groundwater by calculating the H′ and D values was evaluated (Fig. 4).
As the recharge time progressed, the D value in the observation wells showed an overall tendency to stabilize, decline and then re-stabilize, while the H′ value did not vary obviously. Specifically, the D value varied from 0.90–0.91 to 0.09–0.10, while the H′ value varied from 2.29–2.47 to 2.33–2.30, indicating that the function and status of the dominant microbial community populations in groundwater decreased after recharge water was injected, but the microbial diversities did not vary obviously. This occurred because, as the proportion of recharge water in the groundwater increased, the environment of original groundwater began to change, and microorganisms could not immediately adapt to the new environment. In addition, the previously dominant microbial community populations in groundwater decreased. However, as the recharge water was injected, microorganisms were added as well, so variations in microbial diversities were not apparent. After all the recharge water was injected into the recharged aquifer, the microbial habitat gradually stabilized, as shown by regular variations in the D and H′ values.
These results indicate that, after recharge water arrived, the microbial community richness and population diversity underwent a gradual dynamic change. Plotting the variation curves of the D value as a function of time revealed three obvious stages in the entire recharge cycle (Fig. 4).
In summary, the hydrochemical composition of the original groundwater changed when the recharge water reached recharge wells, and changes in dissolved organic carbon, dissolved oxygen, NO3 −, total iron and SO4 2− influenced the groundwater quality, which was reflected in the D value. The variations in D value indicate that there are marked variations in microbial habitat. Dynamic changes in micro flora occurred after 25 days of artificial recharge for J4. After 70 days of artificial recharge for J4, the hydrochemical composition of groundwater in the recharged aquifer gradually stabilized. When the groundwater environment reached a new equilibrium, the D value also stabilized.
Correlation between groundwater components and bacterial community diversity
The principal component regression analysis function in SPSS is widely used to analyze the correlation between variable values (Liu et al. 2003). To identify the correlation between the simpson indices and elements in groundwater, principal component regression analysis was used to calculate the correlation indexes (Table 2). The results revealed that the Simpson index was highly correlated with dissolved oxygen and NO3 −, but showed low correlation indexes with dissolved organic carbon and Fe, indicating that this index was mainly affected by dissolved oxygen and NO3 −.
Confirmation of bacterial community by DGGE
Variations in microbial population diversity and community structure in the recharged aquifer are shown in the DGGE fingerprints of groundwater samples (Fig. 5). In the DGGE fingerprint, the number and intensity of the DNA bands reflect the number of microbial species and the abundance of bacterial species in groundwater, respectively. As the number of total DNA bands increases, the number of microbial species present in groundwater also increases, with a higher intensity of a specific DNA band indicating higher abundance of the corresponding bacterial species (Nimnoi et al. 2010; Delgado et al. 2013; Kushida 2013).
The results showed that the composition and abundance of bacterial species in groundwater varied between sampling points and between time intervals at the same sampling points. These findings demonstrate that continuous injection of recharge water leads to variations in microbial diversity and community structure of the original groundwater.
To identify the bacterial species showing the greatest homology with test sequences retrieved from groundwater, 15 DNA nucleotide sequences were selected after DGGE separation and PCR re-amplification and compared with available sequences of known bacteria deposited in the GenBank database using the BLAST tool. Generally, bacteria with 16S rDNA sequence similarities of less than 95 % are considered to be different genera, whereas those with 16S rDNA sequence similarity less than 98 % are considered to be different species (Kushida 2013; Sun et al. 2013). The 15 groups of nucleotide sequences of the 16S rDNA genes detected in groundwater were compared with closely related bacteria (Table 3). As shown in the table, the main bacterial communities reduce total nitrogen, total phosphorous and chemical oxygen demand, which corresponded to the correlation index that calculated based on the SPSS method.
Conclusions
In this study, variations in groundwater components concentrations were analyzed during artificial recharge. The results showed that the variations could be divided into three stages at 25 and 70 days. Specifically, analysis of the groundwater bacterial community diversity based on the Simpson and Shannon–Wiener indices during artificial recharge showed that, after recharge water arrived, the microbial community richness and population diversity underwent a gradual dynamic change. Correlation analysis conducted demonstrated that the Simpson index had high correlation indexes with dissolved oxygen and NO3 −, but low correlation indexes with dissolved organic carbon and Fe. These findings indicate that the Simpson index was mainly affected by dissolved oxygen and NO3 −, which was confirmed by PCR-DGGE. Moreover, the main bacterial communities reduce total nitrogen, total phosphorous and chemical oxygen demand, which were reflected in the calculated correlation indexes.
References
Alidina M, Li D, Ouf M, Drewes JE (2014) Role of primary substrate composition and concentration on attenuation of trace organic chemicals in managed aquifer recharge systems. J Environ Manage 144:58–66
An YL, Zhang LY, Liu N, Zhou AX, Zhang TD, An YK (2012) Field scale analysis on structural changes of microbial community and its relationships with environmental factors in nitrobenzene-contaminated groundwater during air sparging remediation. Environ Eng Manag J 11:1687–1696
Barnett SR, Howles SR, Martin RR, Gerges NZ (2000) Aquifer storage and recharge: innovation in water resources management. Aust J Earth Sci 47:13–19
Bouwer H (2002) Artificial recharge of groundwater: hydrogeology and engineering. Hydrogeol J 10:121–142
Burke V, Greskowiak J, Asmuß T, Bremermanna R, Taute T, Massmann G (2014) Temperature dependent redox zonation and attenuation of wastewater-derived organic micropollutants in the hyporheic zone. Sci Total Environ 482–483:53–61
Campos-Gaytan JR, Kretzschmar T, Herrera-Oliva CS (2014) Future groundwater extraction scenarios for an aquifer in a semiarid environment: case study of Guadalupe Valley Aquifer, Baja California, Northwest Mexico. Environ Monit Assess 186:7961–7985
Crump BC, Kling GW, Michele B, Hobbie JE (2003) Bacterioplankton community shifts in an arctic lake correlate with seasonal changes in organic matter source. AEM 69:2253–2268
Delgado S, Rachid CTCC, Fernández E, Rychlik T, Alegría Á, Peixoto RS, Mayo B (2013) Diversity of thermophilic bacteria in raw, pasteurized and selectively-cultured milk, as assessed by culturing, PCR-DGGE and pyrosequencing. Food Microbiol 36:103–111
Dillon P (2005) Future management of aquifer recharge. Hydrogeol J 13:313–316
Du SH (2012) Groundwater quality variation affected by artificial recharge in Hutuo River bed. Appl Mech Mater 170:2158–2161
Du SH, Su XS, Zhang WJ (2013) Effective storage rates analysis of groundwater reservoir with surplus local and transferred water used in Shijiazhuang City, China. Water Environ J 27:157–169
Galloway DL, Burbey TJ (2011) Review: regional land subsidence accompanying groundwater extraction. Hydrogeol J 19:1459–1486
Kedziorek MM, Geoffriau S, Bourg ACM (2008) Organic matter and modeling redox reactions during river bank filtration in an Alluvial Aquifer of the Lot River, France. Environ Sci Technol 42:2793–2798
Kruawal K, Sacher F, Werner A, Muller J, Knepper TP (2005) Chemical water quality in Thailand and its impacts on the drinking water production in Thailand. Sci Total Environ 340:57–70
Kushida A (2013) Design and evaluation of PCR primers fot denaturing gradient gel electrophoresis analysis of plant patasitic and fungivorous nematode communities. Microbes Environ 28:269–274
Leite AMO, Mayoa B, Rachid CTCC, Peixoto RS, Silva JT, Paschoalin VMF, Delgado S (2012) Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol 31:215–221
Li D, Alidina M, Ouf M, Sharp JO, Saikaly P, Drewes JE (2013) Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (B dissolved organic carbon) concentrations. Water Res 47:2421–2430
Liu RX, Kuang J, Gong Q, Hou XL (2003) Principal component regression analysis with SPSS. Comput Methods Progr Biomed 71:141–147
Lu YZ, Yan BX (2011) Microbial adsorption capacity of heavy metals for sediment main chemical components. China Environ Sci 31:105–2011
McNab WW, Singleton MJ, Moran JE, Esser BK (2009) Ion exchange and trace element surface complexation reactions associated with applied recharge of low TDS water in the San Joaquin Valley, California. Appl Geochem 24:129–137
Nimnoi P, Pongsilp N, Lumyong S (2010) Genetic diversity and community of endophytic actinomycetes within the roots of Aquilaria crassna Pierre ex Lec assessed by Actinomycetes-specific PCR and PCR-DGGE of 16S rRNA gene. Biochem Syst Ecol 38:595–601
Pophare AM, Lamsoge BR, Katpatal YB, Nawale VP (2014) Impact of over-exploitation on groundwater quality: a case study from WR-2 Watershed, India. J Earth Syst Sci 123:1541–1566
Qian JZ, Zhan HB, Wu YF, Li FL, Wang JQ (2006) Fractured-karst spring-flow protections: a Case Study in Jinan, China. Hydrogeol J 14:1192–1205
Shi XF, Zhang WJ, Wang HM, Jiao X (2013) The comparison study of water—rock interaction before and during the artificial recharge. 2013 third international conference on intelligent system design and engineering applications, pp 1411–1416
Stuyfzand PJ (2015) Trace element patterns in Dutch coastal dunes after 50 years of artificial recharge with Rhine River water. Environ Earth Sci 73:7833–7849
Su XS, Xu W, Du SH (2014a) Responses of groundwater vulnerability to artificial recharge under extreme weather conditions in Shijiazhuang City, China. J Water Supply: Res Technol-Aqua 63:224–238
Su XS, Xu W, Du SH (2014b) In situ infiltration test using a reclaimed abandoned river bed: managed aquifer recharge in Shijiazhuang City, China. Environ Earth Sci 71:5017–5025
Sun SY, Guo ZG, Yang RL, Sheng ZG, Cao P (2013) Analysis of microbial diversity in tomato paste wastewater through PCR-DGGE. Biotechnol Bioprocess Eng 18:111–118
Terry C, Hazen LJ, Geralyne L, Carl BF (1991) Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microb Ecol 22:293–304
Valhondo C, Carrera J, Ayora C, Tubau I, Martinez-Landa L, Nodler K, Licha T (2015) Characterizing redox conditions and monitoring attenuation of selected pharmaceuticals during artificial recharge through a reactive layer. Sci Total Environ 512:240–250
Vandenbohede A, Houtte EV, Lebbe L (2009) Sustainable groundwater extraction in coastal areas: a Belgian example. Environ Geol 57:735–747
Werner AD, Bakker M, Post VEA, Vandenbohede A, Lu CH, Ataie-Ashtiani B, Simmons CT, Barry DA (2013) Seawater intrusion processes, investigation and management: recent advances and future challenges. Adv Water Resour 51:3–26
Worrall F, Kolpin DW (2004) Aquifer vulnerability to pesticide pollution combining soil, land-use and aquifer properties with molecular descriptors. J Hydrol 293:191–204
Xu W, Du SH (2014) Information entropy evolution for groundwater flow system: a case study of artificial recharge in Shijiazhuang City, China. Entropy 16:4408–4419
Xu XW, Huang SL (2011) Transport-transformation of polycyclic aromatic hydrocarbons in surface waters. Environ Sci Technol 34:26–33
Zhai YZ, Wang JS, Huan H, Zhou J, Wang W (2013) Characterizing the groundwater renewability and evolution of the strongly exploited aquifers of the North China Plain by major ions and environmental tracers. J Radioanal Nucl Chem 296:1263–1274
Zhang WJ, Gao L, Jiao X, Yu J, Su XS, Du SH (2014) Occurrence assessment of earth fissure based on genetic algorithms and artificial neural networks in Su-Xi-Chang land subsidence area, China. Geosci J 18:485–493
Zhang WJ, Huan Y, Yu XP (2015) Multi-component transport and transformation in deep confined aquifer during groundwater artificial recharge. J Environ Manage 152:109–119
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41103045, 41472215). The authors are grateful for the support provided by the “985 Project” of Jilin University and the China Scholarship Council. We are also thankful to the staff at the Shanghai Institute of Geological Survey for their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, W., Huan, Y., Liu, D. et al. Influences of microbial communities on groundwater component concentrations during managed artificial recharge. Environ Earth Sci 75, 84 (2016). https://doi.org/10.1007/s12665-015-4959-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4959-5