Abstract
Sediment contamination by heavy metals can result in significant damage to the ecological water environment. Sediment dredging is a useful way to reduce the adverse effects of heavy metal pollution in freshwater. The dredging depth is a key parameter in environmental dredging engineering. In this paper, we propose an innovative method called the critical-risk-depth method for calculating the environmental dredging depth that has been specifically designed for removal of river sediments contaminated by heavy metals. To determine the critical risk depth for dredging, the heavy metal concentrations at different sediment depths and their potential ecological risks must be tested and evaluated. The first step of the method involves analyzing sediments to determine the lateral and vertical distribution of heavy metals. In the next step, Hakanson’s potential ecological risk index is used to assess the ecological risk of heavy metals at different sediment depths. Finally, the recommended environmental dredging depths are calculated based on the potential risk for change in the vertical distribution and the given threshold level for the potential critical risk from heavy metals. We carried out a case study to determine the dredging depth for river sediment in Pinghu. The sediment analysis results show that the contents of Cd, Zn, and Pb are excessive when compared with the local soil background levels. Because of the accumulation effect of heavy metals in sediments, the heavy metal contents tend to decrease with sediment depth, but this trend may change as a result of human activities and other river dredging events. There is a high potential ecological risk level from heavy metal pollution in sediments in the study area, and the recommended environmental dredging depths of the ten rivers range from 35 to 100 cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals can cause great damage to the ecological environmental system because of their environmental persistence, resistance to bacterial decomposition, their potential for enrichment and amplification in aquatic organisms, and their ability to destroy normal physiological and metabolic activities of living organisms. To maintain a healthy river environment, the content of heavy metals should be strictly controlled (Raziuddin and Khan 1987; Papagiannis et al. 2004; Ebrahimpour and Mushrifah 2010; Paula et al. 2013). As a major sink of heavy metal pollutants, sediment can release heavy metals to the overlying water by diffusion and desorption in certain conditions, and has become a secondary source of pollution in aquatic systems (Vallee and Ullmer 1972; Wang et al. 2010). In particular, once point source pollutants that drain into a river are effectively controlled, sediment becomes a major internal source of river water pollution (Pempkowiak et al. 1999). Sediment dredging is an effective means to manipulate the endogenous pollution, which can effectively reduce the concentration of heavy metals in sediments by removing the contaminated river sediment, lower the concentration gradient at the soil and water interface, and reduce the flux of heavy metals that can be released from river sediments. Therefore, the sediment depth is a very important measurement in attempts to reduce the adverse effects from heavy metal pollution in the river ecological environment. In fact, the sediment dredging depth is the key parameter that has to be established in environmental dredging engineering for the purposes of improving water quality. While the dredging depth needs to be sufficiently deep to achieve its purpose of effectively removing pollutants from sediment, if the depth is too deep, dredging may damage ecosystems that are difficult to restore. Even though much effort, involving numerous studies, has been expended in recent decades to establish robust methods to remove pollutants from sediments by environmental dredging (Edwards et al. 1995; Van et al. 2001; Spencer et al. 2006), there are still no technical standards or norms to determine a reasonable environmental dredging depth. It is widely accepted that the environmental dredging depth should be determined by analyzing the distribution of the target pollutants in the sediment profile and determining their ecological risk, and combining this information with the potential for release from different dredging layers. In this paper, we therefore propose the critical-risk-depth method that can be used to determine a reasonable ecological dredging depth based on analysis of the heavy metal distribution in sediment and Hakanson’s potential ecological risk index. We applied this method to rivers in Pinghu to determine the environmental dredging depth of river sediment. The results from this study will help develop strategies for river pollution control and ecological dredging of rivers in Pinghu, and will also provide reference information for other similar areas.
Materials and methods
Description of study area
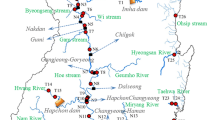
Pinghu has a river density of 4.29 km/km2 and is located in the northeast of Zhejiang Province, China. The rivers are part of the Taihu Lake system. Because the city is at the end of the river system, industrial effluents containing heavy metals from upstream and pollutant discharges from the local area mean that the water quality of the rivers in Pinghu are either classified as Grade V or worse than Grade V. The monitoring data show that the main pollutants in the rivers are CODMn, NH3–N, and TP, average concentrations of which were 7.21, 2.31, and 0.38 mg/L, respectively, in 2014. Further, the average concentrations of Cu, Zn, Pb, Cd, and Ni also indicate poor water quality. The specific research area and the sampling sites are presented in Fig. 1.
Study area and location of sampling sites. S1 Sampling site 1 in Songbeihe River; S2 Sampling site 2 in Duifengbang River; S3 Sampling site 3 in Xujiabang River; S4 Sampling site 4 in Wushayan River; S5 Sampling site 5 in Huangjiahui River; S6 Sampling site 6 in Yixianggang River; S7 Sampling site 7 in Donggang River; S8 Sampling site 8 in Hanjiaqiao River; S9 Sampling site 9 in Tengjiaqiao River; S10 Sampling site 10 in Changtang River
Sample collection and processing
In November 2013, 10 core-shaped sediment samples between 40 and 100 cm long were collected from the chosen sampling sites in 10 rivers using a two rotary pipe sampler made of iron (Fig. 2). The diameter of the inner pipe is 20 mm and the diameter of the outer pipe is 23 mm. The length of the sediment collector is from 0 to 4000 mm. Several extension pipes, each 1000 mm long, can be screwed to the sampler depending on the water depth and sediment depth at the sampling location. Samples are collected by first rotating the inner pipe with the L-shaped handle to ensure that the openings of the inner pipe and the outer pipe are completely closed. Second, the sediment sampler is lowered to the required vertical depth and then the L-shaped handle of the inner pipe is rotated so that the openings of inner pipe and the outer pipe are completely open. At this point, the sediment will be fully compressed into the sampler. Third, the L-shaped handle of the inner pipe is turned to close the sampler after the lumen of the inner pipe is filled with the sediment. Fourth, the sediment sampler is lifted up and the L-shaped handle of the inner pipe is rotated to ensure that the inner pipe and the outer pipe are completely open, and the sediment samples are collected at regular intervals (for example, an interval of 100 mm) depending on the specific requirements of the research. The sediment core samples were divided into 10-cm sections from the surface to the bottom. In total, 86 samples were collected. The samples were first air dried for several days, and then stones, animal remains, and other debris were removed from the samples. The dried samples were ground and passed through a 100-mesh sieve to obtain a homogenous powder. The ground and sieved samples were stored in airtight plastic bags inside a desiccator until analysis. Water samples were collected from the 10 sampling sites in pre-cleaned plastic bottles and stored in a refrigerator at 4 °C until laboratory analysis.
Sample analytical methods
The sample analysis process comprised two stages: the sample pretreatment and the detection of heavy metals. The sediment sample pretreatment and the water sample pretreatment were carried out following Chinese standard methods, respectively (GB/T 17138 1997; GB/T 7475 1987). As outlined in standard method GB/T 17138 1997, a weighed dried sediment sample (0.4 ± 0.0002 g) was placed into a clean Teflon crucible and digested with 6 mL hydrochloric acid [HCl (36 %)] at 100 °C on a hot plate until 2 mL remained. When cooled, the remaining sample was digested using a combination of 5 mL nitric acid [HNO3 (69 %)], 5 mL hydrofluoric acid [HF (48 %)], and 3 mL perchloric acid [HClO4 (70 %)] at between 120 and 150 °C on a hot plate until it was nearly dry. Water samples were processed following the method outlined in GB/T 7475 1987. One hundred microliters of water was put directly into a beaker with 6 mL hydrochloric acid [HCl (36 %)], and placed on a hot plate at 100 °C until nearly dry. The digested solution of sediment and water was diluted with deionized water and filtered quantitatively into a 50-mL volumetric flask. The metal concentrations (Cu, Zn, Pb, Cd, and Ni) in all samples (sediment and water) were determined by an atomic absorption spectrometer (AA-6300, Shimadzu, Japan), using air/acetylene flame absorption spectrometry. Acetylene gas with a pressure of 0.09 MPa was used. Air with a pressure of 0.4 MPa was used as the auxiliary gas. The parameter settings are listed in Table 1.
Method for calculating the environmental dredging depth
The method for calculating the environmental dredging depth for removing heavy metal contamination comprises two steps. First, the single potential ecological risk index (\(E_{\text{r}}^{i}\)) and the integrated potential ecological risk index (RI) of heavy metals at different sediment depths are calculated. Second, Eqs. (4)–(7) are used to calculate the dredging depth based on the vertical distribution of \(E_{\text{r}}^{i}\) and RI, respectively.
Assessment of potential ecological risk
The Potential Ecological Risk Index (Hakanson 1980), developed by the Swedish scientist Hakanson to evaluate the damage to the environment from heavy metals in sediment, is used to assess the heavy metal pollution. It integrates the concentrations of heavy metals, their toxic responses and ecological factors, and shows the combined effect of many types of heavy metals. The potential risk index can be calculated as follows:
where \(C_{\text{f}}^{i}\) is the contamination coefficient for a certain heavy metal and \(C_{\text{h}}^{i}\)is the measured value of the heavy metal. \(C_{n}^{i}\) is a reference value for heavy metals, and in this study the local background value listed in Table 2 is used as the reference value. \(E_{\text{r}}^{i}\) is the potential ecological risk index for heavy metal pollution from one metal. \(T_{\text{r}}^{i}\) is the toxic response factor of heavy metals that indicates the hazards of heavy metals on the human and aquatic ecosystems and reflects the levels of heavy metal toxicity and ecological sensitivity to the heavy metal pollution. RI represents the potential ecological risk index of multiple heavy metals.
Calculation of the environmental dredging depth
The dredging depth, based on the potential ecological risk index of heavy metal pollution from a single metal (\(E_{\text{r}}^{i}\)), can be calculated using Eqs. (4) and (5).
In the above equations, \(h^{a}_{ \hbox{max} }\) is the environmental dredging depth, \(h^{i}_{0}\) is the critical risk depth; \(E_{\text{r}}^{i}\)(h) is the potential ecological index of metal i at depth h in the sediment, and C 0 is the controlled risk level of a single heavy metal. In this study, C 0 is 80, which indicates that the level of risk is controlled below moderate.
The dredging depth based on the integrated potential ecological risk index (RI) can be calculated using Eqs. (6) and (7).
where \(h^{b}_{ \hbox{max} }\) is the environmental dredging depth, h 0 is the critical risk depth, and RI(h) is the potential ecological risk index of heavy metals at depth h. C 0 is the potential risk threshold level of heavy metals; in this study, C 0 is 600, which indicates that it is below the severe level.
Result and discussion
Concentrations of heavy metals in sediment
Based on Eq. (1), the concentrations of heavy metals in surface and different depths of sediments (\(C^{i}_{h}\)) should be measured. The concentrations of Cu, Zn, Pb, Cd, and Ni in surface sediments are presented in Table 2. Wang et al. (2007) studied the soil geochemical baseline and environmental background values in Zhejiang Province. In this study, we adopted the environmental background levels of heavy metals from Wang et al. (2007) to evaluate the pollution level. The concentrations of Cu were lower than the background levels at all sampling sites, the concentrations of Pb and Ni were higher than the background levels at some sampling sites, and the concentrations of Zn and Cd were higher than the background levels at all sampling sites. The standard exceedance rates of Zn, Pb, Cd, and Ni are 100, 70, 100, and 60 %, respectively. The maximum concentrations of Zn, Pb, Cd, and Ni were 7.96, 4.11, 33.03, and 0.91 times greater than the background values, respectively, while the average concentrations of Zn, Pb, Cd, and Ni were 2.37, 0.94, 23.76, and 0.21 times greater than the background values, respectively. Out of all the metals, pollution from Cd is the most serious. The pollution levels decrease in the order Cd > Zn > Pb > Ni > Cu. The average coefficients of variation (CV) of Cu, Zn, Pb, Cd, and Ni are 0.17, 0.87, 0.85, 0.18, and 0.37, respectively. The formula for the CV is CV = S n /L n , where S n and L n are the standard deviation and average value of the concentrations of a heavy metal at all sampling sites. The coefficients of variation are relatively small, indicating that the heavy metals are uniformly distributed in the sediments.
The heavy metal concentrations at different sediment depths were used to produce 10 vertical depth distribution profiles of heavy metals at the 10 sampling sites. We chose sampling sites S4 and S7 as examples. The vertical distributions of heavy metals in the sediments of S4 and S7 are shown in Fig. 3. The concentrations of Cu, Zn, Pb, Cd, and Ni decrease as the depth increases. In the upper layer, the concentrations show a lot of variation, while the variation in the concentrations decreases rapidly with depth. In the lower layers, the concentrations do not change much, and gradually become steady. However, because of the influence of human industrial activities and river regulation, there are various irregular patterns in the vertical distribution of the heavy metals. For example, the heavy metal concentrations in river sediment increase suddenly in the middle layer. Therefore, the vertical distribution of heavy metals in river sediment reflects the polluting conditions and the river regulations at different times.
Correlation coefficients between Cu, Zn, and Cd concentrations in surface sediments and those in the overlying water are 0.425, 0.032, and −0.481, respectively, indicating significant relationships between the concentrations of Cu and Cd in surface sediments and the overlying water. Cu in surface sediments and in the overlying water at each sampling site is compared in Fig. 4a, which shows that higher Cu concentrations sometimes occur simultaneously in sediment and water. This infers that, when the concentrations of Cu in sediment exceed a certain threshold, Cu will be released from sediment to water under appropriate circumstances (such as pH and temperature). However, Zn concentrations in sediment and water are not correlated, as shown in Fig. 4b, which suggests that the heavy metal content in water is determined not only by sediment, but is also influenced by other factors, such as flora and fauna, microorganisms, organic matter, and pH.
Potential ecological risk of heavy metals in sediments
Results of calculations of the single potential ecological risk index (\(E^{i}_{\text{r}}\)) and the integrated potential ecological risk index (RI) based on Eqs. (2) and (3) are presented in Table 3. The maximum and average values of \(E^{i}_{\text{r}}\) and RI at each sampling sites are represented by ‘max’ and ‘avg’ in Table 3, respectively. When \(E^{i}_{\text{r}}\) < 40, 40 ≤ \(E^{i}_{\text{r}}\) < 80, 80 ≤ \(E^{i}_{\text{r}}\) < 160, 160 ≤ \(E^{i}_{\text{r}}\) < 320, and \(E^{i}_{\text{r}}\) ≥ 320, the degree of ecological risk (\(E^{i}_{\text{r}}\)) of heavy metals is low, moderate, higher, high, and serious, respectively. When RI < 150, 150 ≤ RI < 300, 300 ≤ RI < 600, and RI > 600, the potential ecological risk (RI) of heavy metals is low, moderate, severe, and serious, respectively (Hakanson 1980).
All of the potential ecological risk indexes (\(E^{i}_{\text{r}}\)) for Cd exceeded 320 (Table 3), and fall into the serious risk category. The \(E^{i}_{\text{r}}\) values for Cu, Zn, and Ni are less than 40, and indicate low risk. The \(E^{i}_{\text{r}}\) of Pb is between 10 and 60, which indicates low to moderate risk. The potential ecological risk values (\(E^{i}_{\text{r}}\)) decrease in the order: Cd > Pb > Ni > Zn > Cu. RI values are between 500 and 1000 (Table 3), which indicates that the integrated potential ecological risk at all of the 10 sampling sites is either severe or serious. The average contribution proportions of \(E^{i}_{\text{r}}\) of Cu, Zn, Pb, Cd, and Ni to RI are 0.28, 0.43, 1.77, 94.23, and 0.77 %, respectively, out of which Cd contributes the most. This shows that Cd is the element with the potential to cause most ecological damage and therefore Cd concentrations are the main factor to consider in sediment dredging.
Calculation of the environmental dredging depth
There are obvious accumulation effects of Cu, Zn, Pb, Cd, and Ni (Fig. 3), which indicate that river sediments in Pinghu have been polluted by heavy metals. The aim of dredging is to decrease the amount of heavy metals in river sediment and to reduce the flux of heavy metal release into the overlying water. The key issue in sediment dredging therefore is how to determine a reasonable dredging depth. The dredging depth of river sediments in the study area can be determined easily and quickly using the critical-risk-depth method proposed in this study.
An example of the method for calculating the dredging depth at S10 is shown in Fig. 5. As shown in Fig. 5, \(h^{a}_{ \hbox{max} }\) is 70 cm and \(h^{b}_{ \hbox{max} }\) is 47 cm. The dredging depths for the 10 rivers are listed in Table 4, which shows that the dredging depth to regulate heavy metal polluted sediment in the research area ranges from 35 to 100 cm.
Conclusion
This paper reports the development and application of the critical-risk-depth method for calculating the environmental dredging depth to effectively remove river sediments that are contaminated with heavy metals. This method is based on the pollution level of heavy metals and the potential ecological risk at different sediment depths. We applied the method to the 10 rivers in Pinghu, Zhejiang Province, China, to describe and demonstrate the computational processes of the critical-risk-depth method. The results indicate that the sediment in the 10 rivers is severely polluted by heavy metals. The average concentrations of Cu, Zn, Pb, Cd, and Ni in the surface sediments are 16.18, 370.28, 74.21, 5.10, and 49.61 mg kg−1, respectively, all of which exceed the local soil background levels. Comparison shows that pollution from Cd and Zn is more severe than the pollution from the other metals, with average concentrations that are 23.76 and 2.37 times higher than the background levels, respectively. Heavy metal concentrations decrease with sediment depth; however, the concentration change patterns fluctuate because of various human activities and river regulations through different periods of time. There is a high potential ecological risk from heavy metals in the sediments. The risk from Pb is slight/medium, while the risks from Cu, Zn, and Ni are slight. In contrast, the risk from Cd is extremely high. Therefore, Cd is the element from which there is most ecological risk and is the key element that will control sediment dredging. The recommended environmental dredging depths of the 10 rivers range from 35 to 100 cm. This study demonstrates that the critical-risk-depth method can provide useful guidance for river dredging for environmental protection.
References
Ebrahimpour M, Mushrifah I (2010) Seasonal variation of cadmium, copper, and lead concentrations in fish from a freshwater lake. Biol Trace Elem Res 138:190–201
Edwards SC, Williams TP, Bubb JM, Lester JN (1995) The success of elutriate tests in extended prediction of water quality after a dredging operation under freshwater and saline conditions. Environ Monit Assess 36(2):105–122
GB, T 17138 (1997) Soil quality-determination of copper, zinc-Flame atomic absorption spectrophotometry. Chinese Standards Press (in Chinese), Beijing
GB, T 7475 (1987) Water quality-determination of copper, zinc, lead and cadmium-Atomic absorption spectrometry. Chinese Standards Press (in Chinese), Beijing
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Res 14(8):975–1001
Papagiannis I, Kagalou I, Leonardos J et al (2004) Copper and zine in four freshwater fish species from Lake Pamvotis (Greece). Environ Int 30(3):357–362
Paula W, Evertpm RB, De Camila LK et al (2013) Metals in water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem J 106:61–66
Pempkowiak J, Sikora A, Biemacka E (1999) Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere 39(2):313–321
Raziuddin AM, Khan AU (1987) Heavy metals in water, sediments, fish and plants of river Hindon, U. P., India. Hydrobiologia 148(2):151–157
Spencer KL, Dewhurst RE, Penna P (2006) Potential impacts of water injection dredging on water quality and ecotoxicity in Limehouse Basin, River Thames, SE England, UK. Chemosphere 63(3):509–521
Vallee BL, Ullmer DD (1972) Biochemical effects of mercury, cadmium and lead. Annu Rev Biochem 41(10):91–128
Van den Berg GA, Meigers GA, Van der heijdt LM et al (2001) Dredging-related mobilization of trace metals: a case study in the Netherlands. Water Res 35(8):1979–1986
Wang QH, Dong YX, Zhou GH et al (2007) Soil geochemical baseline and environmental background values of agricultural regions in Zhejiang Province. J Ecol Rural Environ 23(2):81–88 (in Chinese)
Wang S, Jia Y, Wang S et al (2010) Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay. J Environ Sci China 22(1):23–31
Acknowledgments
This study was supported by Natural Science Foundation of Zhejiang Province (No. Y13E090021), Planning Project for Important Issues from Department of Water Resources of Zhejiang Province (No. 317013-2013-0471).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, T., Tian, Yj., Liu, Jb. et al. Calculation of the environmental dredging depth for removal of river sediments contaminated by heavy metals. Environ Earth Sci 74, 4295–4302 (2015). https://doi.org/10.1007/s12665-015-4515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4515-3