Abstract
The geochemical and isotope analysis of groundwaters from the Murunkan basin in north western Sri Lanka was carried out to examine their evolution and recharge sources in order to shed light to enhance the current knowledge of the hydro-geochemical processes in a karst geological setting. A total of 40 water samples from ground and surface water bodies were collected from the Miocene limestone terrain, nearby metamorphic and from unconsolidated Quaternary terrains for major anions, cations and environmental isotopes ratios (δ18OVSMOW and δ2HVSMOW). Distinct geochemical differences were noted between waters from limestone terrain and nearby metamorphic terrain indicating the modification of groundwater flow paths. Bicarbonate-chloride rich water is dominated in the limestone terrain in which water flows through a less mineralized aquifer system and is modified by the sea water intrusion. Groundwater in the metamorphic terrain is modified by dissolving of Ca–Mg rich mineral phases and subsequent ion exchange processes. The environmental isotopes of groundwater from both limestone and metamorphic terrains vary from −0.38 to −6.68 ‰ and −2.41 to −42.3 ‰ for δ18OVSMOW and δ2HVSMOW, respectively. However, slightly enriched isotope signatures and low d-excess values from limestone terrain indicate an excessive evaporation compared to that of the metamorphic terrain where rapid infiltration occurs through the overlying highly permeable grumusols soil layers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater resources are of vital importance in all parts of the world under any climatic condition as nearly 30 % of fresh water on the earth occurs as groundwater (Gleick 2000). It has been already identified that climate change and global population growth cause a substantial impact on the global water resources (Vörösmarty et al. 2000). Simultaneously, intensive exploration of groundwater resources is evident in most of the arid and semi-arid regions of the world for agricultural and domestic needs. From among these groundwater resources, coastal aquifers provide water to more than one billion people who live near the coastal zone around the World (Post 2005; Small and Nicholls 2003). In these regions groundwater resources are depleting drastically due to both lack of recharge and saline water intrusion (Chandrajith et al. 2013). Rising sea level due to predicted global climatic changes could also create an additional burden on these coastal aquifer systems (Loáiciga et al. 2012). Therefore, adequate assessment, protection and management of coastal aquifer systems require better understanding of its behaviors and hydrogeochemical characteristics. In recent years, multi-tracer investigations coupled with stable isotope analyses are commonly used to understand groundwater movement, mixing patterns between different groundwater sources, salinity origin and recharge periods (Gammons et al. 2013; Khaska et al. 2013; Pu et al. 2013; Siebert et al. 2012; Wang and Yakir 2000; Chandrajith et al. 2012, 2013). Such information is essential to improve groundwater resource management, particularly in tropical equatorial regions. Applications of stable environmental isotopes in hydrological investigations has also evolved rapidly in the past few decades as conventional hydrogeochemical studies alone are not sufficient to investigate source areas of recharge water and hydrodynamics.
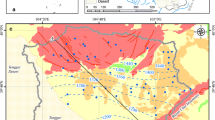
Sri Lanka is a tropical island, with an area of 65,630 km2, located in the Indian Ocean with a 1,340 km long costal belt. It also has well demarcated climatic zones, known as wet zone and dry zone. As a vital resource, groundwater is gaining increasing importance in the supply of water to rural communities, particularly in the drier regions of Sri Lanka where surface waters are very scarce or compromised by poor quality. Particularly in the coastal belt of Sri Lanka, groundwater resource management is crucial due to rapid urbanization and consequent over exploitation (Chandrajith et al. 2013). Although over 90 % of the island is dominated by aquifers developed on Precambrian metamorphic rocks, the narrow coastal belt along the north and northwest of the island is characterized with karstified sedimentary limestone aquifers. These coastal sedimentary aquifers have been considered as highly productive, but extremely vulnerable to pollution. During the last few years, extensive development activities including resettlements and agricultural development have been initiated in the northern region of Sri Lanka that leads to overexploitation of groundwater resources from karstified coastal sedimentary aquifers. In the north and northwestern coastal region few well-known karstified aquifer basins are located (Fig. 1) (Basnayake 1988; Davis and Herbert 1988).
Therefore, an attempt is made to characterize one such karstified sedimentary aquifer basin in Sri Lanka located in the northwestern coastal belt using geochemical and isotope characters. Knowing the hydrodynamic characteristics of aquifer systems will lead to determine the predominant geochemical processes taking place along the inferred horizontal groundwater flow paths. Finally, knowledge on the groundwater behavior and its chemical quality will allow the water managers to properly govern the aquifers, fully utilize the system and provide good potable water to communities. Although the selected region is hydrogeologically important, extremely limited studies and investigations have been carried out to date.
Geological and hydrological settings
The sedimentary limestone beds of Miocene age occupies nearly one tenth of the land area of the island of Sri Lanka. Groundwaters in these sedimentary beds are confined to a number of isolated basins (Fig. 1). Fault zones in limestone sequences form hydrogeological barriers segregating aquifers into a series of isolated blocks (Basnayake 1988). The Miocene limestone belt forms the largest sedimentary aquifer system in the north and north-western coastal zone of Sri Lanka covering an area of about 5,000 km2. In this stretch, seven isolated hydrogeological basins were identified, named as Madurankuli, Vanathavillu, Silavathurai, Murunkan, Mulankavil, Paranthan-Killinochchi and Mulathivu basins (Davis and Herbert 1988). These aquifer systems show a high degree of heterogeneity and anisotropy, which makes them behave differently from the metamorphic crystalline rock aquifers which are found elsewhere in the country.
This study mainly earmarked the Murunkan hydrogeological basin which is located 212 km north to the capital Colombo in the Mannar district. The basin is bound by the Malwatu Oya River to the South, the Indian Ocean to the West, the Nayaru River to the north and Madhu-Mannar road to the east covering an area of 686 km2 (Fig. 2). The basin is one of the most utilized aquifer systems in the northern region that supplies water to the Mannar Island. There are many high yielding wells constructed to extract groundwater from the Murunkan basin. A recent well drilled in the Murunkan basin yielded over 7,000 L/min of good quality water (Wickramaratne and Davies 2011). Some karstified features can be observed in the limestone stratigraphy that was created by sea level fluctuations in the post Miocene age (Davis and Herbert 1988). Within this region, the Precambrian metamorphic basement rocks are unconformably overlain by a sandstone stratum possibly belonging to the Jurassic period. Borehole data indicated that these rocks are not continuous (Basnayake 1988). Up in the sequence, sandstone layers gradually change from cream to yellow colour, hard siliceous to soft fossiliferous limestone. Alluvial sediments were then deposited on westerly dipping limestone sequences. Further up on the sedimentary sequences in the Murunkan region, non-marine Quaternary deposit of grumusols and latosols can also be observed (Basnayake 1988) in which Latosols are deposited unconformably on limestone (Fig. 3). The basin is characterized with large scale block faulting system. A down faulted limestone trough occurs in the northeast and southwest direction across the giant tank (reservoir); the main surface water body in the region is fed by monsoonal rain and used mainly for paddy cultivations; a dominant lineation trend can be observed across the giant tank in the northwest to a southeast direction. Malwatu Oya River may have flow through this lineation in the geological past (Davies and Selvarathnam 1982). Two main soil types are predominant in the study area. The eastern part of the Giant tank is composed of highly permeable red–yellow Latosols or red earth formations. The average thickness of the soil layer is about 2.5 m while the occurrence of ferruginous gravel layers can be observed in certain locations. The western part of the region is composed mainly of grey colored grumusols derived from alluvial deposits washed into the flat area along river channels that are highly utilized for paddy cultivation because of their impermeable nature. In this part, a wide range of transmissivity values that vary from 650 to 2,000 m2/day were observed (Basnayake 1988). Towards the coast, quaternary deposits such as dune sands and alluvial deposits can be observed. In spite of its small land area of Sri Lanka, the distribution of average rain fall varies remarkably from 1,000 mm in the drier parts to over 5,500 mm in the wettest part. However, the study region is characterized by a semi-arid climate in which the annual rain fall is less than 800 mm per annum from which over 90 % will fall in November and December through the winter monsoon. Karstic carbonate rock outcrops slope gently towards the west favors higher infiltration of rain water recharging the groundwater basins. Perennial rivers, Malwatu Oya and Nayaru control the drainage network of the study area. From a hydrogeological point of view, three major water-bearing systems can be distinguished in and around the region as (1) limestone aquifer system, (2) water bearing zones in the metamorphic terrain and (3) aquifers developed on alluvial or lacustrine sedimentary formations.
East–West geological cross-section through the study area showing hydrogeological connections and geological structure (Davies and Selvarathnam 1982)
Materials and methods
Water samples were collected mainly from production deep and shallow wells that extract water from aquifer systems in and around the Murunkan basin (Fig. 4). Depth of deep wells in the selected area varies from 20 to 30 m while shallow wells are 5–12 m deep. A total of 40 samples were collected from production deep wells (n = 16), hand pump deep wells (n = 10), shallow wells (n = 9), nearby Malwatu Oya River (n = 4) and Giant tank (n = 1) during a single sampling event carried out in summer 2013. An effort was made to obtain a uniform distribution of sampling locations throughout the area as much as possible; however, due to the uneven distribution of population in the study area, this exercise proved to be difficult in some cases. In production wells with installed pumps, water was pumped out for several minutes before collecting the sample. Hand pumps were also operated for about 10 min before the sampling. A battery driven submersible sampling pump that was placed several meters below the water surface was used to collect samples from dug wells and also from some production wells. Geographic locations of all sampling points were recoded using a hand held GPS device. Depth to the water table and other important geological parameters were also recorded in every sampling location.
In situ determination of pH, electrical conductivity (EC)/total dissolved solids (TDS), water temperature and salinity were carried out using filed devises. The alkalinity was determined titrimetrically using Hach® Field titration kit with 0.16 N sulfuric acid cartridges. Samples collected for anion (chloride, sulfate, fluoride, phosphate and nitrate) and cation (Na, K, Ca, Mg, Fe and Mn) analyses were filtered through 0.45 µm disposable membrane syringe filters in situ in which the samples collected for cation analysis were acidified with ultrapure nitric acid. For oxygen and hydrogen isotope ratio analyses, 15 mL of water was collected from each location into screw capped high density polyethylene tubes that were wrapped using parafilm to avoid any possible evaporation. All samples were kept in the dark and at 4 °C until the analyses were performed. Hardness and chloride in collected water samples were measured by the titration method while the other anionic parameters were determined using a Hach® DR2700 spectrophotometer. All cations were measured using a Varian 240FS Atomic Absorption Spectrophotometer (AAS). Bicarbonate and Carbonate contents were calculated using pH and alkalinity values. The δ18O and δ2H of water samples were determined with Picarro L2130i wavelength-scanned cavity ring-down infrared spectroscopy instrument at the Isotope Laboratory of Technical University of Darmstadt, Germany. The results are expressed in δ per-mille (δ‰) (Clark and Fritz 1997), reported as relative differences (δ values) with respect to Vienna Standard Mean Ocean Water (V-SMOW) where δ = (R sample−R standard)/R standard in which R is the ratio of 2H/1H and 18O/16O. Analytical precision of the stable isotope analyses is in the order of 0.037 ‰ for δ18O and 0.100 ‰ for δ2H.
Results and discussion
Physical and chemical characteristics of groundwater
During this study, water samples were obtained from wells located in the limestone aquifer terrain (21), metamorphic aquifer terrain (8), alluvial aquifers (4) and sand dune (2) while five surface water samples were obtained from the reservoir and the river. Table 1 gives the main geochemical characteristics of sampled waters. All groundwater in the area is neutral to slightly alkaline with pH ranging from 6.52 to 7.93, while slightly higher pH was observed from water samples from the alluvial aquifer (pH 7.53–7.93). Slightly lower pH levels are predominant in the north-eastern recharge areas, while it increases alone groundwater flow paths towards the coast. Surface water samples that were obtained from Malwatu Oya River and Giant tank have also given higher pH values compared to that of the groundwater. Wider variations of TDS values were noted in different aquifers in the study area where TDS in limestone aquifers were between 109 and 2,120 mg/L (Table 1). As expected, high TDS values were obtained in samples collected closer to the sea, indicating possible intrusion of saltwater into the aquifer systems. Considerably low TDS values were observed in samples obtained from the metamorphic terrain (76–710 mg/L) and the alluvial aquifers (647–771 mg/L) suggesting a low level of mineralization. Surface water samples in the region are dominated by relatively lower TDS values ranging from 381 to 674 mg/L. Usually TDS less than 1,000 mg/L are considered as potable water (WHO 1996).
In decreasing order, bicarbonates, chloride and sulfates are the dominant anions in groundwater examined in which the metamorphic aquifer showed slightly lower HCO3 − and Cl− contents compared to the limestone terrain. The mean chloride content of groundwater samples is 353 mg/L. Although no health-based guideline values have been given for chloride in drinking water, chloride contents above 250 mg/L can induce a taste in water (WHO 2008). Heavy use of high chloride water can cause soil salinization as these wells are being used extensively for agricultural purposes. Among other water quality parameters, fluoride and nitrate in drinking water are more important due to their public health concerns. The fluoride concentrations of all groundwater samples ranged from 0.02 to 0.90 mg/L with the mean value of 0.53 mg/L. However, the fluoride in the metamorphic terrain is slightly lower (mean 0.41 mg/L) compared to that of the limestone terrain. However, most of these values are below the WHO recommended limit of 0.6 mg/L for tropical countries (WHO 2008). Although the dry zone of Sri Lanka is characterized with high fluoride groundwater and associated dental diseases, in the study regions, however, most groundwaters are well within the WHO recommended levels. The nitrate–N and ortho-phosphate (PO4 3−) contents in most of the groundwater collected from three types of aquifers vary from 0.3 to 1.8 mg/L and 0.13 to 1.5 mg/L, respectively, in which DW06 samples showed the highest values for both parameters, possibly an indication of anthropogenic pollution.
The dominant cation in study aquifers is Na+ followed by Ca2+ in the limestone aquifer but Mg2+ in the metamorphic aquifer. In all groundwater samples (Na+ + K+) exceeds (Ca2+ + Mg2+). Based on the Piper trilinear plot, a majority of the groundwater samples collected from the limestone terrain are plotted in the mixed zone and in the Na-sulfate–chloride region (Fig. 5); however, only a few samples fall in the Ca–Mg-bicarbonate zone. Waters that circulate through the carbonate aquifer indicate possible intrusions of sea water or leaching of residues of saline soils into the groundwater. High evaporation under semi-arid condition that prevails in the region can considerably increase the soil salinity. The Ca2+ and Mg2+ ions most likely are due to weathering and dissolving of minerals such as calcite, dolomite and gypsum in the limestone aquifer. Groundwater from the metamorphic terrain is predominantly of Ca–Mg-HCO3 − type, but some samples lie in the mixed zone. The excess alkaline earth elements are possibly leached from weathering of silicate minerals, such as calcic-feldspar, pyroxenes and amphiboles, that are dominant in high-grade rocks of Sri Lanka.
Geochemical evolution of groundwater
The Na–Cl relationship has been widely used to identify groundwater evolution and mixing mechanisms particularly in arid and semi-arid regions (Vengosh and Rosenthal 1994; Dixon and Chiswell 1992; Hidalgo and Cruz-Sanjulián 2001). The Na/Cl molar ratio ranges from 0.86 to 1.0 in groundwater that flows through the active hydrological cycle (Hem 1985; Vengosh and Rosenthal 1994). The scatter plots of the variation of Na+ and Cl− in the study region (Fig. 6a), shows good linear relationships in which most wells are plotted very close to or below the isolines. The Na/Cl molar ratio becomes approximately equal to one, if the mixing between sea water and fresh water occurred in the aquifer (Vengosh and Rosenthal 1994) or halite dissolution is responsible for Na (Meybeck 1987). However, the low Na+ and Cl− values obtained from most groundwater indicate that these parameters are probably derived from water–rock interactions. In the limestone terrain, particularly the few deep wells located close to the coastline showed higher values of both Na+ and Cl− possibly due to sea water intrusion. Weathering of Ca–Mg bearing silicates occurs in the metamorphic terrain while calcite dissolution can provide these ions from the limestone and subsequently modified by cation exchange processes. The dominancy of HCO3 − and high Ca2+/Mg2+ molar ratio (>1.0) (Fig. 6b) in most groundwater samples in the region provides further evidence for rock-water interactions that governs the hydrogeochemical composition. Katz et al. (1997) inferred that higher Ca2+/Mg2+ molar ratio (>2) indicates the dissolution of silicate minerals, while the ratio between 1 and 2 indicate the dissolution of calcite (Rajmohan and Elango 2004). The groundwater from the limestone aquifer shows the Ca2+/Mg2+ molar ratio between 0.63 and 3.87 (mean 1.37) except the sample HP-07. As illustrated in Fig. 6c, the (Ca2++Mg2+) content increases with the increasing Cl ̄; however, the relationship is more prominent and linear in the metamorphic terrain. This may be due to reverse ion exchange that occurs between clay minerals and the solution in which dissolved Na exchange for both Ca2+ and Mg2+ (Rajmohan and Elango 2004). The linear relationships between Cl− and Mg2+ (Fig. 6e) indicate the cation exchange process that takes place in coastal freshwater aquifers with the sea water intrusion as given by Appelo and Postma (2005);
where, X represents ion exchange sites in aquifer materials. The reverse reactions occur when fresh groundwater flushes out saline groundwater in the aquifer. Hence, high Mg2+/Cl− ratio is an indicator for the influence of saline water.
Except for a few wells in the limestone terrain, all other wells show a very good linear relationship between Cl− and SO4 2− indicating their common source (Fig. 6g). Most groundwater from all study aquifer systems showed a Cl−/SO4 2− molar ratio close to 7.5. All wells in the study terrain fall below the isoline 1:1 of (Ca2++ Mg2+) versus HCO3 − plot (Fig. 6h). A fairly similar scatter pattern is also obtained in (Ca2++ SO4 2−) versus HCO3 − plot (Fig. 6i). Very strong correlation between alkali earths and HCO3 − (r = +0.963; p = 0.0001) can also be observed in water from the metamorphic aquifer. The lower Ca2++ Mg2+/HCO3 − ratio in both limestone and hard rock terrain indicates a depletion of alkaline earths over HCO3 −, which possibly occurs due to cation exchange processes. Particularly in the limestone aquifers, due to enrichment of Na, replacement of Mg2+ can occur through cation exchange process (Appelo and Postma 2005). Although the relative content of Ca2+ and HCO3 − increases with dissolution of calcite, the evaporation process will decrease ions by precipitation of CaCO3 and returns to the sea water composition that is dominated by Na+ and Cl− (Appelo and Postma 2005). The Na+/Cl− ratio would be unchanged if the evaporation process is dominant, assuming that no mineral species are precipitated, (Jankowski and Acworth 1997).
Davies and Selvarathnam (1982) mapped the groundwater flow paths in the region using piezometers (Fig. 4) and indicated that the main groundwater flow is towards the limestone terrain from the direction of the crystalline metamorphic complex. This flow continues through the limestone terrain across the giant tank and finally flows towards the ocean (flow 1 in Fig. 4). However, some other minor flows were also observed in the north-west coast from Adampanthalvu ridge (flow 2 in Fig. 4) to the Malwatu Oya River from the metamorphic terrain (flow 3 in Fig. 4). Due to the gentle dipping of limestone beds towards the sea, a steep flow gradient is created from the center of the terrain. The geographic distribution of the major ionic geochemical parameters in the study area also indicates clearly that chloride is the dominating ion towards the coast while bicarbonate dominates in the upper part of the flow (Fig. 7). Na++K+, Cl− and SO4 2− increases towards the coast in the flow path while HCO3 −, Ca2+ and Mg2+ are dominated in the upper part of the flow path. Therefore, the hydrogeochemical evolution in the Murunkan limestone basin takes place from less saline water that converts to saline water towards the coast with increasing contribution from Na+, Cl− and SO4 2− that contribute from sea water intrusions. Along the groundwater flow path carbonate mineral dissolution, enrichment of Na due to sea water intrusion and consequent ion exchange produces the entire geochemical composition of the aquifer system. In the upper part of the flow paths and particularly in the hard rock formations, weathering of minerals and consequent leaching, ion exchange processes control the concentration of Ca, Mg and Na concentrations. In general, excessive evaporation under high ambient temperature conditions would cause an enrichment of all chemical species in groundwater.
Geochemical variation of groundwater along the flow path 1 A-B (as show in Fig. 2)
Isotope characteristics
The isotope signature of the groundwater collected from different hydrogeological domains in the Murunkan region is discussed with the regional precipitation. The isotope composition of atmospheric precipitation was obtained from the database at Global Network of Isotopes in Precipitation of the framework of the global network of isotopes in precipitation (IAEA/WMO 2004) at International Atomic Energy Authority (IAEA) since no other isotope date sources are available for precipitation of Sri Lanka. Average monthly isotope values (δ18O and δ2H) recorded in meteorological stations at Mannar, Puttalam and Anuradahapura for the period of 1983–1994 were used to obtain the local meteoric water line (LMWL). All the monthly weighted rainwater data fits a least-squares regression line of the following equation:
The LMWL obtained for the northwestern part of the dry zone of Sri Lanka is almost similar to that of the Global meteoric water line (GMWL); \( \delta^{2} {\text{H}} = 8.0\delta^{18} {\text{O}} + 10 \), derived by Craig (1961). Isotope composition of different water samples collected from the study terrain is plotted in Fig. 8 with the GMWL and LMWL. The mean δ18O and δ2H signatures of water samples from metamorphic aquifer are −5.05 and −31.13 ‰, respectively, whereas for the limestone aquifer, the values are −3.24 and −21.4 ‰, respectively. One sample collected from the metamorphic aquifer (PW_11) showed enriched isotope signatures possibly due to excessive evaporation. The same sample showed the highest Mg content from among other samples. Compared to the groundwater from limestone terrain, metamorphic aquifers show more depleted isotope signatures. The mean δ18O and δ2H values are −3.40 and −21.06 ‰, respectively, for the samples from the alluvial aquifer. In the limestone terrain, both PW_04 and HP_03 that are located close to the sea (<2 km) show enriched isotope signatures. Both wells also showed enriched chloride contents, indicating the admixing of sea water with groundwater. Some samples (e.g., PW_14, PW_01 and PW_02) that are located in the alluvial terrain indicates the possible mixing of aquifer water with river water. Groundwater flow direction of the alluvial aquifer also provides evidence for this mixing. Majority of the data obtained from Murunkan regions plotted below both global and local meteoric water lines, indicating partial evaporation during the precipitation or during the infiltration with the influence of high ambient temperature conditions. For the limestone terrain the regression line obtained was δ2H = 5.97δ18O − 2.00 (R 2 = 0.997) while it is δ2H = 7.17δ18O + 5.05 (R 2 = 0.971) for the metamorphic terrain.
In the metamorphic terrain the slope of the regression line (7.17) is somewhat closer to the slope of the GMWL as described by Craig (1961), but the intercept (5.05) is considerably lower. Dansgaard (1964) described this intercept as the deuterium-excess (d-excess), defined by d = δ2H − 8 δ18O, which is a very useful indicator for explaining secondary processes influencing groundwater. High d-excess values indicate more evaporated moisture that has been added to the atmosphere while low values are associated with the fractionation caused by evaporation (Gat and Matsui 1991). The calculated d-excess values for the groundwater in the limestone terrain range from 1.1 to 11.8 ‰ with an average of 4.57 ‰. Except for three samples (HP_04, 07 and PW_10), all samples showed less than 10 ‰ of d-excess with an average of 3.44 ‰. On the other hand, samples from metamorphic terrain indicate higher d-excess values that ranged from 7.29 to 11.5 ‰ with the mean of 9.24 ‰. This sharp deviation of d-excess values between two main aquifer systems in the Murunkan region is possibly due to different infiltration rates that governs the groundwater.
It is important to note that the isotope compositions significantly vary in wells built in latosols and grumusols soil regions (Fig. 9). Particularly the north-east recharge area of the study region comprises latosols, which show relatively high permeability compared to that of grumusols (Davies and Selvarathnam 1982) that which occupies mainly in the western part of the study area. The grumusols are rich with clay and infiltration is relatively low. Therefore, this part of the land gets flooded even with moderate rainfall. It is likely that in the metamorphic terrain, rain water infiltrates vigorously without any modifications. The evaporation affects under restricted infiltration conditions, however, makes significant changes in the isotope signatures in groundwater from the limestone terrain as shown by lower d-excess and more enriched δ18O and δ2H values.
Conclusions
The Murunkan basin is one of the most important aquifer systems in Sri Lanka that supplies drinking water to the nearby community and also to the Mannar Island. These hydrogeochemical and environmental isotope studies were carried out to evaluate the recharging mechanism and potential geochemical processes involved in the recharge. The composition of groundwater is modified by mineral dissolution during the subsurface flow and enhanced by seawater intrusion in the coastal region. Stable isotope composition of δ18O and δ2H of groundwater closely follows the meteoric composition but is slightly modified by the evaporation processes. Enriched isotope signatures and lower d-excess values obtained from groundwaters of the limestone terrain suggest a high vapor flux through excessive evaporation under semi-arid condition and also subjected to slow infiltration. However, in the metamorphic terrain only slight evaporation occurs at or near the soil surface before percolating into depth. Groundwater in the coastal belt, particularly in the limestone terrain where discharge areas are located, is vulnerable to salinization by sea water. Therefore, proper management of water resources in the Murunkan basin is highly recommended. Detailed investigations of this nature are warranted to understand important geochemical processes affecting water quality in other limestone aquifer basins in northern and northwestern Sri Lanka.
References
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution. Taylor & Francis, Boca Raton
Basnayake BMSB (1988) Groundwater potentials in the sedimentary rock of Sri Lanka. In: Fernando LJD (ed) Felicitation volume. Geological Society of Sri Lanka, Peradeniya, pp 71–77
Chandrajith R, Barth JA, Subasinghe N, Merten D, Dissanayake C (2012) Geochemical and isotope characterization of geothermal spring waters in Sri Lanka: evidence for a steeper than expected geothermal gradients. J Hydrol 476:360–369
Chandrajith R, Chaturangani D, Abeykoon S, Barth JAC, van Geldern R, Edirisinghe V, Dissanayake CB (2013) Quantification of groundwater-seawater- interaction in a coastal sandy aquifer system—a study from panama. Sri Lanka Environ Earth Sci 72(3):867–877
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press/Lewis Publishers, Boca Raton
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16(4):436–468
Davies J, Selvarathnam R (1982) The groundwater resources of the Murunkan area. North-West Land Water Resources Development Project Sri Lanka. Water Resources Board, Groundwater Division, Colombo
Davis J, Herbert R (1988) Hydrogeology of the miocene sedimentary belt of Sri Lanka. J Geol soc Sri Lanka 1:45–63
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland. Aust J Hydrol 135(1):259–274
Gammons CH, Pape BL, Parker SR, Poulson SR, Blank CE (2013) Geochemistry, water balance, and stable isotopes of a “clean” pit lake at an abandoned tungsten mine, Montana, USA. Appl Geochem 36:57–69
Gat J, Matsui E (1991) Atmospheric water balance in the Amazon Basin: an isotopic evapotranspiration model. J Geophys Res Atmos 1984–2012 96(D7):13179–13188
Gleick PH (2000) A look at twenty-first century water resources development. Water Int 25(1):127–138
Global network of isotopes in precipitation-the GNIP database (2004). http://isohis.iaea.org. Accessed 10 Oct 2013
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254. Department of the Interior, US Geological Survey, Washington, D.C., pp 263
Hidalgo MC, Cruz-Sanjulián J (2001) Groundwater composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza basin, southern Spain). Appl Geochem 16(7):745–758
Jankowski J, Acworth RI (1997) Impact of Debris-flow deposits on hydrogeochemical processes and the developement of dryland salinity in the Yass River Catchment, New South Wales. Aust Hydrogeol J 5(4):71–88
Katz BG, Coplen TB, Bullen TD, Davis JH (1997) Use of chemical and isotopic tracers to characterize the interactions between ground water and surface water in mantled karst. Ground Water 35(6):1014–1028
Khaska M, Le Gal La Salle C, Lancelot J, team A, Mohamad A, Verdoux P, Noret A, Simler R (2013) Origin of groundwater salinity (current seawater vs. saline deep water) in a coastal karst aquifer based on Sr and Cl isotopes. Case study of the La Clape massif (southern France). Appl Geochem 37:212–227
Loáiciga HA, Pingel TJ, Garcia ES (2012) Sea water intrusion by sea-level rise: scenarios for the 21st century. Ground Water 50(1):37–47
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Post V (2005) Fresh and saline groundwater interaction in coastal aquifers: is our technology ready for the problems ahead? Hydrogeol J 13(1):120–123
Pu T, He Y, Zhang T, Wu J, Zhu G, Chang L (2013) Isotopic and geochemical evolution of ground and river waters in a karst dominated geological setting: a case study from Lijiang basin, South-Asia monsoon region. Appl Geochem 33:199–212
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins. South India. Environ Geol 46(1):47–61
Siebert C, Rosenthal E, Möller P, Rödiger T, Meiler M (2012) The hydrochemical identification of groundwater flowing to the Bet She’an-Harod multiaquifer system (Lower Jordan Valley) by rare earth elements, yttrium, stable isotopes (H, O) and Tritium. Appl Geochem 27(3):703–714
Small C, Nicholls RJ (2003) A global analysis of human settlement in coastal zones. J Coastal Res 19(3):584–599
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: its bearing on the water crisis in the country. J Hydrol 156(1):389–430
Vörösmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289(5477):284–288
Wang XF, Yakir D (2000) Using stable isotopes of water in evapotranspiration studies. Hydrol Process 14(8):1407–1421
WHO (1996) Guidelines for drinking-water quality, 2nd ed. Health criteria and other supporting information, vol 2. World Health Organization, Geneva
WHO (2008) Guidelines for drinking-water quality, 3rd Edition, Recommendations, vol 1. World Health Organization, Geneva
Wickramaratne U, Davies J (2011) Reconnaissance hydrogeology in Madu area for high-yielding groundwater aquifer. J Geol Soc Sri Lanka 14:55–63
Acknowledgments
AT gratefully acknowledges a Grant from the Deutscher Akademiescher Austausch Dienst (DAAD), Germany, for this work. The authors thank the valuable comments and suggestions of Professors C.B.Dissanayake and Rohan Weerasooriya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thilakerathne, A., Schüth, C. & Chandrajith, R. The impact of hydrogeological settings on geochemical evolution of groundwater in karstified limestone aquifer basin in northwest Sri Lanka. Environ Earth Sci 73, 8061–8073 (2015). https://doi.org/10.1007/s12665-014-3962-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3962-6