Abstract
Heavy metal pollution index (HPI), is a rating method and an effective tool to assess the water quality with respect to heavy metals. Twenty-seven groundwater samples were collected from the municipal drinking water supply in Damascus Oasis. The metals such as cadmium (Cd), lead (Pb), copper (Cu) and zinc (Zn) were considered. The metal concentrations showed a dominance in the order of Zn > Cu > Pb > Cd, respectively. The value of HPI based on the mean concentration was found to be 8.58, which is far below the critical pollution index value of 100. Most of the groundwater samples (74 %) have an HPI lower than the mean value of 8.58. Whereas, 26 % of the samples exceed this mean and only one sample reaches the limit of low pollution by heavy metal. Spatial distribution of HPI is in well agreement with the metals’ spatial distribution and showed the high values of HPI in the south eastern part of Damascus city to the center and especially in the northeastern part of the study area. This indicates the effect of industrial zone, agriculture and urban activity. It indicated that the groundwater at the northeast of the studied area is likely affected by leaching of heavy metal from the sewage water treatment station.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the scarcity of surface water in many regions around the world and the rapid increase in the population, there has been sharp increase in the potable water demand. Groundwater is becoming an important source of drinking water supply. The value of groundwater depends not only in its availability but also in its consistent good quality. Groundwater contamination is one of the most important environmental problems in the present world where metal contamination has major concern due to its high toxicity even at low concentration (Marcovecchio et al. 2007; Momodu and Anyakora 2010). Overexploitation of groundwater resources and discharge of untreated wastewater induces degradation of groundwater quality.
Heavy metals enter in groundwater from variety of sources; it can either be natural or anthropogenic (Reza and Singh 2010; Adaikpoh et al. 2005). Usually in natural environments, the concentration of the metals is very low and is mostly derived from the minerals and the weathering of geological formations and soils of that area (Karbassi et al. 2008; Reiners et al. 1975; Charles et al. 1994). Main anthropogenic sources of heavy metal contamination are mining, disposal of untreated and partially treated effluents as well as metals from different industries such as pharmaceutical products and indiscriminate use of heavy metal containing fertilizer and pesticides in agricultural fields (Karbassi and Amirnezhad 2004; Hatje et al. 1998; Amman et al. 2002; Nouri et al. 2008). Municipal solid wastes can also contribute considerable amount of metals to the groundwater through disposable household materials, plastics, paints and inks, body care products, medicines and household pesticides (Bardos 2004).
Trace metal contaminations are important due to their potential toxicity for the environment and human beings (Lee et al. 2007; Adams et al. 2008; Vinodhini and Narayanan 2008). Some of the metals like Cu and Zn are essential as micronutrients for the life processes in animals and plants, while many other metals such as Cd and Pb have no known physiological activities (Kar et al. 2008; Suthar and Singh 2008; Aktar et al. 2010). Metals are nondegradable and can accumulate in the human body system, causing damage to nervous system and internal organs (Lee et al. 2007; Lohani et al. 2008).
The present study aimed to investigate the groundwater quality status with respect to heavy metal concentrations in Damascus Oasis. Heavy metal pollution index (HPI) was used to assess the influence of overall pollution and illustrate the spatial distribution of heavy metal concentration and the pollution index in the groundwater of the studied area.
Materials and methods
Study area
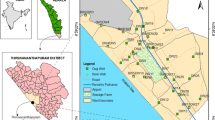
Damascus Oasis lies between latitudes 33°24′–33°37′N and longitudes 36°15′–36°38′E with an area of about 1,200 Km2 and altitude ranged between 550 and 750 m above sea level. The Oasis represents the lower part of Barada and Awaj basin, where Oteibeh and Hijaneh Lakes are located in the eastern part representing the natural drainage system of surface water. Damascus city is located in the western part of the Oasis. The drainage pattern is controlled by Barada and Awaj Rivers which are the main rivers of the hydrographic network in the basin. Barada River is fed by both Barada Spring with an average discharge of 3.12 m3/s and the Cretaceous Karistic Fijeh Spring which has an average discharge of 7.7 m3/s (Selkhozpromexport 1986).
The climate is Mediterranean type, which is characterized by rainy cold winter and hot dry summer (Soumi and Chayeb 1989). The mean annual rainfall is 221 and 136 mm/y as measured in Mazzeh and Damascus airport meteoric stations, respectively (Homsi et al. 1989).
Quaternary sediments of the Damascus depression in the western part are composed of pebble, gravel and conglomerates. Fine sand and silty soils are encountered in the center and become more recent sediments consisting of loamy and clayey lacustrine deposits in the eastern part (Ponikarov 1966).
Quaternary alluvial aquifer system consisting of pebble, gravel, sand, silt and clay has 400–450 m thickness. This aquifer compiles several sub-aquifers (multi layers) that differ in lithological characteristics. The aquifer is generally unconfined but in some places becomes semi-confined by clay lenses. The upper part (10–20 m) of this aquifer is mostly made up of alluvial–proluvial materials, mainly of loam and clays. The hydraulic conductivity sharply varies from 3.7 to 142 m/d, and thus the transmissivity (165–3,700 m2/d) (Selkhozpromexport 1986). The water quality is generally good (TDS < 0.9 g/l), but the aquifer system is severely being exploited throughout many wells (>25,000) for drinking and irrigation purposes. Groundwater flow direction is from west to east in parallel with the flow direction of Barada River. The hydraulic head changes from 660 m.a.s.l., near Damascus city, to less than 530 m.a.s.l. in the vicinity of Oteibeh Lake that corresponds to 0.004 ± 0.002 of hydraulic gradient (Abou Zakhem and Hafez 2001).
The total population in Damascus Oasis is about 5 million, accounting for approximately 20 % of the total population of Syria. Thus, various socioeconomic activities take place in this area such as urbanization, industry and agriculture. There are more than 12,000 factories ranging from micro-scale to large-scale that belong to private and public sectors. Number of chemical, metal and textile manufactories are approximately 40 % of total factories but they produce huge load of heavy metals especially, chromium, cadmium and lead. Chromium generates mainly from leather processing, metal calving and battery production. Moreover, cadmium is discharged from smelters, iron and steel plants, and battery production. While, the main sources of lead are smelting and processing of lead (Ministry of Environment 2001; World Bank Group 1998). The most highly contaminated area that worth to be mentioned is the Kabbas (DMKB) tannery located in the eastern suburb and Hoch Balss (DMHB) industrial zone in the southern suburb of Damascus city. This area consists of several types of industries, but most critically it includes a large cluster of tanneries. Leather processing is carried out in around 160 private tanneries and four public ones. Other industries as food processing industries, textiles, printing, electroplating industries and car-repairing workshops are major sources of oil pollution and heavy metals.
The first sewage treatment plant in Damascus Oasis has started operation in 1998. It is located at Adra area, 20 km northeast of the city. The plant has good biological treatment but there is no special treatment for inorganic matters specially heavy metals. The wastewater treatment plant is designed for 2.25 million of the population and it works now only to 60 % of its capacity. This means that 62 % of the total domestic wastewater is without treatment so far (Ministry of Housing 2000). The treated water was used for irrigation transmitting massive amount of heavy metals to the soil and finally to the groundwater. According to Ministry of Housing, heavy metals are present in surface water, groundwater and sediment. However, very high concentrations of heavy metal are detected in surface water samples located in contaminated area. The detected peaks were 40.39 mg/L for Cr, 0.506 mg/L for Cd, and 0.272 mg/L for Pb in 1998 at Zablatani Kabbas (DMKB) and Babila (KBA) (Ministry of Environment 1996, 2001) where the tanneries are considered as point pollution by dumping of industrial wastewater into drainage network, without treatment. The sampling has covered all suspected pollution areas.
Sampling and analysis
Twenty-seven groundwater samples were collected from the municipal drinking water supply in Damascus Oasis. The sampling locations were selected on the basis of residential areas, different land use pattern, including industrial and agriculture zones (Fig. 1). Water samples were collected from these wells during October and November, 2001. Physical parameters including pH, temperature, electrical conductivity (EC) and total alkalinity (ALK) were measured in situ. Groundwater samples were collected in polypropylene bottles and acidified with nitric acid to a pH below 2 to minimize precipitation and adsorption on container walls. Heavy metal concentrations (Pb, Cd, Cu and Zn) were measured using the anodic stripping voltammetric (ASV) method (Khandekar et al. 1988; Alvarez et al. 2007; Sonthalia et al. 2004) with (693 VA) processor, Metrohm (Swiss made). Multi-mode electrode, hanging mercury dropping electrode (HMDE) was used with Ag/AgCl as reference electrode and platinum as auxiliary electrode. ASV method provides good sensitivity, low detection limits, good precision, and stability and widely used for contaminated samples: lake, groundwater, tap water, wastewater and contaminated soil.
The samples are measured ten time and the detection limits is 3 SD (standard deviation). The detection limits were obtained as 1 µg L−1 (Pb), 0.25 µg L−1 (Cd), 1 µg L−1 (Cu) and 2.5 µg L−1 (Zn). The precision of the proposed method was estimated by calculating the relative standard deviation (RSD %) which was 5, 6, 4 and 5 %, respectively.
Heavy metal specification
Lead (Pb) is a heavy metal, belonging to the carbon group, mostly used in the manufacture of lead acid storage batteries. Lead is also released from smelting, motor vehicle exhaust fumes and from corrosion of lead pipe work (Gowd and Govil 2008). Lead is rarely present in tap water as a result of its dissolution from natural sources; rather, its presence is primarily from household plumbing systems containing lead in pipes, solder, fittings or the service connections to homes (WHO 1993).
Cadmium (Cd) is a minor metallic element naturally occurring in the earth’s crust and waters. It is used as a pigment for corrosion-resistant plating on steel and also used in stabilizing the plastic. Cadmium compounds are widely used in batteries. Cadmium is released to the environment in wastewater, and diffuse pollution is caused by contamination from fertilizers and local air pollution. Contamination in drinking water may also be caused by impurities in the zinc of galvanized pipes and solders and some metal fittings, although levels in drinking water are usually less than 1 µg/L. Exposure to certain forms and concentrations of cadmium is known to produce toxic effect on humans. The maximum permissible limit of Cd in drinking water is 5 µg/L in Syrian standard Ministry of Environment (1994) and the guideline value is 3 µg/L of WHO (1993).
Copper (Cu) is a malleable metallic element used in large quantities as an electrical conductor such as brass and bronze. Industries such as steel, plastic and blast furnaces cause the accumulation of Cu in water. Agrochemicals and waste dumps also act as important sources. Copper levels in drinking water are usually low at only a few micrograms per liter, but copper plumbing may result in greatly increased concentrations. Concentrations can reach several milligrams per liter following a period of stagnation in pipes. Copper is an essential element, and the intake from food is normally 1–3 mg/day. In adults, the absorption and retention rates of copper depend on the daily intake; as a consequence, copper overload is unlikely. Acute gastric irritation may be observed in some individuals at concentrations in drinking water above 3 mg/L (WHO 1993). The maximum permissible limit of Cu in drinking water is 1,000 µg/L in Syrian standard Ministry of Environment (1994) and the guideline value is 2,000 µg/L of WHO (2008).
Zinc (Zn) is an essential trace element found in virtually all food and potable water in the form of salts or organic complexes. The diet is normally the principal source of zinc. Although levels of zinc in surface and groundwater normally do not exceed 0.01 and 0.05 mg/L, respectively, concentrations in tap water can be much higher as a result of dissolution of zinc from pipes. However, drinking water containing zinc at levels above 3 mg/L may not be acceptable to consumers. The maximum concentration limit of Zn in drinking water is 3,000 µg/L in Syrian standard Ministry of Environment (1994) and in the WHO (2008) guideline.
Indexing approach
Heavy metal pollution index is a method of rating that shows the composite influence of individual heavy metal on the overall quality of water (Sheykhi and Moore 2012). In this indexing, weights (W i ) between 0 and 1 were assigned for each metals. The rating is based on the relative importance individual quality considerations and defined as inversely proportional to the recommended standard (S i ) for each parameter. Water quality and its suitability for drinking purpose can be examined by determining its quality index (Mohan et al. 1996; Prasad and Kumari 2008; Prasad and Mondal 2008).
The HPI is calculated with the following equation:
where W i is the unite weight of ith parameters, Q i is the sub index of the ith parameter, n is the number of parameters considered. Weighted arithmetic index method has been used for calculation of HPI.
The unit weight (W i ) has been found out using formula:
where K is the proportionality constant and S i is the standard permissible value of ith parameter.
The subindex of (Q i ) of the parameter is calculated by:
where M i is the monitored value of heavy metal of ith parameter, I i is the ideal value of ith parameter which is taken from the Syrian drinking water specification (Syrian standards for drinking water quality), (Ministry of Environment 1994); S i is the standard value of the ith parameter, in ppb (µg/L). After completion of the result, the concentration of each pollutant was converted into HPI. The higher HPI value causes greater damage to the health. Generally, the critical heavy metal pollution index HPI value is taken to be 100.
Results and discussion
In the present study, metals such as Pb, Cd, Cu and Zn were considered. Heavy metal concentrations of groundwater samples, the guideline values as specified by the WHO (2008) and the maximum concentration limit (MCL) as defined by Syrian standard for drinking water quality (Ministry of Environment 1994) are summarized in Table 1.
Table 2 presents a summary of descriptive statistics of the metal concentrations in the groundwater samples (µg/L).
Figure 2 shows a box plot of the studied heavy metal concentration illustrating their statistical parameters.
A relatively low concentration of lead varied between 1 and 4.8 µg/L with a mean value of 1.53 µg/L was recorded in the study area (Fig. 2). No samples exceeded the permissible limit of 10 µg/L of Syrian standard for drinking water (Ministry of Environment 1994) and the WHO (1993, 2008, 2011).
Results show a range between 0.25 and 0.40 µg/L of cadmium with an average of 0.27 µg/L (Table 2; Fig. 2). All the samples were noted to be lower than the Syrian permissible limit of drinking water (Ministry of Environment 1994) and the guideline value of WHO (1993).
In the present study, the concentration of copper ranged from 0.1 to 18.9 µg/L with an average value of 4.1 µg/L (Fig. 2). The highest Cu concentration of 18.9 was found in the northeast of the studied area near the sewage water treatment station. It may be attributed to domestic sewage water and runoff from extensive farmed areas. All the groundwater samples listed below the maximum permissible limit. The low values of Cu indicate that there is no significant source of pollution.
The concentration of zinc (Zn) in the study area varied between 5 and 93 µg/L with an average value of 25.51 µg/L (Fig. 2) and 75 % of the samples has a concentration below 34 µg/L. All groundwater sample concentrations do not exceed the maximum permissible limit of 3,000 µg/L.
Heavy metal pollution index (HPI) is an effective method to characterize the groundwater pollution. It represents the composite influence of metals on the overall quality of water (Reza and Singh 2010; Reza et al. 2011). This method was used in the evaluation of surface water quality for heavy metals of Diyala River in Iraq (Abdullah 2013), of the Subarnarekha River (India) (Manoj et al. 2012) and of Kor River in southwest Iran (Sheykhi and Moore 2012). Yankey et al. (2013) studied the evaluation of heavy metal pollution index of groundwater in the Tarkwa mining area, Ghana. Kumar et al. (2012) presented an appraisal of heavy metals in groundwater in Chennai city using a HPI model.
In the present study, metals such as Pb, Cd, Cu and Zn were considered. The HPI for the study area is determined by incorporating the mean concentration values of recorded heavy metals. The details of the calculation are presented in Table 3.
The mean HPI resulted in this study was 8.58 and the HPI was also calculated separately for each individual groundwater samples. Deviation and deviation percentage (%) of individual HPI values compared to the mean are calculated (Table 1).
Heavy metal pollution index can be classified into three categories (Kumar et al. 2012) like low (<19), medium (19–38) and high (>38). The HPI in our study classified the groundwater samples in the category of low pollution by heavy metal (Fig. 3).
Figure 3 shows that most of the water samples (74 %) have an HPI lower than the mean value of 8.58. Whereas, 26 % of the samples exceed this mean and only one sample reaches the limit of low pollution by heavy metal (HPI = 19.19). This suggested the effect of Adra sewage water treatment station on the groundwater quality in that region. Generally, HPI for the mean concentration value was found to be 8.58, which is far below the critical pollution index value of 100, above which the overall pollution level should be considered unacceptable for drinking water (Prasad and Kumari 2008; Prasad and Mondal 2008), thus indicating that groundwater samples are not critically polluted with respect to heavy metals.
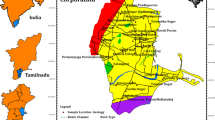
Figures 4 and 5 show the spatial distribution of Cu and Zn concentration in the groundwater of Damascus Oasis, respectively. In general, higher concentrations are exhibited in the south and eastern part of Damascus suburb to the center and especially in the northeastern part of the study area. These areas are slightly contaminated with the sewage water as well as the industrial effluents. High concentration of Cu and Zn in the south and eastern part of Damascus suburb (DGR, DHQ, DMT, DMHB and DHT) is largely controlled by industries such as steel, leather, plastic and blast furnaces; agrochemical fertilizers and pesticides cause the accumulation of Cu and Zn in groundwater. However, the highest Cu and Zn concentration was found in the northeast of the studied area. It indicated that the groundwater in this area was likely affected by leaching of heavy metal from the sewage water treatment station (Adra station) (Melhem and Higano 2010; Shawaf 2000).
Spatial distribution of HPI is plotted in Fig. 6. This map is in well agreement with the other metals’ spatial distribution (Figs. 4, 5). However, HPI distribution represents a global illustration of overall groundwater pollution by the considered heavy metal. It showed the high values of HP in the south and eastern part of Damascus suburb to the center. These areas are affected by industrial zone, agriculture and urban activity. The highest (HPI = 19.19) was found in the northeastern part of the study area. This area was likely affected by leaching of heavy metal from the sewage water treatment station in Adra area. The plant has good biological treatment but there is no special treatment for inorganic matters particularly for heavy metals. Groundwater pollution can be separated into two categories: nonpoint (diffuse) pollution and point pollution. The first one is diffuse pollution related to the irrigation with treated and non-treated wastewater. The main factors which effectively contribute to shallow groundwater pollution process are soil texture, unsaturated zone thickness, irrigation type and irrigation return flows. The second is point pollution linked to the industrial zone and urban activities.
Conclusion
The present study reveals that the HPI based on the mean concentration value of (Cd, Pb, Cu and Zn) was found to be 8.58, which is far below the critical pollution index value of 100. Most of the groundwater samples (74 %) have an HPI lower than the mean value. Whereas, 26 % of the samples exceed this mean and only one sample reaches the limit of low pollution by heavy metal. Spatial distribution of HPI is in well agreement with the metals’ spatial distribution and showed the high values of HPI in the south and eastern part of Damascus city to the center and especially in the northeastern part of the study area. This indicates the effect of industrial zone, agriculture and urban activity. It indicated that the groundwater at the northeast of the studied area was likely affected by leaching of heavy metal from the sewage water treatment station in Adra area. It was concluded that drinking water in Damascus Oasis is considered as a good quality with respect to considered heavy metals.
Heavy metal pollution index is an effective tool to characterize the groundwater pollution with respect to heavy metals. It represents the composite influence of metals on the overall quality of water.
Continuous monitoring of groundwater quality with respect to heavy metals is warranted, taking into consideration the sharp increase of the socioeconomic activities in the studied area.
References
Abdullah EJ (2013) Evaluation of surface water quality indices for heavy metals of Diyala River-Iraq. J Nat Sci Res 3(8):63–69
Abou Zakhem B, Hafez R (2001) General hydro-isotopic study of direct infiltration and evaporation process through the unsaturated zone in Damascus Oasis, Syria, IAEA-Tec.Doc.-273, Vienna, pp 131–170
Adaikpoh EO, Nwajei GE, Ogala JE (2005) Heavy metals concentrations in coal and sediments from River Ekulu in Enugu, coal city of Nigeria. J Appl Sci Environ Manag 9(3):5–8
Adams RH, Guzmán Osorio FJ, Zavala Cruz J (2008) Water repellency in oil contaminated sandy and clayey soils. Int J Environ Sci Tech 5(4):445–454
Aktar MW, Paramasivam M, Ganguly M, Purkait S, Sengupta D (2010) Assessment and occurrence of various heavy metals in surface water of Ganga River around Kolkata: a study for toxicity and ecological impact. Environ Monit Assess 160(1–4):207–213
Alvarez AM, Alvarez JRE, Alvarez RP (2007) Heavy metal analysis of rain waters: a comparison of TXRF and ASV analytical capabilities. J Radioanal Nucl Chem 273(2):427–433
Ammann AA, Michalke B, Schramel P (2002) Speciation of heavy metals in environmental water by ion chromatography coupled to ICP-MS. Anal Bioanal Chem 372(3):448–452
Bardos P (2004) Composting of mechanically segregated fractions of municipal solid waste, a review. Sita Environmental Trust, Falfield
Charles TD, James KO, Akeiverfeldt (1994) Biogeochemistry of small catchments: a tool for environmental research. In: Moldan B, Cemy J (eds) Trace metals speciation and cycling, 13th edn. Wiley, UK
Gowd SS, Govil PK (2008) Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ Monit Assess 136:197–207
Hatje V, Bidone ED, Maddock JL (1998) Estimation of the natural and anthropogenic components of heavy metal fluxes in fresh water Sinos river, Rio Grande do Sul state, South Brazil. Environ Tech 19(5):483–487
Homsi M, Bouni M, Kurdi A (1989) Damascus basin climate and its role in economical plans and the climatic changes. National meteorology report 35 (unpublished report)
Karbassi AR, Amirnezhad R (2004) Geochemistry of heavy metals and sedimentation rate in a bay adjacent to the Caspian Sea. Int J Environ Sci Tech 1(3):191–198
Karbassi AR, Monavari SM, Nabi Bidhendi GR, Nouri J, Nematpour K (2008) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147(13):107–116
Kar D, Sur P, Mandal SK, Saha T, Kole RK (2008) Assessment of heavy metal pollution in surface water. Int J Environ Sci Tech 5(1):119–124
Khandekar RN, Tripathi RM, Rahnnath R, Mishra VC (1988) Simultaneous determination of Pb, Cd, Zn, Cu in surface soil using differential pulse anodic stripping voltametry. Indian J Environ Health 30:98–103
Kumar PJS, Delson PD, Babu PT (2012) Appraisal of heavy metals in groundwater in Chennai city using a HPI model. Bull Environ Contam Toxicol 89:793–798. doi:10.1007/s00128-012-0794-5
Lee CL, Li XD, Zhang G, Li J, Ding AJ, Wang T (2007) Heavy metals and Pb isotopic composition of aerosols in urban and suburban areas of Hong Kong and Guangzhou, South China evidence of the long-range transport of air contaminants. Environ Pollut 41(2):432–447
Lohani MB, Singh S, Rupainwar DC, Dhar DN (2008) Seasonal variations of heavy metal contamination in river Gomti of Lucknow city region. Environ Monit Assess 147(1–3):253–263
Manoj K, Kumar Padhy P, Chaudhury S (2012) Study of heavy metal contamination of the river water through index analysis approach and environmetrics. Bull Environ Pharmacol Life Sci 1(10):7–15
Marcovecchio JE, Botte SE, Freije RH (2007) Heavy metals, major metals, trace elements. Handbook of water analysis. CRC Press, London
Melhem R, Higano Y (2010) Policy measures for river water management in Barada Basin, Syria. Ministry of Environment, Damascus
Ministry of Environment (1994) Drinking water quality standards. Higher council for Environment and safety, Damascus (Arabic)
Ministry of Environment (1996) Syrian standard for wastewater discharged to sewerage system. Ministry of Environment Document, Damascus (Arabic)
Ministry of Housing (2000) Adra treatment plant. The Ministry of Housing documents, Damascus
Ministry of Environment (2001) National overview of chemicals pollution and safety. Ministry of Environment Document, Damascus (Arabic)
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A 31(2):283–289
Momodu MA, Anyakora CA (2010) Heavy metal contamination of ground water: the Surulere case study. Res J Environ Earth Sci 2:39–43
Nouri J, Mahvi AH, Jahed GR, Babaei AA (2008) Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environ Geo 55(6):1337–1343
Ponikarov VP (1966) Explanatory notes on the geological map of Syria, scale 1/200.000, sheet VII (Damascus) Techno-export
Prasad B, Kumari S (2008) Heavy metal pollution index of ground water of an abandoned open cast mine filled with fly ash: a case study. Mine Water Environ 27(4):265–267
Prasad B, Mondal KK (2008) The impact of filling an abandoned opencast mine with fly ash on ground water quality: a case study. Mine Water Environ 27(1):40–45
Reiners WA, Marks RH, Vitousek PM (1975) Heavy metals in subalpine soils of New Hampshire. Oikos 26:264
Reza R, Singh G (2010) Assessment of heavy metal contamination and its indexing approach for river water. Int J Environ Sci Tech 7(4):785–792
Reza R, Singh G, Jain Manish K (2011) Application of heavy metal pollution index for ground water quality assessment in Angul district of Orissa, India. Int J Res Chem Environ 1(2):118–122
Selkhozpromexport (1986) Water resources use in Barada and Awage basins for irrigation of crops, vol II, book 2, USSR, Moscow, pp 484
Shawaf S (2000) The contamination of Barada River. Ministry of Irrigation documents, Damascus (Arabic)
Sheykhi V, Moore F (2012) Geochemical characterization of Kor River water quality, Fars Province, Southwest Iran. Water Qual Expo Health 4:25–38
Sonthalia P, McGaw E, Show Y, Greg M, Swain GM (2004) Metal ion analysis in contaminated water samples using anodic stripping voltammetry and a nanocrystalline diamond thin-film electrode. Anal Chimica Acta 522:35–44
Soumi G, Chayeb R (1989) Water need and plant irrigation techniques. UNDP, FAO, SYR/86/015, Technical report, Agriculture Ministry, pp 33
Suthar S, Singh S (2008) Vermicomposting of domestic waste by using two epigeic earthworms (Perionyx excavatus and Perionyx sansibaricus). Int J Environ Sci Tech 5(1):99–106
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int J Environ Sci Tech 5(2):179–182
WHO (1993). Guidelines for drinking water quality. World Health Organization, Geneva. 2nd edn. vol 1: Recommendations, p 188
WHO (2008) Guidelines for drinking water quality, vol 1, 3rd edn. World Health Organization, Geneva, p 515
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva, p 340
World Bank Group (1998) Pollution prevention and abatement handbook, World Bank
Yankey RK, Fiank JR, Osae S, Ahialey EK, Duncan AE, Essuman DK, Bentum JK (2013) Evaluation of heavy metal pollution index of groundwater in the Tarkwa mining area, Ghana. Elixir Pollut 54:2663–12667
Acknowledgments
The authors would like to gratefully acknowledge Prof. Dr. I. Othman, director general of Atomic Energy Commission of Syria (AECS), for his guidance and support. Special thanks to Dr. K. M. Kulkarni technical officer of the CRP n° SYR.11517 (IAEA). Thanks to S. A. Baker, S. Ibrahim, H. Issa and O. Shayiah in the AECS laboratory for heavy metal analysis. Thanks to B. Katta for mapping work and M. Yaya for the field work achievement. Finally, thanks to the laboratory team and colleagues in Geology Department (AECS). This study was done under the framework of CRP project no. SYR.11517 in cooperation with International Atomic Energy Agency (IAEA) entitled “Application of isotopes to the assessment of pollutant behavior in the unsaturated zone for groundwater protection”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou Zakhem, B., Hafez, R. Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci 73, 6591–6600 (2015). https://doi.org/10.1007/s12665-014-3882-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3882-5