Abstract
In order to explore the change process and law of carbon sinks flux in the middle and upper reaches of the Xijiang River basin, the research workers discuss the influencing factors of carbon sinks flux in the study area under natural process and impact of human activity. The hydrologic station at Wuzhou was taken as the research object. River water was sampled and measured regularly from April 2011 to March 2012 three times per month (intensive measurements and sampling were carried out during the heavy rainfall period), and then river water samples were analyzed. The following results are shown: (1) The chemistry of water revealed that the middle and upper reaches of the Xijiang River basin comprised the HCO3 −Ca type; in total anions, both HCO3 − and Ca2+ comprised 69 % and 75.9 %, respectively, as a result of carbonate rock weathering. (2) The content of NO3 − and SO4 2− was high, which might be the result of human activities, such as agriculture and industry. Moreover, sulphuric acid is involved in dissolution of carbonate minerals, and missing sink that is caused by exogenous acid is indispensable. (3) The annual total carbon flux was 6664.45 × 106 kg CO2; the carbon flux in flood season and non-flood season accounted for 58.25 and 41.75 % of carbon sinks of karst water, respectively. Correspondingly, the intensity of carbon sinks was 1694.48 and 1214.53 kg/mon km2, respectively. (4) Discharge was the primary factor of the intensity of carbon sinks and carbon flux; other influential factors, such as inorganic carbon and biological pump, had less influence on the intensity of carbon sinks and carbon flux. These conclusions are theoretically important and practically significant for estimating the carbon sink in Xijiang basin accurately. This study is a scientific basis for exploiting and protecting water resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, the process and mechanism of global carbon cycle and its resources environment effect has become one of the core issues in the study on global change; and one of the most important issues was that CO2 budget in global atmosphere was not balanced. In other words, there was a huge missing sink. Since the 1990s, the experimental results showed that the missing sink has reached 1.8–3.4 Pg C/y (Tans et al.1990; Watson et al. 1992; Sundquist 1993; Melnikov and O’Neill 2006; Lal 2008). Along with the development of research, the carbon sink effects caused by rock weathering had become known, especially carbonate rock. The corrosion of carbonate rock can consume CO2 in the atmosphere, which was considered a potential atmospheric CO2 sink, and based on biological pump effect; and its stability was more stable than the cognition of previous studies (Maier-Peimer 1993; Dean and Gorham 1998; Cassar et al. 2004; Liu et al. 2011; Liu 2012). Meanwhile, the consumption of the atmospheric CO2 by silicate weathering has been considered as a net carbon sink in the global carbon cycle (Gaillardet et al. 1999; Tao et al. 2011; Wu et al. 2012).
In the process of karstification, CO2, which is a major greenhouse gas in Earth’s atmosphere, is transformed into dissolved inorganic HCO3 −. Finally, it flowed into the ocean through the effect of biological pump. Based on the interaction of water–rock–atmosphere–biosphere carbonnate weathering (karstification) is an important way to form carbon sinks which takes part in global carbon cycle and is closely relation to the global change (Liu and Dreybrodt 2012; Jiang et al. 2013). Being an important part of the global water cycle, rivers played a key role in the bio-geochemical cycle of elements; it was an important channel of mass and energy exchange between land and sea, and its hydrochemical characteristics reflected geochemical behavior of elements in the basin, petrochemistry weathering, the degree of mechanical denudation, and the amount of CO2 consumed by rock weathering (Gaillardet et al. 1999; Dupré et al. 2003). Meanwhile, the river system had stronger influx, transporting and reconstruction effects on carbon in the basin, and also affected the carbon budget in coastal waters. It had drawn widespread attention of scientists (Gao and Shen 1998; Raymond 2003; Yao and Gao 2005; Yao et al. 2008). The middle and upper reaches of the Xijiang River basin comprised the study area. The serial observations were made for one consecutive hydrologic year, once a month from April 2011 to March 2012 and intensive observations were made during the heavy rainfall period. The data revealed the main changing mechanism of hydrochemistry and temporal-spatial variability in the middle and upper reaches of the Xijiang River basin. In the meantime, estimation of the export flux of dissolved inorganic carbon in the basin was also studied. The study results provide the scientific basis for evaluating the water resources and for proving the contribution of dissolved inorganic carbon export flux of continental river basin.
General situation of the Xijiang basin
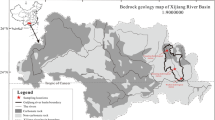
The Xijiang River, as one of the main streams of the Pearl River system, is in eastern Guangdong province. It flows through subtropical damp-heat monsoon climate area, and its full length is 2,214 km. The total area of the Li River basin is 35,000 km2, and the runoff volume was 2,300 million m3 per year. Great seasonal runoff varied, and flood season was from April to September, accounting for about 72–78 % in total of a year. The annual mean temperature was between 12 °C and 14 °C, and its annual change was not very obvious. The underlying rock layers in the basin were dominated by sedimentary rocks and magmatic rocks. The carbonate rock covers the largest area, it is 44 % of total river basin and distributes in the middle and upper catchment of Xijiang River basin. The dissolving or weathering processes on surface and under ground is strong. Most of magmatic rocks were granitoid rocks, and mainly distributed in the eastern part of Guangxi and Guangdong province, China. Lateritic red soil and red soil were the main soils in the basin. It is 600–800 m above sea level of Yungui Plateau in China. From over 700 to 1,200 m above sea level, mountains in Northwest Guangxi are covered with yellow soil. There was lime soil in limestone areas; there was also paddy soil in valley plain and basin. The main type of land use is agricultural activity in the study area. Gui River, one of the main tributaries of the Pearl River basin flows through Xing′an, Guilin, Yangshuo, Pingle, Zhaoping, and terminates at the estuary of Wuzhou section and is about 300 km away from the Pearl River mouth. Wuzhou hydrologic station (sampling and measuring point) is the debouchure of the middle and upper reaches of the Xijiang River basin, which accounts for about 94 % discharge and 91 % catchment area of the Xijiang River basin. (The Compilation Committee 1991; The Compilation Committee of Zhaoping County 1992; Yuan et al. 1994).
Materials and methods
Experiments were carried out at the Wuzhou hydrologic station of Chinese geological carbon sink monitoring network in this paper. The article adopts the method of combining instrument field testing, in situ titrating with measurements of samples in laboratory. Water samples were monitored and collected regularly from April of 2011 to March of 2012 three times per month (intensive measures and sampling were made during the heavy rainfall period). During the observation period, research workers carried out monitoring and sampling 38 times. The sampling location is shown in Fig. 1. The pH value, EC (electrical conductivity), temperature, HCO3 − and dissolved oxygen of 12 samples (the research workers only have monthly average discharge rate data) were measured on the spot in routine monitoring. This was due to the above indices, which depended mainly on the shifting of equilibrium of carbonic acid system in the karst water. However, the equilibrium of the system was destroyed once groundwater flowed out of the original system, and then contacted with the atmosphere, resulting in the greatly increased water pH value until it reached a new balance with the atmosphere.
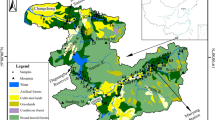
The study areas and geological map of the main streams of the basin (Guijiang) (Yao et al. 2008)
In order to accurately and factually reflect the hydrogeochemical information with the karst system, direct determination must be done to monitor the parameters that change easily, such as pH value, EC, water temperature, HCO3 − and dissolved oxygen (DO; Liu 1990; He 1996). The portable water multi-parameter analyzer (Multi3430, Germany) was used for field monitoring the pH value, temperature, EC, DO and other parameters in Xijiang River. Here, EC is automatically compensated to value of 25 °C; the accuracy of pH value, water temperature, EC and DO is 0.01 pH unit, 0.1 °C and 1 μs/cm, 0.1 mg/L. HCO3 − concentration is determined by alkalinity kit (Merck Company, Germany) in field, its resolution is 0.01 mmol/L. Discharges were measured by velocity measurement of YSD5 type that was made in Xinyuan Technology Company in Shanxi province, and the measuring accuracy was 0.01 m3/s.
At the same time, the objective was to analyze in the laboratory the hydrochemical characteristics, pH value, EC, water temperature, SO4 2−, NO3 −, F−, Cl−, K+, Na+, Ca2+, Mg2+, NH3 + and HCO3 − of 38 water samples. Water samples were collected and placed into corked polythene bottles, which were washed three times before collecting. Firstly, they were filtered with a 0.45 μm Acetate Fiber Filter, and then placed in pre-sulfided polyethylene bottles. They were finally stored in the refrigerator at 4 °C for testing. HCO3 − concentrations were determined by hydrochloric titration in field trials; each sample was titrated two to three times. The average error was less than 5 %, and the accuracy was 0.1 mmol L−1. The water chemical composition range is shown in Table 1. The cations (K+, Na+, Ca2+, Mg2+ and NH3 +) were analyzed by ion chromatography (ICS1500); the anions (SO4 2−, NO3 −, F− and Cl−) were analyzed by ion chromatograph of MIC (Table 1). Anionic charge (TZ- = HCO3 −+2SO4 2−+NO3 −+F−+Cl−) and cationic charge (K++Na++2Ca2++2 Mg2++NH4 +) the difference between the balance (|(TZ−−TZ+)/(TZ− + TZ+)|) <5 %.
Results and discussion
Physical and chemical parameters
Based on the data of field monitoring (Fig. 2), the temperature of the river water was between 13.1 and 31.1 °C on the spot, and the average was 22.5 °C. The pH value was from 7.39 to 7.80, and the average was 7.57. The concentration of Ca2+ varied in scope 37.9–55.0 mg L−1. The range of HCO3 − values varied from 1.7 to 2.37 mmol L−1. The range of EC was from 241 μs/cm to 318 μs/cm. Discharge, pH value, EC, Ca2+ and HCO3 − had a similar variation tendency, which was manifested as decreased in the flood season from April to September, and increased in the non-flood season from October to March in the next year. However, discharge was controlled by precipitation; therefore, discharge has a positive correlation with precipitation.
The composition characteristics of solvent ions
In order to explain the relative abundance and distribution characteristics of major ions in the river, and to evaluate the relative contribution of total solute composition to rock weathering, the hydrochemistry composition of the middle and upper reaches of the Xijiang River can be expressed by the Piper trilinear diagram. The Piper trilinear diagram represented the percentage of MEQ (milligram equivalent) of Ca2+, Mg2+ and Na+. Three sidelines of the triangle in the right corner, respectively, represented the percentage of MEQ of HCO3 −, SO4 2− and Cl−.
According to the Piper trilinear diagram in the study area (Fig. 3), the main ions in the water in the middle and upper reaches of the Xijiang River were Ca2+ and HCO3 −; and the other anions and cations were relatively less. The hydrochemistry type of the river was characterized by water type HCO3 − Ca. HCO3 − takes up the highest content of anion, which accounts for 69 % of total anions concentration; SO4 2− took second place; and Cl− takes up the lowest percentage; and Ca2+ takes up the highest percentage of cations with 75.90 %, Mg2+ takes second place, and Na+ takes up the lowest percentage. Ca2+ and HCO3 − possibly were from carbonate rock dissolution in the middle and upper reaches of the Xijiang River. There was a close link between concentration of the two ions and karstification intensity, which reflected that hydrochemistry characteristic of the river in the karst area was affected by carbonate rock dissolution.
Effect of meteoric water
In order to intuitively compare the chemical composition, forming reason and their interrelation in different kinds of river water, Gibbs (1970) had designed a semilog coordinate diagram based on the main chemical composition of solute; and the natural solute had been divided into three different types: rainfall controlling type, rock weathering type and evaporation/crystallization type. The ordinate in the map was logarithmic coordinate, which represented total dissolved solids (TDS) (mineralization) in the river water. Abscissa was the ordinary coordinate, which represented the ratio of Na+/(Na++Ca2+) or Cl−/(Cl−+HCO3 −) in the river water. On the Gibbs′ diagram, river water sites were distributed in the bottom right corner of the Piper diagram, which was with low salinity and high ratio (close to 1) of Na+/(Na++Ca2+) and Cl−/(Cl−+HCO3 −). The river water sites fall on the mid-left side, which was with medium content of TDS and a lower ratio (less than 0.5) of Na+/(Na++Ca2+) and Cl−/(Cl−+HCO3 −). River water sites fall on the upper-right corner of the Piper diagram, which was with high content of TDS (such as rivers of arid area) and a lower ratio (close to 1) of Na+/(Na++Ca2+) and Cl−/(Cl−+HCO3 −). According to the above situation, Gibbs (1970) held that the first description reflected the properties of meteoric water; the second description reflected the influence of rock components on the river water; and the third description reflected the influence of evaporation on the river water in the arid area.
The hydrochemistry data of 38 water samples are illustrated in Fig. 4. It is clear from these data that for most samples in the middle and upper reaches of the Xijiang River, the ratio of Cl−/(Cl−+HCO3 −) was from 0.03 to 0.07, and the ratio of Na+/(Na++Ca2+) was from 0.06 to 0.11. The main components all belonged to rock weathering type; it mainly reflected that the hydrochemistry characteristic of river was affected by rock dissolution in the karst area. Generally speaking, the hydrochemistry of the river water was mainly controlled by weathering of rock in Xijiang River basin, and meteoric water may have had little and even no influence on hydrochemistry of river water in Xijiang River basin.
Lithological influences
Carbonate rock was widely distributed in the middle and upper reaches of the Xijiang River basin (Fig. 1). Carbonate rock was liable to karstification in the presence of CO2 and H2O; its reaction equation (Liu et al. 2007) was as follows:
Limestone and dolostone were dissolved to form Ca2+, Mg2+ and HCO3 −; the dissolution rate of dolostone was quicker than limestone. Mg2+ was usually less than Ca2+ in the river water; therefore, HCO3 − and Ca2+ were usually the main anions and cations of the river in the study area.
According to the plots of Ca2+/Na+ versus Mg2+/Na+, Ca2+/Na+ versus HCO3 −/Na+ ratios for the river waters (Fig. 5), the ratios of Ca2+/Na+, Mg2+/Na+ and HCO3 −/Na+ were relatively high; most of them fall on the right corner of Piper diagram. Therefore, the hydrochemistry characteristic and lithology of Xijiang River was mainly controlled by carbonate rock; some of them were affected by silicate rocks, but not affected by evaporite. This was because the carbonate rock was widely distributed in the middle and upper reaches of the Xijiang River basin; in Guangxi Autonomous Region, carbonate strata were exposed with an outcrop area of 78,000 km2 in Devonian and Triassic. Among them, Guijiang River basin was the main stream in Xijiang River basin, and the carbonate rock occupies 9,885 km2 in the Guijiang River basin (Huang et al. 2011; Cao et al. 2011).
Effects of human activities
NO3 − of river water mainly originated from agricultural nitrogen fertilizers, and SO4 2− mainly originated from industrial nitrogen fertilizers and atmospheric deposition (Han and Liu 2005). The change in the ratios of NO3 −/Na+ and SO4 2−/Na+ mainly reflected the effects of agricultural activities and industrial activities. As shown in Fig. 6a, all samples fall in the middle of industrial activities, atmospheric input and agricultural activities. The possible reason is that Wuzhou hydrologic station is the dividing point of upper, middle, and lower reaches of Xijiang River, which controlled the incoming water in the upper and middle reaches of the river. The water in the main streams and tributaries mixed together, which is due to human activities intermingled with atmospheric input.
According to formula (1) and formula (2), namely, carbonate dissolution equation of carbonate minerals, HCO3 −, Ca2+ and Mg2+ were the main products, and the equivalent ratio between (Ca2++Mg2+) and HCO3 − was 1.0. Figure 6b shows that the negative is only HCO3 − + SO4 2−; the equivalent ratio between (Ca2++ Mg2+) and (HCO3 −+ SO4 2−) is approximate to 1.0 in the Xijiang River water. The results indicated that Ca2+ and Mg2+ were balanced by bicarbonate and sulfate ions, and sulfuric acid was an important species involved in dissolution of carbonate mineral. On the basis of existing research, Han and Liu (2005) suggested that SO4 2− mainly originated from nitrogen fertilizers of industrial activities and atmospheric deposition. Table 1 indicated the SO4 2− values were at higher levels in the river water of study area as compared with Gui River, which was less affected by human activities, with the concentration of SO4 2− from 0.085 to 0.163 mmol/l, NO3 − from 0 to 0.110 mmol/l (Tang et al. 2014). Human activities could make the SO4 2− content increase and provide power for dissolution of carbonate rocks. Moreover, sulfuric acid was involved in dissolution of carbonate mineral. Missing carbon sink which was caused by exogenous acid had been indispensable (Gao et al. 2001; Han and Liu 2001, 2004; Liu et al. 2008; Yu et al. 2012).
The characteristics of carbon sinks flux
The carbon sink mainly was the inorganic carbon source on karstification and was controlled by its range of pH values; inorganic carbon can be approximately expressed by HCO3 − concentration; the hydrochemistry-runoff method was used to estimate the amount of discharge, and then was used to calculate the carbon sink intensity. Its reaction formula was shown as follows:
In the equation, C SF was carbon sink that was formed by karst processes; Q was discharges; HCO3 − was the content of bicarbonate ions; M(CO2)g and M(HCO3 −) was molecular weight of CO2 and HCO3 −, respectively.
In the equation, C SI was carbon sink intensity (kg/mon km2); C SF was carbon sink that formed by karst processes; S was a catchment area of Wuzhou hydrologic station with 327,006 km2.
Carbon sink flux and carbon sink intensity in the upper and middle reaches of Xijiang River was calculated by formula (3) and formula (4); the results are shown in Fig. 7. The annual carbon sink flux was 6664.45 × 106 kg CO2; there was 3324.63 × 106 kg CO2 in flood season; and 2382.96 × 106 kg CO2 in the non-flood season. The average annual carbon sink intensity was 1694.48 kg/mon km2; there was 1698.35 kg/mon km2 in flood season, which accounts for 58.25 % of the annual carbon sink of karst water. There was 1214.53 kg/mon km2 in non-flood season, which accounts for 41.75 % of the annual carbon sink of karst water.
Analysis of the influencing factors
As shown in Fig. 8, the change of carbon sink intensity, carbon sink and discharge were similar. It shows that discharge was the main control factor of carbon sink flux by combining the above analysis.
The relationship among HCO3 − concentration, carbon sink intensity and carbon sink flux was more complex, which was related to a different reaction mechanism (He et al. 1997; Liu et al. 2004; Hu et al. 2011). HCO3 − concentration in water varied in a wide range, and there was an inverse correlation between HCO3 − concentration and discharge in flood season; and it was easy to be affected by the dilution effect and the diffusion boundary layer effect. Because of decreased discharge in the non-flood season, HCO3 − concentration affected by other factors (Drysdale et al. 2003; Zhang 2011; Nimick et al. 2011) was more obvious; it showed an incomplete inverse correlation between HCO3 − concentration and discharge.
Beside the discharge and HCO3 −, there are two ways for the influence of inorganic carbon in water by algal photosynthesis in a karst water environmental system. One way was to consume DIC (dissolved inorganic carbon) by synthesis of organic carbon itself; the other way was calcite precipitation consumed DIC in water, namely biological pump effect. These can be regarded as net carbon sink effect of algae in a karst environment (Liu and Liu 2010). At normal state, there is a strong photosynthesis in aquatic plants, which was always reflected as increasing DO, decreasing HCO3 −, and vice versa (Lü et al. 2006). On the field monitoring, the result in October 2011 and March 2012 accorded with the above laws, but other months were not obvious, and even the opposite situation appeared in May and June of 2011, January and February of 2012. The phenomena in May and June of 2011 were due to water dilution effects and degasification by higher temperature and disturbance of river water. In January and February of 2012, aquatic plants grew slowly, and temperature and light intensity was lower in the winter; therefore, photosynthesis was not significant. The biological pump effect had little influence on carbon sink intensity and carbon sink flux.
Conclusion
-
(1)
The chemistry of water revealed that the middle and upper reaches of the Xijiang River basin comprised the HCO3 −Ca type; in total anions, both HCO3 − and Ca2+ comprised 69 % and 75.9 %, respectively, as a result of carbonate rock weathering.
-
(2)
The content of NO3 − and SO4 2− was high compared with Gui River (less affected by human activities); it is possibly affected by human activities, such as agriculture and industry. Moreover, sulphuric acid was involved in dissolution of carbonate minerals; missing sink that was caused by exogenous acid had been indispensable.
-
(3)
The total carbon sink flux was 6664.45 × 106 kg CO2 in a hydrological year; the carbon sink flux was 3324.63 × 106 kg CO2 in flood season and 2382.96 × 106 kg CO2 in non-flood season. The mean annual carbon sink intensity was 1694.48 kg/mon km2; the carbon sink in karst water was 1698.35 kg/mon km2 in flood season and 1214.53 kg/mon km2 in non-flood season, which accounted for 58.25 and 41.75 % of carbon sinks in karst water, respectively.
-
(4)
Discharge was the primary factor of the intensity of carbon sinks and carbon flux; the relationship among HCO3 − concentration, carbon sink intensity and carbon sink flux was more complex, which related to different reaction mechanism; the biological pump effect had little influence on carbon sink intensity and carbon sink flux.
References
Cao JH, Yang H, Kang Z (2011) Preliminary regional estimation of carbon sink flux by carbonate rock corrosion: a case study of the Pearl River Basin. Chin Sci Bull 56(35):3766–3773
Cassar N, Laws EA, Bidigare RR et al. (2004) Bicarbonate uptake by southern ocean phytoplankton. Global Biogeochem Cycles 18(2). doi:10.1029/2003GB002116
Dean WE, Gorham E (1998) Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26:535–538
Drysdale R, Lucas S, Carthew K (2003) The influence of diurnal temperatures on the hydrochemistry of a tufa depositing stream. Hydrol Process 17:3421–3441
Dupré B, Dessert C et al (2003) Rivers chemical weathering and Earth’s climate. Comptes Rendus Geosci 335(16):1141–1160
Gaillardet J, Dupré B, Louvat P et al (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30
Gao QZ, Shen C (1998) River carbon flux and continental erosion. Adv Earth Sci 13(4):369–375 (In Chinese)
Gao QZ, Shen C, Sun Y (2001) Chemical weathering in Zhujiang. River drainage 30(3):223–230 (In Chinese)
Gibbs R (1970) Mechanisms controlling world water chemistry—evaporation-crystallization process. Science 170:1088–1090
Han GL, Liu C (2001) Hydrogeochemistry of Wujiang River water in Guizhou province China. Chin J Geochem 20(2):240–248
Han GL, Liu C (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21
Han GL, Liu C (2005) Hydrogeochemistry of rivers in Guizhou Province, China: constraints on weathering in karst terrain. Adv Earth Sci 20(4):394–406 (In Chinese)
He SY (1996) Field study methods in karst geochemistry and some examples. Carsologica Sinica 15(1–2):200–206 (In Chinese)
He SY, Xu SY, Zhang M (1997) Observation on soil CO2 concentration, hydrochemistry, and their relationship with karst processes. Carsologica Sinica 16(4):319–324 (In Chinese)
Hu Y, Jiang Y, Li L (2011) Preliminary study on short-time carbon absorption in epikarst spring basin—a case of the Shuifang Spring in Jinfo Mountain, Chongqing. Carsologica Sinica 30(2):169–174
Huang J, Liu P, Qin X (2011) The characteristics of karst carbon sink in the Guijiang catchment. Carsologica Sinica 30(4):437–442 (In Chinese)
Jiang YJ, Hu Y, Schirmer M (2013) Biogeochemical controls on daily cycling of hydrochemistry and δ13 C of dissolved inorganic carbon in a karst spring-fed pool. J Hydrol 478:157–168
Lal R (2008) Carbon sequestration. Phil Trans R Soc B 363:815–830
Liu ZH (1990) Necessities of measuring pH in site in the study of karst hydrogeochemistry. Carsologica Sinica 9(4):310–317 (In Chinese)
Liu ZH (2012a) New progress and prospects in the study of rock-weathering-related carbon sinks. Chin Sci Bull 57(2–3):95–102 (In Chinese)
Liu ZH (2012b) Recent advance and prospect of carbon sink caused by rock weathering. Chin Sci Bull 57(2–3):95–102
Liu ZH, Dreybrodt W (2012) Comparison of carbon sequestration capacity between carbonate weathering and forests: the necessity to change traditional ideas and methods of study of carbon sinks. Carsologica Sinicas 31(4):345–348
Liu Y, Liu Z (2010) Experimental study on the utilization of DIC by Oocystis solitaria Wittr and its influence on the precipitation of calcium carbonate in karst and non-karst waters. Carbonates Evaporites 25(1):21–26
Liu Z, Li Q, Wang J (2004) Ground water resource assessment under the conditions to control ground subsidence—a case study in Shanghai city. Carsologica Sinica 23(3):169–176 (In Chinese)
Liu ZH, Dreybrodt W, Wang H (2007) An important CO2 sink may be generated by global hydrological cycle. Chin Sci Bull 52:2418–2422 (In Chinese)
Liu C, Jiang Y, Tao F (2008) Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China. Geochimica 04:404–414 (In Chinese)
Liu ZH, Dreybrodt W, Liu H (2011) Atmospheric CO2 sink: silicate weathering or carbonate weathering. Quat Sci 31:426–430 (In Chinese)
Lü B, Liu Z, Liao C (2006) The influence of aquatic plants variation of hydrochemistry in Karst dynamic system—a case in the Guilin Karst experimental site. Carsologica Sinica 25(4):335–340 (In Chinese)
Maier-Peimer E (1993) The biological pump in the greenhouse. Glob Planet Change 8:13–15
Melnikov NB, O’Neill BC (2006) Learning about the carbon cycle from global budget data. Geophys Res Lett 33:L02705. doi:10.1029/2005GL023935
Nimick DA, Gammons CH, Parker SR (2011) Diel biogeochemical processes and their effect on the aqueous chemistry of streams: a review. Chem Geol 283(1–2):3–17
Raymond PA (2003) Cole JJ (2003) Increase in the Export of Alkalinity from North America’s Largest River. Science 301:88–91
Sundquist ET (1993) The global carbon dioxide budget. Science 259:934–941
Tang WK, Tao Z, Gao QZ (2014) Biogeochemical processes of the major ions and dissolved inorganic carbon in the Guijiang river. Environ Sci 35(6):2099–2107
Tans PP, Fung IY, Takahashi T (1990) Observational constraints on the global atmospheric CO2 budget. Science 247:1431–1438
Tao Z, Gao QZ, Wang ZG et al (2011) Estimation of carbon sinks in chemical weathering in a humid subtropical mountainous basin. Chinese Sci Bull 56:3774–3782
The Compilation Committee (1991) The history of Zhujiang. Guangzhou: Guangdong Science and Technology Press, 150 pp (In Chinese)
The Compilation Committee of Zhaoping County (1992) The history of Zhaoping County. Nanning: Guangxi People’s Publishing House pp 87–88 (In Chinese)
Watson RT, Meria Filho LG, Sanhueza E et al (1992) The supplementary report to the IPCC scientific assessment (in climate change). Cambridge Press, Cambridge, pp 29–46
Wu WH, Zheng HB, Yang JD et al (2012) Review and advancements of studies on silicate weathering and the global carbon cycle. Geol J China universities 18(2):215–224 (In Chinese)
Yao G, Gao Q (2005) The feedback and response of the riverine carbon cycle to global changes. Progress Geography 24(5):50–60 (In Chinese)
Yao G, Gao Q, Wang Z (2008) Seasonal and spatial variations of dissolved inorganic carbon in the lower reaches of the Xijiang River and its export flux. Geochimica 37(3):258–264 (In Chinese)
Yu S, He S, Yang H (2012) Research on carbon source effect of acid rain in a typical carbonate rock area,Guangxi. Earth Environ 40(1):44–49 (In Chinese)
Yuan DX, Zhu D, Weng J (1994) Karstology in China. Beijing: Beijing Geological Press, 207 pp (In Chinese)
Zhang C (2011) Time-scale of karst processes and the carbon sink stability. Carsologica Sinica 30(4):368–371 (In Chinese)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (41202185), the Project of the China Geological Survey (12120113005100), the Project of Natural Science Foundation of Guangxi (2014GXNSFBA118228) and Project of Institute of karst geology, CAGS (201320, 201429).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, S., Du, W., Sun, P. et al. Study on the hydrochemistry character and carbon sink in the middle and upper reaches of the Xijiang River basin, China. Environ Earth Sci 74, 997–1005 (2015). https://doi.org/10.1007/s12665-014-3771-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3771-y