Abstract
The concentrations of Ca, some essential (Fe, Zn, Mn, and Cu) and non-essential nutritive elements (Cd, Pb, and Ni) were measured in the edible parts (mantle and adductor muscles) of Tridacna maximx collected from south Quseir City (Red Sea). The general trend of metal contents of the different parts follows the order; Ca > Fe > Zn > Pb > Mn > Cu > Cd > Ni. The tissues before cooking recorded the highest average concentrations of Cu, Pb, Fe, Cd, Mn, and Ni (2.658, 5.250, 34.375, 1.464, 3.207, and 0.886 ppm, respectively) relative to tissues after cooking and the water of cooked tissues (WCT). The total cooked tissues recorded higher average contents of Zn and Ca (17.282 and 1,114.679 ppm) than the uncooked tissues. Calcium recorded the highest concentration in the ECT of adductor and mantle muscles (2,081.126 ± 177.39 and 1,893.326 ± 394.28 ppm). Mantle recorded higher concentrations of Pb, Mn, Ni, and Ca (7.489 ± 4.65, 4.241 ± 1.13, 0.980 ± 0.60, and 1,039.362 ± 177.42 ppm, respectively) than adductor muscle before cooking. Ca concentration levels in the WCT increased after cooking tissues especially in adductor muscles. This may attributed to the liberation of larger amount of calcium in ionic form in water. The clams may have intracellular regulatory mechanisms to keep their concentrations in equilibrium, subsequently; the recorded metal concentrations are in the safe limits for human consuming, where these concentrations did not exceed the clam’s capacity of regulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bivalves are valuable sentinel organisms (Farrington et al. 1982, 1983) as they greatly concentrate many chemical pollutants from seawater and sediment. Bivalve clams are used as bio-indicators and sedentary filter feeders as they usually have the ability to accumulate heavy metals in their tissues without metabolizing them appreciably (Gunther et al. 1999; Nasci et al. 1999; Olivier et al. 2002; Blaise et al. 2002; Fung et al. 2004; Zhou et al. 2003). The bioavailability, clam sizes, hydrodynamics of the environment, changes in tissue composition, and reproductive cycle influence the metal concentrations (Boyden and Phillips 1981). In comparison to fish and crustacean, bivalves have very low level of activity of enzymes capable of metabolizing persistent organic pollutants and reflect the magnitude of environmental contamination more accurately (Phillips 1980; Kumari et al. 2006). Most metals are generally concentrated many times over within organism’s soft tissues especially mantle and during the period of shell growth, the level in the soft parts as well as shell increased appreciably (Carriker et al. 1982; Thorn et al. 1995; Huanxin et al. 2000; Yasoshima and Takano 2001). Otchere (2003) pointed out that, element concentrations in mollusks in the same location differ between different species and individuals due to species ability to regulate and accumulate metals. Szefer et al. (1999) reported that, the soft tissues and byssi of Mutilus edulis are good bio-indicators for identification of coastal areas exposed to metal contaminations.
Tridacna maxima is the most wide-ranging giant clam species, being found from the east coast of Africa to as far east as the Red Sea and eastern Polynesia. It is recognizable by its brightly colored mantle (blue, green, and brown) and boring habit (Ellis 1999, 2000). Giant clams flesh have been traditionally used as subsistence food source (Ellis 1999). Adductor muscles and mantles are consumed as food in raw, cooked, or dried forms. Giant clam shells are sold as souvenir items (Leung et al. 1993).
Many authors studied the metal levels in the edible mollusks; in tissues and shells of Cerithidea cingulata, Crassostrea madrasensis, and Meretrix meretrix (Kesavan et al. 2010), tissue of Mytilus edulis (Pellerin and Amiard 2009; Szefer et al. 1999), and Paphia malabarica (Kumari et al. 2006). The concentration level of some elements in Tridacnidae were studied in tissues (Ishii et al. 1992; Adjei-Boateng et al. 2011; Madkour et al. 2011) and shells (Madkour 2005).
The present study aims to evaluate the concentration levels of Ca, some essential nutritive elements (Fe, Zn, Mn, and Cu) and non-essential nutritive (Cd, Pb, and Ni) in the most edible parts (mantle and adductor muscles) of the common giant clam Tridacna maxima compared to the metal safe limits to be used as food for human.
Materials and methods
Materials
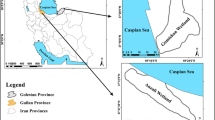
Thirty specimens of giant clam Tridacna maxima were collected from inshore zone of the Red Sea in area extended between 10 (26º0′42.78″, 34º16′24.81″) and 17 km (25º58′9.76″, 34º21′56.43″) south of Quseir city (Fig. 1). The investigated site is away from the direct human impacts as well as the flash floods. The maximum recorded depth in this site does not exceed 5 m.
Elements determination in the soft tissues (adductor and mantle muscles)
The mantle and adductor muscles of each specimen were separated from the animal tissues then subdivided into two parts. The first part (mantle and adductor) was freezed, while the second part (mantle and adductor) was dried directly at 70 °C for 48 h. For each sample, the first parts of mantle and adductor muscles were cooked in distilled water (200 ml for each sample) for about 50–60 min. The cooked parts (4.4519 ± 1.07 and 7.7958 ± 1.97 g) of adductor and mantle muscles, respectively (±standard deviations, SD) were dried at 70 °C for 48 h to remove the moisture content. The dried parts (Table 1) are ranged between 1.12 and 2.33 g (adductor muscle with average 1.5276 ± 0.36) and between 1.78 and 3.88 g (mantle muscles with average 2.484 ± 0.63). The freshly dried parts and the dried parts of the cooked samples were ground using an agate mortar for 5 min to <80 mesh in order to estimate the complete homogeneity of the samples. Generally, about 0.5 g of all powdered samples (cooked and uncooked) were digested in 10 ml of hot conc. HNO3 to near dryness and then diluted to 25 ml with de-ionized water (Chester et al. 1994). The waters of cooked tissues (WCT) were evaporated to dryness then digested in 10 ml of hot conc. HNO3 to near dryness and then filtered and diluted to 25 ml with de-ionized water (Mohammed and Dar 2010). The digested mantles and adductors as well as the WCTs were used to determine the metal contents (±SD) in the selected parts and liberated metals from the cooked parts. Calcium, some essential nutritive elements (Fe, Zn, Mn, and Cu) and non-essential nutritive (Cd, Pb, and Ni) were determined in the digested samples using atomic absorption spectrophotometer (AAS model GPC A932 Ver 1.1). The measurement accuracies were checked by applying three replicates in each measurement and the recorded metal concentrations in the uncooked and cooked tissues were expressed in μg/gm dry wt. and in ppm for the WCT.
The statistical analyses of the different samples (mantles and adductors) before and after cooking as well as the WCT were applied using the multiple comparisons (Bonferroni) of the ANOVA test using SPSS (Ver. 11). The standard deviation was calculated using software surfer 8.
Results
Heavy metal concentration of giant clam T. maxima before cooking
Iron recorded the highest concentration of the essential elements before cooking in the mantle (36.859 ± 20.44 ppm), while the adductor muscle recorded 31.891 ± 20.59 μg/gm dry wt., followed by Zn which recorded 14.497 ± 3.97 and 14.394 ± 2.37 μg/gm dry wt. (in the adductor and male muscles, respectively). Generally, mantle recorded the higher mean concentrations of metals Pb, Mn, Ni, and Ca (7.489 ± 4.65, 4.241 ± 1.13, 0.980 ± 0.60, and 1,039.362 ± 177.42 μg/gm dry wt., respectively) than the adductor muscle (Table 2) while adductor muscle recorded a relatively high concentration for Cu, Zn, and cd (3.061 ± 1.51, 14.497 ± 3.97, and 1.856 ± 0.92 μg/gm dry wt., respectively).
Heavy metal concentration of giant clam T. maxima after cooking
The metal concentrations are almost the same pattern after cooking where, Cu, Fe, Pb, Mn and Ca recorded the highest concentrations (2.240 ± 0.71, 33.504 ± 7.38, 3.862 ± 5.66, 4.026 ± 0.99 and 1,122.285 ± 200.13 μg/gm dry wt., respectively) in mantle tissues (Table 2) while the adductor muscle recorded a slightly high metal concentrations for Zn and Ni (17.328 ± 4.95 and 1.112 ± 1.09 μg/gm dry wt., respectively). Cd recorded nearly equal level in both adductor and mantle muscles (1.0 μg/gm dry wt.).
A general trend in metal concentration of adductor and mantle muscles were observed for Ni, Cu, Cd, Pb, Mn, and Fe; where Fig. 2 illustrated that, metals concentration of tissues before cooking is slightly higher than the cooked tissues, but the Zn and Ca concentrations increases in tissues after cooking (Fig. 2).
Heavy metal concentration in WCT
The WCT showed different patterns in the metal concentrations, mantle recorded higher Cu, Cd, Pb, Mn, and Fe concentrations (1.706 ± 0.50, 0.184 ± 0.31, 2.620 ± 0.87, 3.632 ± 1.48, and 31.382 ± 9.63 ppm, respectively) than those of the adductor muscles (Table 2; Fig. 2). Inversely, the adductor muscles recorded relatively high Zn, Ni, and Ca concentrations (6.644 ± 3.44, 0.855 ± 0.41, and 2,081.126 ± 177.39 ppm, respectively). Generally, Fe recorded the highest values among the essential and non-essential elements for tissues (before and after cooking) and the WCT.
Metal concentrations of total tissue before and after cooking and the WCT
The average concentration of heavy metals in the total tissue of T. maxima before and after cooking as well as the WCT showed a general trend in their concentration and accumulation (Table 3) as following graduation for the essential (Cu, Fe, Mn, and Zn) and non-essential elements (Ni, Cd, and Pb) as well as Ca as a major constituting element: Ca > Fe > Zn > Pb > Mn > Cu > Cd > Ni. Generally the average concentration of Cu, Pb, Fe, Cd, Mn, and Ni showed higher values (2.658, 5.250, 34.375, 1.464, 3.207, and 0.886 μg/gm dry wt., respectively) in the total tissues before cooking than the cooked tissues and the WCT. While the cooked tissues showed higher concentrations for Zn and Ca (17.282 and 1,114.679 μg/gm dry wt.) than uncooked tissues.
Calcium is one of the major constituting element for the giant clam shells, where it recorded the highest concentration in the WCT of mantle and adductor muscles (2,081.126 ± 177.39 and 1,893.326 ± 394.28 ppm) with average of the total WCT 1,987.226 ppm followed by the cooked tissues (mantle 1,122.285 ± 200.13 μg/gm dry wt. and adductor muscle 1,107.072 ± 133.79 μg/gm dry wt.) with mean of 1,114.679 μg/gm dry wt. of the total tissue. The tissues before cooking recorded the lowest concentrations (715.492 ± 383.84 μg/gm dry wt. for adductor muscle, 1,039.362 ± 177.42 μg/gm dry wt. for mantle, and 877.427 μg/gm dry wt. for the total tissues). ANOVA-multiple comparison test (Bonferroni test) illustrated that, there are many significantly differences in the heavy metal concentrations between the uncooked and the cooked tissues for Cu, Zn, and Ca at P values of 0.023, 0.006, and 0.014, respectively (where P is significant at 0.05). On the other hand, another significant differences were observed (Table 4) between the cooked tissues and their WCTs for Zn at 0, Cd at 0.002, and Ca at 0 (where P = 0.05).
Discussion
The trace metals can be divided into essential elements and non-essential elements, the essential elements occur normally in all organisms. The high doses of the essential elements can be poisonous and causes hazardous effects on organisms (Kesavan et al. 2010). The non-essential elements do not have any positive effects on organisms and they are harmful already in low doses. Adjei-Boateng et al. (2011) illustrated that, metal concentrations in the clam tissues were highly variable over the sampling period and seemed to be influenced by the reproductive cycle of the clam. On contrary, Denton et al. (1999) pointed out, the metal concentration in the soft tissues of the studied mollusks are relatively low especially for lead, nickel and cadmium.
The elements (Cu, Fe, Mn, and Zn) are essential nutrition elements for most marine organisms (Kesavan et al. 2010), where copper is essential for respiration and other enzymatic functions as well as the importance of other elements, where the high doses causes changing to poisonous and can cause hazardous effects on organism. During the present study, the concentrations of the essential elements were low and within the safe limit proposed by many authors (Table 5). The low concentrations may be due to the availability of these metals in the environment (Richardson et al. 2001; Palpandi et al. 2010), where the studied site was not impacted with exception of presence of few small fishing boats in the area (not exceed 5). The effectiveness of metal uptake may differ in relation to ecological need and metabolism of animals and concentration of the heavy metals in water, food, and sediment as well as some other factors such as salinity, temperature and interacting agents (Roesijiadi and Robinson 1994). The present results suggested that, the level of contamination of these metals does not exceed the clam’s capacity of regulation (Amiard et al. 1987; Durou et al. 2005).
In the present study, the recorded concentrations of the Fe and Zn, as essential elements, were within the safe limits proposed by FAO/WHO (1984); Eisler (2010a, b); Ishii et al. (1992) where the biological and physiological roles of these clam may be the main reason for their concentrations variability (Phelps et al. 2003; Ferreira et al. 2004). The clams also have intracellular regulatory mechanisms (Luoma and Rainbow 2008; Ferreira et al. 2004; Luoma and Rainbow 2008; Madkour et al. 2011) to keep their concentrations in the clam tissues in equilibrium, where the concentration levels of Fe and Zn in the present study do not exceed the clam’s capacity of regulation (Wang et al. 2002). The non-essential elements (Pb, Cd, and Ni) do not have any positive effects on organisms and they may harmful in low concentrations. They can inhibit an essential elements and cause enzymatic disturbances to the body. The safe limits of these heavy metals were proposed by FAO/WHO (1984) for some bivalves and gastropods reached to 10 ppm for Pb and 0.5 ppm/day for a meal contain Cd and 80 ppm for Ni. On the other hand, the safe limits for these elements in the edible bivalves including Tridacna for human consuming were 10 ppm for Pb (according to Eisler 2010a, b; WHO, World Health Organization 1999), 2.4–3.5 ppm for Cd (Ishii et al. 1992), and 80 ppm for Ni (Sankar et al. 2006; Eisler 2010a, b). The recorded concentrations in the present study illustrated that, these elements were within the acceptable safe limit and permissible for human consuming as a food (Amisah et al. 2011) with exception of Cd which recorded a relatively higher concentration (0.09–1.46 ppm) in T. maxima than the recorded values of FAO/WHO (1984); Gregori et al. (1996) and Blasco et al. (1999) in some bivalves but lies within the safe limits of other bivalves including Tridacnidae. However, the main reason for the moderately low concentration of most metals may be related to the intracellular regulatory mechanisms that keep their concentrations in the clam tissues in equilibrium and not increase (Madkour et al. 2011) and may be due to the change in the metabolic rates of bivalves and their bioavailability to accumulate heavy metals (Otchere 2003). Moreover, the present study is in accordance to (Vazquez et al. 1993) who suggested that, the metal levels in the surroundings are low and are not interfering with normal metabolic processes of T. maxima.
The risk associated with the consumption of T. maxima were ascertained by comparing the studied metals concentrations of the tissue before and after cooking to the WHO, World Health Organization (2000) safety reference standards and other references listed in Table 5. The recorded heavy metals in present study revealed that, the metal concentrations were within the permissible limits for human consumption. Cooking process may play the major role in the recorded differences of metal concentrations before and after cooking as well as in the WCT; so, the increasing in water concentrations may be attributed to the metals liberation from the cooked tissues.
The high concentrations of calcium in the tissues may be related to the biological and physiological processes of the animal tissues in the formation of calcium carbonate of the shell because of the inorganic phase of calcium carbonate contributes 98 % of the shell mass (Palpandi et al. 2010). On the other hand, the WCT recorded very high Ca concentrations that may reflect the bioaccumulation in tissues the marine organisms such as, bivalves, gastropods, corals, and fish (Kesavan et al. 2010).
The concentration levels of Cu, Pb, Fe, Cd, Mn, and Ni are decreased in the cooked tissues may be due to the ability of these metals to liberate in the WCT. Inversely, Ca and Zn were increased because of the metals tend to concentrate in the cooked tissues where the cooking process may play a vital role in condensing these two essential elements for the animal growth and nutritional. Moreover, Ca and Zn may have the ability to associate with non-metal phosphorus in a phosphate form [Ca3 (Po4)2 and Zn3 (Po4)2]. The increased concentrations after cooking may be related to the metal containing tissues (Ishii et al. 1992). The increase in the Ca concentration in the WCT of adductor muscles than the mantles after cooking may be attributed to the liberation of larger amount of calcium from adductor muscles in ionic form in water with higher ratios than mantle. In addition to the used water (distilled water) contents of calcium that causes their elevation with relatively high values. The recorded significance decrease of Cu (P = 0.023) in the cooked tissues is attributed to the liberation of few amount from this element in ionic form, while the significance increase of Zn and Ca (P = 0.006 and P = 0.014, respectively) in the cooked tissues may be due the probability of formation of calcium phosphate and zinc phosphate, phosphorous was found in complicated bonds of phosphate form where [Ca3 (Po4)2 and Zn3 (Po4)2] as illustrated by Ishii et al. (1992).
Finally, the heavy metals in the giant clams T. maxima are in the safe acceptable limits and can be regulated by their soft tissues. Therefore, the giant clams should be cultured for human exploiting, where the adductor muscle and mantle are consumed as food in row, cooked, or dried forms (Leung et al. 1993).
Conclusion
Adductor and mantle muscles before cooking recorded higher concentrations of Cu, Pb, Fe, Cd, Mn, and Ni than after cooking and the WCTs, inversely the cooked tissues recorded higher Zn and Ca concentrations than uncooked tissues.
General trend of metal contents in the different tissue parts follows the order of Ca > Fe > Zn > Pb > Mn > Cu > Cd > Ni. The metal concentrations of Ca and Zn increased in tissues after cooking. This is may be related to the ability of these metals to diffuse in or from the cooked tissues and the used water. These metals may associated with non-metal phosphorus; probably forming an insoluble divalent metal phosphate [Ca3 (Po4)2 and Zn3 (Po4)2].
The recorded metal concentrations in the edible parts of T. maxima were in the safe acceptable limits of consuming subsequently, we recommended by wide scale of culturing for human exploiting.
References
Adjei-Boateng D, Obirikorang KA, Amisah S, Madkour HA, Otchere FA (2011) relationship between gonad maturation and heavy metal accumulation in the clam, galatea paradoxa (Born 1778) from the volta estuary ghana. Bull Environ Contam Toxic 87:626–632
Amiard JC, Amiard-Triquet C, Berthet B, Metayer C (1987) Comparative study of the patterns of bioaccumulation of essential (Cu, Zn) and Non-essential (Cd, Pb) trace metals in various estuarine and coastal or-ganisms. J Exp Mar Biol Ecol 106:73–89
Amisah S, Obirikorang KA, Adjei-Boateng D (2011) Bioaccumulation of heavy metals in the volta clam, Galatea Paradoxa (Born, 1778) in relation to their geo-accumulation in benthic sediments of the volta estuary, ghana. Water Qual Expo Health 2:147–156
Blaise C, Gagn′e F, Pellerin J, Hansen P-D, Trottier S (2002) Molluscan shellfish biomarker study of the Qu′ebec, Canada, Saguenau Fjord with the soft-shell clam Mya arenaria. Environ Toxicol 17:170–186
Blasco J, Arias AM, Saenz V (1999) Heavy metals in organisms of the river Guadalquivir estuary, possible incidence of the Aznalcollar disaster. Sci Total Environ 242(3):249–259
Boyden CR, Phillips DJH (1981) Seasonal variation and inherent variability of trace elements in oysters and their implication for indicator studies. Mar Ecol Prog Ser 5:29–40
Butler CA, Timperley M (1996) Fertilised farmland as a source of cadmium in oysters. Sci Total Environ 181:3–44
Carriker MR, Swann CP, Ewart JW (1982) An explanatory study with the protein microprobe of the ontogenetic distribution of 16 elements in the shell of living oysters (Crassostrea virginica). Mar Biol 69:235–246
Chester R, Lin FG, Basaham AS (1994) Trace metals solid state speciation changes associated with the down-column fluxes of oceanic particulates. J Geol Soc Lond 151:351–360
Denton GRW, Concepcion LP, Wood HR, Eflin VS, Pangelinan GT (1999) Heavy metals, pcbs and pahs in marine organisms from four harbor locations on guam. A Pilot Study. Water and Environmental Research Institute of the Western Pacific Uni. of GUAM. Technical report, pp 158
Durou C, Mouneyrac C, Amiard-Triquet C (2005) Tolerance to metals and assessment of energy reserves in the polychaete Nereis Diversicolor in clean and contami-nated estuaries. Environ Toxicol 20(1):23–31
Eisler R (2010a) Compendium of trace metals and marine biota. vol. 1: plants and invertebrates. Elsevier Publisher. pp 610
Eisler R (2010b) Trace metal concentrations in marine organisms. Pergamon Press Inc., New York, pp 140–325
Ellis S (1999) Aquafarmer information sheet lagoon farming of giant clams (Bivalvia: Tridacnidae). Center for tropical and subtropical aquaculture publication no. 139: pp 6
Ellis S (2000) Nursery and grow-out techniques for giant clams (Bivalvia: Tridacnidae). Center for tropical and subtropical aquaculture publication no. 143:99
FAO/WHO (1984) Joint FAO/WHO Food standers program, codex alimentarius commission contamination. CAC/Vol. XV11.FAO, Roma and WHO, Geneva
Farrington JW, Risebrough RW, Parker PL, Davis AC, Delappe B, Winters JK, Boatwright D, Eraq NM (1982) Hydrocarbons, polychlorinated biphenyls and DDT in mussels and oysters from the US coast, 1976-1978 (The Mussel Watch) WHOI, Tech Rep WHOI-82-42
Farrington JW, Goldberg ED, Risebrough RW, Martin JH, Bowen VT (1983) US “Mussel Watch” 1976–1978: an overview of the trace metal, DDT, PCB, hydrocarbon and artifical radio- nuclide date. Environ Sci Technol 17:490–496
Ferreira GA, Machado ALS, Zalmon IR (2004) Temporal and spatial variation on heavy metal con-centrations in the bivalve Perna Perna (Linnaeus, 1758) on the northern coast of Rio de Janeiro State, Brazil. Brazilian Arch Bio Technol 47(2):319–327
Fung CN, Lam JCW, Zheng GJ, Connell DW, Monirith I, Tanabe S, Richardson BJ, Lam PKS (2004) Mussel-based monitoring of trace metal and organic contaminants along the east coast of China using Perna viridis and Mytilus edulis. Environ Pollut 127:203–216
Gregori I, Pinochet H, Gras N, Munoz L (1996) Variability of cadmium, copper, and zinc levels in molluscs and associated sediments from Chile. Environ Pollut 92(3):359–368
Gunther AJ, Davis JA, Hardin DD, Gold J, Bell D, Cricks JR, Scelfo G, Stephenson M (1999) Long-Term bioaccumulation monitoring with transplanted bivalves in the san francisco estuary. Mar Pollut Bull 38(3):170–180
Huanxin W, Lejun Z, Presley BJ (2000) Bioaccumulation of heavy metals in oyster (Crassostrea virginica) tissue and shell. Environ Geol 39(11):1216–1226
Ishii T, Okoshi K, Otake T, Nakahara M (1992) Concentrations of elements in tissues of four species of Tridacnidae. Nippon Suisan Gakkaishi 58(7):1285–1290
Kesavan K, Rajagopal S, Ravi V, Shanmugam A (2010) Heavy metals in three molluscs and sediments from vellar estuary, southeast coast of india. Carpathian J Earth Environ Sci 5(2):39–48
Kumari LK, Kaisary S, Rodrigues V (2006) Bio-accumulation of some trace metals in the short-neck clam Paphia malabarica from Mandovi estuary, Goa. Environ Int 32:229–234
Leung P, Shang YC, Wanitprapha K, Tian X (1993) Production economics of Giant Clam (Tridacna species) culture systems in the U.S.-Affiliated Pacific Islands. Publication of Center for Tropical and Subtropical Aquaculture, 114: pp 40
Luoma SN, Rainbow PS (2008) Metals contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge
Madkour HA (2005) Distribution and relationships of heavy metals in the giant clam (tridacna maxima) andassociated sediments from different sites in the egyptian red sea coast. Egypt J Aquat Res 31(2):45–59
Madkour HA, Obirikorang KA, Amisah S, Otchere FA, Adjei-Boateng D (2011) Relationship between Heavy Metal Concentrations in Bottom Sediments and the Clam, Galatea Paradoxa (Born 1778) from the Volta Estuary, Ghana. J Environ Protect (JEP) 2:720–728
Mohammed TAA, Dar MA (2010) Ability of corals to accumulate heavy metals, Northern Red Sea Egypt. J Environ Earth Sci 59(7):1525–1534
Nasci C, Da Ros L, Campesan G, Van Vleet ES, Salizzato M, Sperni L, Pavoi B (1999) Clam transplantation and stress-related biomarkers as useful tools for as-sessing water quality in coastal environments. Mar Pollut Bull 39(1):255–260
Olivier F, Ridd M, Klumpp D (2002) The use of trans-planted cultured tropical oysters (Saccostrea Commer-cialis) to monitor Cd levels in North Queensland Coastal waters (Australia). Mar Pollut Bull 44(10):1051–1062
Otchere FA (2003) Heavy metals concentrations and burden in the bivalves (Anadara (Senilia) Senilis, Crassostrea tulipa and Perna Perna) from Lagoons in Ghana: model to describe mechanism of accumulation/excretion. Afr J Biotechnol 2(9):280–287
Palpandi C, Kesavan K, Shanmugam A (2010) Gastropod shells used as a biomonitor. Scholars research library. Annals of. Biol Res 1(1):53–60
Pellerin J, Amiard J (2009) Comparison of bioaccumulation of metals and induction of metallothioneins in two marine bivalves (Mytilus edulis and Mya arenaria). Comp Biochem Physiol C 150:186–195
Phelps HL, Wright DA, Mihursky JA (2003) Factors affecting trace metal accumulation by estuarine oysters, Crassostrea Virginica. Mar Ecol Prog Ser 22:197–204
Phillips DJH (1980) Quantitative aquatic biological indicators: Their use to monitor trace metal and organochlorine pollution. Appl Sci Publ, London 136
Rayment GE, Barry GA (2000) Indicator tissues for heavy metal monitoring-additional attributes. Mar Pollut Bull 41(7–12):353–358
Richardson CA, Chenery SRN, Cook JM (2001) Assessing the history of trace metal (Cu, Zn, Pb) contamination in the North Sea through laser ablation—ICP-MS of horse mussel Modiolus modiolus shells. Mar Ecol Prog Ser 2001(211):157–167
Roesijiadi G, Robinson WE (1994) Metal regulation in aquatic animals: mechanism of uptake, accumulation and release. In: Malins DC, Ostrander GG (eds) Aquatic toxicology (molecular, biochemical and cellular perspectives). Lewis Publishers, London, p 539
Sankar TV, Zynudheen AA, Anandan R, Nair PGV (2006) Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerala, India. Chemosphere 65:583–590
Szefer P, Ali AA, Ba-Haroon AA, Rajeh AA, Geldonn J, Nabrzyski M (1999) Distribution and relationships of selected trace metals in mollusks and associated sediments from the Gulf of Aden, Yemen. Environ Pollut 106:299–314
Thorn K, Cerrato RM, Rivers ML (1995) Elemental distributions in marine bivalve shells as measured by synchrotron X-Ray fluorescence. Biol Bull 188(1):57–67
USEPA, United States Environmental Protection Agency (1994) Sediment sampling. SOP#: 2016 (cited in Amisah et al. 2011)
Vazquez GF, Sanchez GM, Virender KS (1993) Trace metals in the oyster Crassostrea virginica of Terminos Lagoon, Campeche Mexico. Mar Pollut Bull 26(7):398–399
Wang WX, Yan QL, Fan W, Xu Y (2002) Bioavailability of sedimentary metals from a contaminated bay. Mar Ecol Prog Ser 240:23–38
WHO World Health Organization (1999) Monographs on selected medicinal plants 1, Geneva
WHO World Health Organization (2000) Safety evaluation of certain food additives and contaminants. WHO food additives series, 44th edn. Cambridge University Press, Cambridge
Yasoshima M, Takano B (2001) Bradybaena similaris (Férrusac) Shell as a Biomonitor of Copper, Cadmium, and Zinc. Bull Environ Contam Toxic 66(2):239–248
Zhou Q-f, Li Z-Y, Jiang G-B, Yang R-Q (2003) Preliminary investigation of a sensitive biomarker of organotin pollution in Chinese coastal aquatic environment and marine organisms. Environ Pollut 125:301–304
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammed, T.A.A., Mohamed, E.M., Ebrahim, Y.M. et al. Some metal concentrations in the edible parts of Tridacna maxima, Red Sea, Egypt. Environ Earth Sci 71, 301–309 (2014). https://doi.org/10.1007/s12665-013-2434-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2434-8