Abstract
Sap flows of four typical species, Populus. russkii Jabl., Populus euphratica Olive., Ulmus pumila L., and Elaeagnus angustifolia L., of artificial shelter forest in a desert area were monitored in all-weather conditions using SF300 Sap flow Meter based on the theory of thermal compensation. Meanwhile, air temperature (T a), leaf temperature (T l), soil temperature (T s), relative humidity (RH), and wind velocity (V w) were simultaneously recorded by an automatic weather station. The results indicate that (1) the diurnal processes of stem sap flows of P. russkii Jabl., P. euphratica Olive., and U. pumila L., but not E. angustifolia L., show an obvious circadian rhythm. Significant differences of stem sap flow rates were found among species, but not genus. The average sap flow rate of P. russkii Jabl. is 13.8-fold of that of E. angustifolia L. The order of sap flux density (SFD) from the largest to the smallest is P. russkii Jabl., P. euphratica Olive, U. pumila L. and E. angustifolia L.; (2) compared with 373 mm, 747 mm irrigation can induce microenvironmental changes that result in the suppression of photosynthesis and transpiration and the decline of stem sap flow rates of the above four species, indicating 373 mm irrigation meets the growth needs of the above species during experiment; (3) sap flow rates are different at different stem positions: the flow rates of P. russkii Jabl., U. pumila L. and E. angustifolia L., but not P. euphratica Olive, decline gradually from cambium to pith; (4) the correlation analysis indicates that stem sap flow is negatively correlated with RH and T s and positively correlated with T a, T l and saturation vapor pressure deficit (VPD). The sap flow rate of P. russkii Jabl. is significantly affected by V w due to its large size and height. In addition, a model was established by stepwise regression analysis to estimate the relationship between the environmental factors and stem sap flows of the above four typical species of shelter forest in the desert area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil–plant–atmosphere continuum (SPAC) is a unified, dynamic, reciprocal and continuous system, where water plays a crucial role. The water consumption and transportation laws of plants, and the intermediate links of the continuum become an active field in ecological and hydrological research. In desert areas, the natural environment is so harsh that a small change in water resources has become a major factor impacting the regional eco-hydrological processes and sustainable development. In order to manage and utilize the limited water resources in desert areas more rationally, understanding botanic water transportation mechanisms and consumption characteristics, and ecological water consumption needed for maintenance of ecological stability are of great practical significance.
Transpiration, the evaporation process of water absorbed by plants from soil into the atmosphere, plays an important role in plant physiology, the hydrological cycle and global energy balance (Bauerle et al. 2002). Transpiration is the process by which moisture is carried through plants from roots to small stomata on the underside of leaves, where it changes to vapor and is released to the atmosphere. In favorable circumstances, the total stomatal transpiration is generally 80–90% or more (Levitt 1980). Plant roots absorb water more than 99.8% of consumption on the transpiration (Xu et al. 2008). For much of the vegetation canopy density, the transpiration is the main part of the evaporation, and forest floor evaporation is only a small part. The water demand of plants is often limited by the water condition of their environments (Zeng et al. 2000). Changes in environmental water conditions will affect the water physiological characteristics of plants, including water consumption by transpiration, water potential (Fu et al. 2005) and photosynthesis (Zhou et al. 2008). Thus, these water physiological characteristics can be used as a basis to diagnose the water state of plants. The sap flow of plants can accurately reflect the plants’ transpiration and is very sensitive to changes in environmental water conditions. Thus it is an important parameter in studies on plants–water relationship (Nadezhdina 1999), and the main criteria for irrigation regulation (Conejero et al. 2007; Fernandez et al. 2008a, b). By monitoring plant sap flow, people can more accurately estimate plant water state and assess the effect of irrigation, therefore, implementing reasonable irrigation while saving water. Because of the inherent fragility of the environment in desert areas, plants in a shelter forest can effectively protect, control, stabilize and improve the ecological environment. Studies on the relationship between environmental factors and the transpiration features of plants in a shelter forest in desert regions will help to understand the law of water consumption in shelter forest, to select water-saving species, to reasonably combine and distribute species, and provide guidance to resolve the contradictions between water supply and demand.
In this study, four typical species in the northwest edge of an artificial ecological shelter forest in Gurbantunggut Desert were studied. Their stem sap flows under different irrigations and environmental factors were dynamically monitored and used to analyze the characteristics of sap flow and water consumption and the impacts of environmental factors. The study provides theoretical support for water utilization of an artificial forest and scientific basis for species breeding and selection in ecological restoration and reconstruction of a shelter forest.
Materials and methods
Overview of the study area
The study area is a 515.27 ha m2 artificial ecological shelter forest located in the lacustrine desert of ancient Lake Manas, northwest edge of Gurbantunggut Desert, Junggar Basin and south Jayer Mountain, Xinjiang, China (Fig. 1). It has typical temperate, continental desert climate, i.e., cold in winter and hot in summer. The hottest month is July with an average temperature of 27.6°C and the coldest month is January with an average temperature of −16.3°C. Its annual average temperature is 8.1°C, average annual sunshine 2,743.6 h, percentage of sunny day 61%, solar radiation 553.4 kJ cm−2, annual accumulated temperature of ≥10°C is 3,968°C, average annual precipitation 105.3 mm, potential transpiration 3,545 mm, ratio of evaporation to precipitation 34:1, and the annual gale days are 71.4 days (with wind greater than 17.2 m s−1). Its major land is desert. The main plant species of its shelter forest are P. russkii Jabl., P. euphratica Olive, U. pumila L. and E. angustifolia L., etc. All of these were planted as monocultures in 25 by 800 m plots 9 years before the study. P. euphratica Olive, U. pumila L. and E. angustifolia L. are native species while P. russkii Jabl. is introduced. Considering the artificial ecological shelter forest is located in the arid area with less natural precipitation, deep shallow groundwater level and high salinity at soil surface, flood irrigation (747 mm) approach is adapted at beginning. Through flood irrigation, the salt of surface soil is dissolved, and with the saltwater infiltration into the deep soil, surface soil salinity reduced. Thus, there was an increase in the survival rate of seedlings. However, many years’ flood irrigation also rapidly lifted the groundwater level and subsequently caused soil secondary salinization.
Setting of the monitored sample field
Sample plots are located at longitude 84°54′27.7″ east, latitude 45°28′51.7″ north and altitude 286 m in the key shelter forest adjacent to the northern area of the studied desert. In early 2007, eight 25 m × 30 m sections with flat terrain, uniformly fertilized soil and even-sized trees were selected as sample fields. Four sections were irrigated with a total of 373 mm water, 66 mm in May, 80 mm each in June, July and August, and 67 mm in October, and the other four sections were irrigated with a total of 747 mm water, 133 mm in May, 160 mm each in June, July and August, and 133 mm in October. Two sections with different irrigations were assigned for each of the typical four plants mentioned above. During the experiments, the trees under two irrigation treatments, morphological feature (Table 1) (plant height, diameter, sapwood area) showed no statistically significant difference (p > 0.05).
Sap flow monitoring and parameter determination
Sap flow was measured using an SF300 Sap Flow Meter (4 probes; Greenspan Technology Pty Ltd, Australia). Its basic principle is to obtain sap flow rate in xylem by measuring the percentage of increase along xylem vessels of the pulse produced by a small pulse source injected into the plant. Trees that grew well under different irrigations and had straight trunk, moderate crown, smooth bark, and no pests and diseases in the sample fields were selected as samples and monitored continuously for 24 h every day in 11 consecutive days from July 11 to July 21, 2008. Four plants of the same species from each sample plot were selected as replicates and their sap flow rates under different irrigation conditions were simultaneously measured using eight SF300T probes. Sap flow rates of P. russkii Jabl., P. euphratica, U. pumila L. Olive and E. angustifolia L. were measured during July 11th to 13th, July 13th to July 15th, July 16th to July 18th, and July 19th to July 21st, respectively, and expressed as the average of the four plants under the same irrigation condition.
The heat pulse rate \( V^{\prime}_{\text{h}} \) was automatically measured every 30 min. The stem sap flow rate V s is calculated using the following formula (Edwards and Warwick 1984):
where F m is the wood volume factor, F l is the water volume factor. F m and F l can be obtained from the following formulas:
where W f is the fresh weight of sapwood, W d is the dry weight of sapwood, W i is the mass of water leached from the same sapwood.
The stem sap flow flux Q is determined by
where A is the cross-sectional area of sapwood at diameter, \( \overline{{V_{\text{s}} }} \) is the average of sap flow rates monitored by using four probes of the SF300 flow meter separately inserted into sapwood at different radial depths. To avoid damage to the sample trees, 24 well-growing plants of each species excluding the sample trees were selected to determine using increment-borer methods for the thickness of the wood at breast height and the breast circumference, which were then converted into diameter of breast height (DBH). Sapwood area was calculated using the sapwood thickness. The bark thickness was also measured simultaneously.
Micrometeorological observations
Micrometeorological data, including air temperature (T a) (ATH-2, PhyTech Ltd, USA), air relative humidity (RH) (ATH-2, PhyTech Ltd, USA), wind velocity (V w) (010C-1, PhyTech Ltd, USA), soil temperature (T s) (SMS-2M, PhyTech Ltd, USA) at 10 cm, and leaf temperature (shade leaves) (T l) (LT-2M, PhyTech Ltd, USA) were monitored continuously and recorded every 30 min through automatic meteorological observation station and synchronized with the monitoring of sap flow. The vapor pressure deficit (VPD) was calculated in accordance with meteorological data to comprehensively express synergistic effects of temperatures and RH.
Results and analysis
Diurnal process of stem sap flow
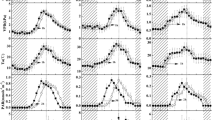
Under sunny weather conditions, diurnal variations of stem sap flows of P. russkii Jabl., P. euphratica Olive and U. pumila L., but not E. angustifolia L., showed obvious diurnal rhythm (Fig. 2), and their start times, peaks and minimum rates of stem sap flow are similar. The stem sap flows of P. russkii Jabl. P. euphratica Olive and U. pumila L. started around 6:30–7:00, basically synchronized with the sunrise. Their flow rates reached peaks between 11:00 and 15:00, started to decrease between 19:30 and 20:30, and reached minimum between 0:00 and 5:30. Overall, the diurnal alternation of flow rates shows a single peak with small fluctuations, consistent with the results of Populus hosiensis C. and Calligonum mongolicum (Chang and Zhao 2004a, b; Xu et al. 2006). The flow rates of the four species were stably maintained at low levels at night, indicating that plant roots still retained a certain degree of water absorption capacity at night to meet water balance and supplement water loss caused by transpiration at daytime (Wang et al. 2002). The stem sap flow of E. angustifolia L. showed no significant circadian rhythm and was always maintained at a relatively low level, consistent with the studies on E. angustifolia L. in the middle and lower regions of Heiher River (Chang and Zhao 2004a, b). The possible explanations for why E. angustifolia L. showed no obvious circadian rhythm are: (1) E. angustifolia L. itself is a very drought-tolerant plant, therefore its water consumption due to transpiration is very low; (2) its branches, leaves, stems and fruits are covered with silver-white scales and stellate hairs, and its leaves are relatively small, thus weakening the impacts of external environment on its transpiration and resulting in a stable, low sap flow rate. The result is in contrast to a previous report (Zhang et al. 2006) showing that sap flow rate of E. angustifolia had clear circadian rhythm. This discrepancy may be caused by a different habitat.
Comprehensive examination of 30 years’ results of individual plants in 65 species of 35 genera indicates that the water consumption of 90% plants is in the range of 10–200 kg (Wullschleger et al. 1998). Table 2 indicates that sap flow rates of P. russkii Jabl. and P. euphratica Olive are similar. These similarities may result from the fact that they belong to the same genus and have similar physiological structures. Thus, under the same weather conditions, they have similar characteristics. However, the initial flow rate of P. russkii Jabl. is faster than that of P. euphratica Olive. It has been shown that morphological characteristics have significant effects on the stem sap flow. The responses of tall plants to environmental changes are faster than short plants (O’Brien et al. 2004). P. russkii Jabl. is taller than P. euphratica Olive.; therefore, the fact that P. russkii Jabl. has greater initial flow rate than P. euphratica olive. can be ascribed to its taller height and quicker response to sunrise. The differences of average daily flow rates of P. russkii Jabl., P. euphratica Olive., E. angustifolia L. and U. pumila L. are significant (p < 0.05). In particular, the differences of average daily flow rates and densities of sap flow flux reached 13.8-fold and 14.3-fold, respectively, between the P. russkii Jabl. and Elaeagnus angustifolia L., indicating the difference between genera is significant. At the same depth, the stem sap flow rates of the four species are orientation dependent, which on the sunny sides are faster (p < 0.05) than those on the shady side. The difference is more prominent when sunlight directly irradiates on the tree at noon, consistent with the previous report (Zhang et al. 2004).
Characteristics of flow rates at different radial depths
Similar to the stem flow rates, the flow rates at different radial depths of P. russkii Jabl., P. euphratica Olive. and U. pumila L., but not E. angustifolia L., show obvious diurnal rhythm, which can be divided into starting, peaking, reducing and low rate maintaining stages. The xylem sap flow rates along the radial depths of P. russkii Jabl., E. angustifolia L., and U. pumila L. show the declining tendencies from the cambium to the pith, in agreement with the research on Acacia mangium (Ma et al. 2007) and Caragana sinica (Xia et al. 2008). Zhang et al. (2004) has presented that the xylem sap flow rates along the radial depths of P. euphratica Olive show increasing tendencies from the cambium to the pith, while the same trend was not found in this study. The order of flow rates is that at radial depth of 14 mm > 5 mm > 10 mm > 19 mm, in other words, the flow rate from cambium to pith first decreases, then increases, and again decreases (Fig. 3).
Sap flow characteristics in different irrigations
The stem sap flows of the four species in the sample plots irrigated with 373 mm water are different from those irrigated with 747 mm water correspondingly. It is noteworthy that their sap flow rates and flux densities decline with the increase of irrigation, as shown in Fig. 2. The sap flow flux densities of P. russkii Jabl., P. euphratica Olive, E. angustifolia L. and U. pumila L. decrease from 0.85, 0.81, 0.06, and 0.24 to 0.81, 0.73, 0.05 and 0.19 L cm−2 day−1, respectively, as shown in Table 2. They declined by 4.7, 9.9, 16.7 and 20.8%, respectively. These phenomena are contrary to the tendencies of stem sap flows of Calligonum mongolicum (Xu et al. 2006), Haloxylon ammodendron (Xie et al. 2008), and Cymnocarpos fruticosus (Yuan et al. 2008) planted in arid areas following the same irrigation changes. The characteristics of stem sap flows of the four species in different irrigation plots are similar. As shown in Fig. 2, at night and weak light conditions, its sap flow rate in the 747 mm irrigation plot is lower than that in the 373 mm irrigation plot, while around noon, its sap flow rate in the 747 mm irrigation plot is higher than that in the 373 mm irrigation plot.
The variation in the stem sap flow rate is the result of interaction among multiple environmental factors. The stem sap flow is caused mainly by transpiration and its rate directly reflects the transpiration rate of the plant. Transpiration of the plants is affected by external environmental factors, and changes with the gradient of vapor pressure between the leaf surface and its surrounding atmosphere. It has been shown that transpiration intensity increases with increased air dryness and temperature (Jiang 2004), and is inversely related to stomatal conductance and VPD (Lambers et al. 1998). Although light has direct and indirect effects on stomatal conductance, researches on plant water utilization have shown that the transpiration decline caused by decreased stomatal conductance is attributed to the increased VPD (Monteith 1995; Whitehead 1998). When RH increases, VPD will diminish correspondingly, leading to the decreases of the water potential difference between the leaf and its surrounding atmosphere, the stomatal conductance on the leaf surface, and the transpiration.
Micrometeorological comparative analysis of the 747 and 373 mm irrigation plots indicates that when the difference in daily average temperature is small (30.2 and 30.26°C), the daily average RH in the 747 mm irrigation plot is relatively great (45.47 and 34.48%), the difference between daily average T l and T a is small (0.09 and 0.68°C). Although the differences in daytime average VPD (2.03 and 2.66 kPa) and in night average VPD (1.28 and 2.00 kPa) are small, they are statistically significant. The correlation analysis of sap flow rate with VPD indicates that the same plant has similar sensitivity to VPD under different irrigation conditions. These micrometeorological factors function synergistically and, to a certain extent, restrict transpiration. In addition, larger moisture content causes poor ventilation in soil, leading to decreases of the soil oxygen content and temperature. In the relatively low temperature and hypoxia conditions, respiration of the plant roots is decreased, metabolism weakened, the water-absorbing capacity reduced, which together reduces the rate of stem sap flow (Gao et al. 2001). With the temperature increases at noon, the rate of sap flow increases. After noon, RH reaches its minimum, at this time the synergistic effect of the above factors diminishes or disappears. Therefore, when soil moisture is high, the rate of stem sap flow will increase. Sap flow rates of Elaeagnus angustifolia L. and P. euphratica Olive in 747 mm irrigation plots shown in the daily variation curves equal to zero may be caused by the reduced transpiration pull, and may be because the flow rate is too slow, and the instrument failed to detect (Burgess et al. 2001). In other words, the decrease in transpiration pull may interrupt the continuous sap flow in the vessels and lead to temporary cavitation of xylem vessels.
Relationship between sap flow rate and environmental factors
Sap flow is affected not only by its own characteristics but also by meteorological factors. Pearson correlation analyses of stem sap flows of the four species and the six synchronically obtained environmental factors V w, RH, T a, T s, T l and VPD, as shown in Table 3, indicate that the stem flow rates are differentially related to different environmental factors. V w is only correlated with the sap flow rate of P. russkii Jabl., which may be due to its greater height and larger wind-accessible surface. RH and T s are negatively correlated with the sap flow rates of all four species. By contrast, T a, T l and VDP are positively related to the sap flow rates of the four species. P. russkii Jabl. are significantly correlated with all the six environmental factors at 0.01 level; their correlative order is T a > T l > VPD > RH > V w > T s. P. Euphratica Olive is evidently correlated with all environmental factors except V w at 0.01 level, their correlative order is T a > T l > VPD > T s > RH. E. angustifolia L. is obviously correlated with T a, T s and VPD at 0.01 level and with T l at the 0.05 level; their correlative rank is T s > VPD > T a > T l. The correlative rank of U. pumila L. with meteorological factors is T a > T l > T s > VPD > RH.
Based on the above analysis, the correlations between the environmental factors and the flow rates were further evaluated with regress stepwise analysis using multiple linear regression method and the confidence level of 95% was set as confidential value to select and exclude dependent variables. The obtained optimal regression equation of stem sap flow rate on meteorological factors are shown below and used to predict the flow rates of the four species:
where SF1, SF2, SF3, and SF4 represent the estimated sap flow rates of P. russkii Jabl., P. euphratica Olive, E. angustifolia L. and U. pumila L., respectively.
Environmental factors show different correlations with different species. In other words, the dominant environmental factors for different species are different. As shown in the formula above, the estimated flow rate values of P. russkii Jabl., P. euphratica Olive and U. pumila L., except E. angustifolia L., are correspondingly close to their measured values, indicating that the equations established by the stepwise regression method can be used to predict the changes of sap flow rates.
Conclusions
-
1.
The characteristics of sap flow can reflect the influences of their own physiological characteristics and environmental factors on themselves. The temporal consistency of the four species except for Elaeagnus angustifolia L. in starting, peaking, declining and minimizing of sap flows indicates that the four species have similar responses to diurnal variation of solar radiation. It is generally acknowledged that diurnal processes of sap flow under drought stress show double peaks or multi-peaks, i.e., there are mid-day depressions in photosynthesis. In this study, in 373 and 747 mm irrigations plots, the diurnal processes of P. russkii Jabl., P. euphratica Olive and U. pumila L. show single peaks, indicating that they were not subject to drought stress and 373 mm irrigation was sufficient for their growth. The reason why the sap flow of E. angustifolia L. did not show circadian rhythm is the insensitivity to the irrigation condition caused by its special physiological characteristics. Water consumptions differ significantly among plants. The result that the four species have different diurnal variation indicates that the stem sap flow among species varies significantly. Even in the same habitat, plants with same life form but belonging to different species differ significantly in their stem sap flow rates. The flow rate of P. russkii Jabl. is 13.8-fold of that of E. angustifolia L.; while for P. russkii Jabl. and P. euphratica Olive, which belong to different species of same genus, their difference in flow rate is smaller, reflecting that the four plants are indeed from different species. For water consumption by transpiration, P. russkii Jabl. has the highest, followed by in turn P. euphratica Olive, U. pumila L. and E. angustifolia L. The differences in water consumption are of great importance for species selection in a shelter forest in desert areas, and in the process of the desert ecosystem restoration and reconstruction.
-
2.
Because of the structural heterogeneity of sapwood, xylem of different depth has different water transporting capacity. The changes of sap flow rates along the different radial depths of the four species show different trends. The rates of P. russkii Jabl., E. angustifolia L. and U. pumila L., decrease along the radial gradient from the cambium to the pith, while that of P. euphratica Olive shows a trend of decrease, increase and decrease again from the cambium to the pith, which possibly result from the different vessel diameters and distributions along radial direction in xylems of different species.
-
3.
When plants are not subject to drought stress, increase in water supply will affect their growth niches. Decrease in VPD, as synergetic effects of environmental factors especially temperature and relative humidity, will lead to the decrease of water potential differences between leaves and air, which, to some extent, impact the transpirations of the four species, as indicated by the decreased stem sap flow rates. This also accounts for the inhibited transpiration of the four species by over irrigation. In other words, the irrigation of 373 mm in this study meets the growth requirements of the four species at the experimental stage. Thus, understanding of the change rules of sap flow in different moisture conditions will help to determine the irrigation amount and timing of a shelter forest and optimize the management of water resources to alleviate the contradictions between water demand and supply in desert areas.
-
4.
Stem sap flow is comprehensively subject to environmental factors among which the dominant factors vary upon species. The regression equations of the above four species indicate that the dominant factors for P. russkii Jabl. are Ta, RH, T1 and VPD; for P. euphratica Olive are T a, T s, and RH; for Elaeagnus angustifolia L. are T s and VPD; and for U. pumila L. are T a, T s, VPD and T l. Compared with other species, P. russkii Jabl. has the most principle factors, indicating that it is most sensitive to the environment. U. pumila L. ranks the second, P. euphratica L. and E. angustifolia L. are the least sensitive. In a certain sense, these sensitivities also reflect their resistances to environmental stress, i.e., E. angustifolia L. > P. euphratica L. > U. pumila L. > P. russkii Jabl.
References
Bauerle WL, Whitlow TH, Pollock CR, Frongillo EA (2002) A laser-diode-based system for measuring sap flow by the heat-pulse method. Agric For Meteorol 110:275–284
Burgess SSO, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001) An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21:589–598
Chang XX, Zhao WZ (2004a) Sap flow of Elaeagnus angustifolia and its relation with growth regime in middle reaches of Heihe river basin. J Desert Res 24:473–478
Chang XX, Zhao WZ (2004b) Sap flow of Gansu poplar in farmland shelter forest during the growing season in desert oasis. Acta Ecol Sin 24:1436–1441
Conejero W, Alarcón JJ, García-Orellana Y, Nicolás E, Torrecillas A (2007) Evaluation of sap flow and trunk diameter sensors for irrigation scheduling in early maturing peach trees. Tree Physiol 27:1753–1759
Edwards WRN, Warwick NWM (1984) Transpiration from a kiwi fruit vine as estimated by the heat pulse technique and the Penman–Monteith equation. Agric Res 27:537–543
Fernandez JE, Green SR, Caspari HW, Diaz-Espejo A, Cuevas MV (2008a) The use of sap flow measurements for scheduling irrigation in olive, apple and Asian pear trees and in grapevines. Plant Soil 305:91–104
Fernandez JE, Romero R, Montano JC, Diaz-Espejo A, Muriel JL, Cuevas MV, Moreno F, Girón IF, Palomo MJ (2008b) Design and testing of an automatic irrigation controller for fruit tree orchards, based on sap flow measurements. Aust J Agric Res 59(7):589–590
Fu AH, Chen YN, Li WH, Zhang HF (2005) Research advances on plant water potential under drought and salt stress. J Desert Res 25:744–749
Gao Y, Zhang RM, Liu J (2001) A study on volume and velocity of stem sap flow of Populus popular’s by heat-pulse technique. Acta Bot Boreal Sin 21:644–649
Jiang GM (2004) Plant physiological ecology. Higher Education Press, Beijing, pp 93–99
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, New York
Levitt J (1980) Responses of plants to environmental stresses, vol. II. II Water, radiation, salt and other stresses, 2nd edn. Academic Press, New York
Ma L, Rao XQ, Zhao P, Lu P, Cai XA, Zeng XP (2007) Diurnal and seasonal changes in whole-tree transpiration of Acacia mangium. J Beijing For Univ 29:67–73
Monteith JL (1995) A reinterpretation of stomatal responses to humidity. Plant Cell Environ 18:357–364
Nadezhdina N (1999) Sap flow index as an indicator of plant water status. Tree Physiol 19:885–891
O’Brien JJ, Oberbauer SF, Clark DB (2004) Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ 27:551–567
Wang HT, Ma LY, Sun PS (2002) Sap flow fluctuations of Pinus tabulaeformins and Platycladus orientalis in late autumn. Sci Silv Sin 38:31–37
Whitehead D (1998) Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiol 18:633–644
Wullschleger SD, Meinzer FC, Vertssy RA (1998) A review of whole-plant water use studies in trees. Tree Physiol 8/9(18):499–517
Xia GM, Kang SZ, Li FS, Zhang JH, Zhou QY (2008) Diurnal and seasonal variations of sap flow of Caraganakorshinskii in the arid desert region of northwest China. Hydrol Process 22:1197–1205
Xie TT, Zhang XM, Liang SM, Shan LS, Yang XL, Hua YH (2008) Effects of different irrigations on the water physiological characteristics of Haloxylon ammodendron in Taklimakan Desert hinterland. Chin J Appl Ecol 19:711–716
Xu H, Zhang XM, Wang YD, Wei J, Liang SM (2006) Study on water consumption of Calligonum arboresens Litv. in shelterbelts along the Tarim Desert highway. Arid Zone Res 23:216–222
Xu XY, Sun BP, Ding GD, Guo SJ, Chai CW (2008) Sap flow patterns of three main sands-fixing shrubs and their responses to environmental factors in desert areas. Acta Ecol Sin 28:895–905
Yuan XH, Wu JY, Sun L, Duan LS, Wang JZ (2008) Evapotranspiration of Dianthus chinensis at different irrigation levels. J Beijing For Univ 30:77–81
Zeng XP, Zhao P, Peng SL (2000) Study on the water ecology of artificial Acacia mangium forest in the Heshan Hill region, Guangdong Province. Acta Phytoecol Sin 24:69–73
Zhang XY, Gong JD, Zhou MX, Si JH (2004) Spatial and temporal characteristics of stem sap flow of Populus euphratica. J Desert Res 24:489–492
Zhang XY, Kang ES, Zhang ZH, Si JH (2006) Research on dynamics of stem sap flow of Elaeagnus angustifolia. J Desert Res 26:146–151
Zhou HH, Chen YN, Li WH, Chen YP (2008) Characterization of photosynthesis of Populus euphratica Olive and its microclimate explanation in Lower Tarim River. J Desert Res 28:665–672
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (Grant Nos: 91025025, 41101102, 40901105, and 41101533). Special thanks are owed to editors and anonymous reviewers whose comments helped to improve the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, JX., Chen, YN., Li, WH. et al. Sap flow characteristics of four typical species in desert shelter forest and their responses to environmental factors. Environ Earth Sci 67, 151–160 (2012). https://doi.org/10.1007/s12665-011-1488-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1488-8