Abstract
The Terme and Karakurt thermal resorts are located in the center of Kirşehir city in central Anatolia. Thermal waters with temperatures of 44–60°C are used for central heating and balneologic purposes. Paleozoic rocks of the Kirşehir Massif are the oldest units in the study area. The basement of the Massif comprises Paleozoic metamorphic schist and marbles which partly contain white quartzite layers of a few tens of cm thickness. The metamorphic schists which are cut by granites of Paleocene age are overlain by horizontally bedded conglomerate, sandstone, claystone, and limestone of upper Paleocene-Eocene age. Among the thermal and cold waters collected from the areas of Terme and Karakurt, those from thermal waters are enriched with Ca–HCO3 and cold waters are of Ca–Mg–HCO3 type waters. The pH values of samples are 6.31–7.04 for the thermal well waters, 6.41 for thermal spring, 7.25 and 7.29 for the cold waters, and 7.52 for the Hirla lake water. EC values are 917–2,295 μS/cm for the thermal well waters, 2,078 μS/cm for thermal spring, and 471 and 820 μS/cm for the cold springs. The lowest TDS content is from water of T10 thermal well in the Terme area (740.6 mg/l). The hot and cold waters of Terme show very similar ion contents while the Karakurt hot waters at western most parts are characterized by distinct chemical compositions. There is ion exchange in thermal waters from the T5 (5), T6 (6), T12 (7), and T1 (8) wells in the Terme area. The thermal waters show low concentrations of Fe, Mn, Ni, Al, As, Pb, Zn and Cu. Waters in the study area are of meteoric origin, and rainwater percolated downwards through faults and fractures, and are heated by the geothermal gradient, later rising to the surface along permeable zones. δ13CVPDB values measured on dissolved inorganic carbon in samples range from −1.65 to +5.61‰ for thermal waters and from −11.81 to −10.15‰ for cold waters. Carbon in thermal waters is derived from marine carbonates or CO2 of metamorphic origin while carbon in cold waters originates from freshwater carbonates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thermal resorts of Terme and Karakurt are located in the Kirşehir city, central Anatolia (Fig. 1). The Terme resort is in the Kuşdilli district and the Karakurt resort is in the Karalar village of the Kirşehir city. Terme geothermal field is the most important one in Kirşehir area, and thermal waters are used for district and green house heating and also for thermal tourism. Geothermal works in the Kirşehir Terme region were started in 1974 by the General Directorate of Mineral Research and Exploration of Turkey (MTA). In Terme, there are 12 thermal water wells drilled by MTA with and 3 thermal wells drilled by the private sector. The temperature, depth and flow rate of these wells are range between 44–60°C, 92 to 600 m and 30 to 185 l/s, respectively. From all of these wells only two wells are used for central heating. There is also one well opened by MTA in the Karakurt region at 15 km distance west of Kirşehir city center. The temperature of this well is 50°C. The Karakurt and Terme resorts which are one of the oldest resorts in Turkey were studied repeatedly (Canik 1982, 19911993; Didik et al. 1994; Gündüz 1995; Kara 1997). Kirşehir massif which is one of the important massives in Anatolia, thermal and mineral waters springs are spotted at different locations.
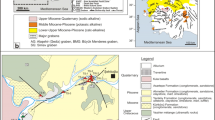
Geological maps of Terme and Karakurt (Kirşehir) area. a Geological map of North-west region of the CACC (after Whitney et al. 2001), b geological map and geological cross section of Terme area (Canik, Pasvanoglu 1993), c Geological map (simplified after Didik et al. 1994) and geological cross section of Karakurt area
In this study, samples were taken from thermal water springs and wells at Kirşehir (Terme and Karakurt) geothermal fluid and isotope, and chemical analyses were conducted. The aim of this study was to examine the physico-chemical characteristics and isotopic properties of the thermal fluid that form the geothermal system.
Materials and methods
The study was conducted in three stages as (i) field observations, (ii) sampling and (iii) data processing and interpretation of results. During the field work, geological maps of the Karakurt and Terme areas were prepared at 1:25,000 scale with data from field works conducted in the years of 2005–2006 and from previous studies (Canik, Pasvanoglu 1993; Didik et al.1994) (Fig. 1). In order to determine chemical characteristics of waters in the geothermal area, samples were collected from four different water groups. A total of ten samples were collected from the regions: one sample from the thermal spring (TS), five samples from thermal wells (TW), two from cold springs (CS) and one sample from surface thermal lake (TL). Information on location of water samples and properties of wells is shown in Table 1. Samples were collected from the Karakurt thermal water well (1); from the Terme wells whose waters are used for district heating (6 and 7); from the Terme wells whose waters are used for balneologic purposes (5, 8 and 9); from the Terme heat center spring (4); and from the Karakurt Şeyh Mustafa (2) and Terme Üçgöz (10) cold water fountains. One sample was also taken from surface water of the Hirla Lake (3) in Terme (Table 1). In addition, travertine in the Terme area was also sampled (sample TT2). Water samples collected for cation and trace element analysis with the exceptional CO3, HCO3, Cl and SO4 were treated with 0.2 ml concentrated HNO3 and added to 100-ml samples. Later, samples for anion analysis were kept unacidified. Similarly, waters were sampled into 100 ml and 1 l polyethylene bottles for oxygen, deuterium, carbon and tritium analyses, respectively.
Major, trace element and tritium analyses were carried out at ACME Laboratories (Canada) by, inductively coupled plasma mass spectrometry (ICP-MS), and at the International Research and Application Center for Karst Water Resources (UKAM) Water Chemistry Laboratories at the Hacettepe University (Ankara). Oxygen-18 (δ18O), deuterium (δ2H) and carbon-13 (δ13C) analyses were conducted at the G.G. Hatch Stable Isotope Laboratories of the Canada Ottawa University. The precision of the analyses is ±0.15‰ for δ18O, ±2‰ for δ2H and ±0.2‰ for δ13C. Precision of analyses in travertines is ±0.1‰ for both δ18O and δ13C. Heavy metal analyses for waters and travertines were carried out by the ICP-MS method; meanwhile, whole-rock analyses (% oxide) of travertines were conducted with the ICP-ES method at ACME Laboratories in Canada.
The pH and EC values of waters were measured with a YSI brand pH-meter at sampling sites. Phreeqc chemical equilibrium software was used for determining the saturation state of minerals in the water. In order to evaluate and identify the chemical composition of waters, results of chemical analyses of the spring waters and wells are presented in different diagrams.
Geology
The Kirşehir Massif (Seymen 1982) also known as Kirşehir Continent (Sengör et al. 1984), the Kırşehir Complex (Lünel 1985), the Central Anatolian Crystalline Complex (Göncüoğlu et al. 1991a, b), and the Central Anatolian Massif (Erkan 1981), consists of metamorphic, ophiolitic and plutonic rocks and is composed of several different structural blocks. The Kaman and Hirkadağ blocks each consists of a metamorphic sequence ranging from greenschist facies (chlorite zone) to upper amphibolite and granulite facies (sillimanite-potassium feldspar zone) (Seymen 1981; Whitney and Dilek 2001; Whitney et al. 2001). The Kirşehir Massif was intruded by calc-alkaline subduction-related granitoids (Akiman et al. 1993).
Kirşehir–Terme
The Central Anatolian Crystalline Complex or the Kirşehir Crystalline Massif (Ketin, 1955; Seymen 1982, 1984; Göncüoğlu et al. 1992, 1993, 1994) is the oldest unit around the Kirşehir resort.
The rocks of the Kirşehir Massif are the oldest unit around the Kirşehir Terme resort and surroundings. The basement is composed of Paleozoic metamorphic schist and marbles which are interbedded with white-colored quartzites with thickness of a few tens of centimeters. The thickness of the metamorphic schists is more than 1,000 m. They are cut by granites of Paleocene age (Canik 1982, 1991). The Eocene conglomerate and sandstones unconformably overlie the older units. In the northern and western parts of the area, Eocene are covered with horizontal bedded Pliocene conglomerate, sandstone, clay and limestone. Quaternary alluvium and travertines are widely exposed in the area (Fig. 1).
Kirşehir–Karakurt
The Karakurt resort in the vicinity of Karalar village, 15 km west of Kirşehir, is one of the oldest thermal resorts in Turkey and, therefore, it has been investigated by various workers (Didik et al. 1994; Kara 1991, 1997; Kuşçu and Erler 1998). The basement of the area is composed of Paleozoic schist, quartzite and marbles of the Kirşehir Massif (Fig. 1). They are unconformably overlain by a sequence of Upper Paleocene-Lower Eocene age consisting of alternating red-colored conglomerate, sandstone and mudstone (Kara 1991). The overlying unit with a thickness of 30 m is composed of gray-colored, fossiliferous sandstones, siltstones, mudstones and limestones of Lutetian age (Birgili et al. 1975). The uppermost part comprises a sedimentary sequence of red-pink, brown-colored conglomerate, sandstone, sandy clay and mudstone and white-yellowish lacustrine limestone of Upper Miocene-Pliocene age (Fig. 1) (Kara 1991). The thrust fault crossing the Çiban Hill is the most important tectonic feature of the area (Kara 1991).
Hydrogeology
Terme resort
The metamorphic schists of the Kirşehir Massif exposing around the Terme resort are mostly impermeable. Permeability is enhanced by fractured-controlled karstification and fractures and fissures in marbles. The marbles are generally cracked and they contain dissolution vugs. The cracks are filled with white-colored calcite. Karstic structures, such as caves, surface solution features of different sizes and geometry, are common at the western part of Terme. Thus, marbles comprise the primary aquifer where substantial amount of hot water can be stored. The Eocene conglomerate and sandstones are loosely cemented and have high efficient porosity and permeability. Conglomerate and sandstones at the contact between Pliocene lacustrine deposits and metamorphic schists and marbles are characterized by high porosity and permeability values (Canik, Pasvanoğlu 1993). Since thermal and mineral waters issuing through the normal faults are oversaturated with respect to calcite, this hydrothermal conduit has been completely plugged by carbonate precipitates. These long-lasting events have given rise to gradual decreasing of spring discharges and extinction of some. The presence of Tertiary intrusion in middle Anatolia (Akiman et al. 1993; Whitney et al. 2001) and Pliocene volcanism (Ercan 1985) in and around the study area caused the geothermic gradient to fall below the normal value of 33 m.
The meteoric waters are heated by geothermic gradient during percolation. These heated waters accumulate within fractures, fissures and karstic voids of marbles which comprise the pressured aquifers. Waters which are heated upon downward percolation rise to the surface via suitable fractures, fissures and karstic voids and form the Terme springs (Fig. 1). These waters rising to the surface for a long geological period are oversaturated with respect to calcite; they form the Terme stone (travertine) at the surface. Some of these waters rising through faults buried under the marbles and Tertiary formations facilitate formation of a hot water aquifer of 25–37°C within the base conglomerates and alluvium of the Pliocene lacustrine deposits.
The temperature of TS (4) at the Kirşehir heat center is 49°C, and the temperatures of waters at the T5 (5), T1 (8) and T10 (9) wells which are all used for balneological purposes are 57, 60 and 44°C, respectively (MTA measurements). Waters from the T6 (6) and T12 (7) wells which are used for heating have the same temperature of 56°C. Based on data from MTA, total discharge rate of waters that are used for heating is 290 l/s and that of waters used for balneology is 305 l/s. The deepest well in this area is T1 (8), with a depth of 500.5 m.
Karakurt resort
Drilling studies conducted around the Karakurt resort showed that marbles of the Kirşehir Massif are the reservoir rocks of thermal waters (Fig. 1). Fractured and karstic voids of these marbles increased their permeability and enabled them to be one of the important aquifers in the region. Paleocene-Eocene conglomerates and sandstones are the other aquifer type units. Thermal waters in the Karakurt resort are issued from a NE-SW extending normal fault. Temperatures of thermal waters in the Karakurt resort are between 31 and 50°C, and the total discharge was measured by MTA as approximately 5.5 l/s. Cold spring waters are discharged from the contact between metamorphic rocks and overlying sedimentary rocks. Their discharge is 0.1–4 l/s. Catchment of these springs is in place so that their waters can be obtained from fountains.
Water chemistry
Results of major ion analyses of thermal and cold waters from the Terme and Karakurt areas are given in Table 2. The EC values of (TW) thermal water wells are between 1,000 and 2,400 μS/cm; 2,170 μS/cm at one thermal spring (4); and between 478 and 883 μS/cm at cold springs (2, 10). The pH values in waters of thermal wells (TW) are 6.31–7.04, in waters of one TS (4), the pH value is 6.41, cold springs (2, 10) have pH values of 7.29 and 7.25, respectively, and the pH value of Hirla lake water (3) is 7.52. The lowest and highest dissolved solid (TDS) contents of the TW waters in the Terme area are 740.6 mg/l (T10; sample no. 9) and 1,761 mg/l (T12; sample no.7), respectively. TDS values of cold water springs are 339.4 and 569.4 mg/l. TDS values measured in TW waters in the Terme area are higher than those from the Karakurt well. According to classification in the Piper (Piper 1944) diagram (Fig. 2), thermal water wells and spring are of Ca–HCO3 while cold waters are of Ca–Mg–HCO3 type.
All thermal waters are dominated by Ca2+ and HCO3 −. The low pH values (pH < 7) of these waters which originate from the marbles of the Kirşehir Massif resulted in increased Ca2+ and HCO3 − concentrations. Na+ is the second most common ion in these waters. Sodium is generally derived from albite-rich volcanic rocks (Hem 1970). However, in our study area, marbles are the main reservoir rock. In addition, schist’s and overlying clastic sedimentary rocks are secondary aquifer units. K+ has the lowest concentration in thermal waters of the Terme Kirşehir region. Magnesium which is abundant in olivine, pyroxene and amphibole-rich rocks has a low abundance in the studied waters. This indicates that rocks of the geothermal system and springs in the recharge area are free of these minerals. In the Terme geothermal field (including the area of the thermal wells), Pliocene conglomerate, sandstone, clay and limestone types of sedimentary rocks and travertine’s are exposed. Drilling data show that marbles are the reservoir rock of geothermal system. Thermal waters are characterized by high concentrations of Ca2+ and HCO3 − and low concentrations of Na+, Mg2+ and SO4 2−. This type of concentration trends may also be due to the low temperature of waters which cannot accomplish a full water-rock interaction.
Chloride which is a characteristic ion for geothermal systems is the second most dominant anion in thermal waters of the Terme Kirşehir area. It shows positive correlations with major ions in waters (Fig. 3). Correlation coefficients of waters computed for Cl− and other major ions are between 0.23 (between Cl− and SO4 2−) and 0.99 (between Cl− and Na+). The reason for the low correlation coefficient between Cl− and SO4 2− is attributed to the fact that composition of Karakurt thermal water is different from that of Terme thermal and cold waters. As shown in Fig. 3 the source of sulfate is different for Terme thermal and cold waters and Karakurt hot water. Examination of ion relations reveals that Terme thermal and cold waters show similar trends while Karakurt thermal waters in the westernmost parts are of diverse composition.
The Ca/Mg ratios of thermal waters are between 2.40 and 4.06 in Terme hot waters; the ratio is 5.98 for Karakurt well water (1) due to marbles of the reservoir rocks (Table 3, Fig. 4). The Ca/Mg ratios of CS waters are between 2.27 and 4.02. The highest Ca/Na values are observed in (2) CS water. The Ca/Na ratios in Terme hot waters are between 1.52 and 4.00. In waters of low Ca/Na ratios, Ca/Mg ratio is high (Table 3), indicating ion exchange process (2Na++ CaX = Ca+2 + Na2X; Langmuir 1997) in waters of Terme T5 (5), T6 (6), T12 (7) and Terme T1 (8) thermal wells. However, the water of Terme T10 (9) well is different from that of other wells as there is cold water mixing with the water (44°C) of this well, which was opened to a depth of 165 m. The Cl/HCO3 ratio of all waters is less than 1 due to high HCO3 contents. While Cl/HCO3 ratio increase in Terme well waters, it decreases in Karakurt well water and cold waters. The water of Karakurt well water (1) is characterized by a different SO4/Cl ratio because of decomposition of mineral with sulfur in the Eocene aged sedimentary rocks at the recharge area of Karakurt geothermal field. The Na/Cl ratios of all waters under investigation are around 1. The hydrochemical facies of the waters are defined on the basis of Base Exchange Index (bei). While thermal well waters of Terme have positive bei values, Karakurt well water, thermal spring and cold springs have negative values. The waters emerging from magmatic and metamorphic rocks have negative values caused by contribution from the alkaline ions due to alteration and decomposition of silicate minerals (Şahinci 1991). Terme thermal waters derived from metamorphic schists and marbles, loosely cemented Eocene conglomerate and sandstones and Pliocene lacustrine deposits.
The saturation index of waters for various minerals was calculated by using Phreeqc chemical equilibrium software (Parkhurst DL Appelo 1999).The results show that Terme Kirşehir thermal waters are slightly saturated with respect to calcite, aragonite, dolomite, quartz and chalcedony, and undersaturated with respect to anhydrite and gypsum. The Karakurt thermal waters are slightly saturated or in equilibrium with respect to calcite, quartz and chalcedony but undersaturated with respect to other minerals (Table 3). Cold water samples 2 and 10 are saturated with respect to calcite, quartz and chalcedony, but undersaturated with respect to other minerals. Scaling of the carbonate minerals is expected for the thermal waters. The Kirşehir region is an important region especially for its thermal water resources and travertine formations. New travertine precipitations are spotted around the Kirşehir downtown heat center. Only calcite (or aragonite) is depositing even though waters are oversaturated with respect to some other minerals. In the travertine analysis from this area, and from oxide identification in these analyses, the highest oxide percentage value is found to be CaO, and the second highest oxide percentage value is Fe2O3. Other oxides such as MgO value is 0.12%. Al is below detection according to Table 4. But Table 2 shows there is more Fe2O3 than MgO. The values in the saturation index confirm these results.
Concentrations of trace element analyzed in travertine conform to those of thermal waters (Table 4). The trace element contents of water from Karakurt (1) are different from those of Terme thermal waters; however, concentrations of As, B, Ba, Li and Sb in Karakurt thermal waters are lower than those of Terme waters while Fe and Mn contents are higher (Table 4). Concentrations of heavy metals such as Pb, Zn and Cu are low in all the waters. Fe content is 42 ppb in Karakurt well water (Table 4). Fe is leached from the minerals, such as biotite during water-rock interaction. Trace element contents of Terme Heat Center Spring (4) are higher than that of Terme T10 well (9). Terme T5 (5), T6 (6), T12 (7) and T1 (8) have similar trace metal concentration. According to ion ratios and trace element contents, Karakurt thermal well has shallow circulated compared with Terme thermal wells. Considering to temperature and chemical composition Terme heat center spring (4) is of an intermediate circulated.
Geothermometry applications
In order to determine reservoir temperature in the Kirşehir field, various silica and cations geothermometers were used (Table 5). Different reservoir temperatures calculated for the Kirşehir waters indicate that concentrations of silica and cations in these waters are affected by chemical processes such as mixing and evaporation and by the use of different constants in geothermometers proposed by various workers. Reservoir temperatures calculated from the cation geothermometers are generally higher than those from the silica geothermometers.
Temperatures estimated by Na–K geothermometers in Fournier (1973), Truesdell (1976), Tonani (1980), Giggenbach (1988), and Arnorsson et al. (1983) yield anomalously high temperatures (Table 5), which is due to the high Ca+2 concentrations of the waters (Fournier and Truesdell 1973). During their rise to the surface, Kirşehir thermal waters may lose some heat due to possible mixing with cold waters along the fracture zones. Therefore, the assumption of Na–K and Feldispar equlibrium may not be correct for mixing waters since the cation concentrations in such waters are controlled by leaching rather than the chemical equlibrium between minerals and Na–K. Temperatures estimated by K–Mg geothermometer in Giggenbach (1988) are lower than outlet temperatures of waters and those of Na–K geothermometers. This is due to interaction of thermal waters with reservoir rocks during their rise to the surface and increasing solubility of Mg+2 with decreasing temperature. In other words, Mg+2 reflect equilibrium condition at shallow depths. In order to eliminate the possible effects of Ca concentrations on the Na-K geothermometer, the Na–K–4/3Ca geothermometer of Fournier and Truesdell (1973) was used in the study and the results obtained by this geothermometer were found to be lower than results obtained by Na–K geothermometers (Table 5). Dilution would cause the Na–K–4/3Ca geothermometer values to be slightly too high. All indications are that this is a low-temperature system. Based on Fig. 5 proposed by Giggenbach (1988) all the data points plot in the area of immature waters (shallow or mixed waters); therefore solute geothermometry is not likely to yield meaningful equilibration temperatures.
Since all these geothermometer calculations are based on pure mineral phases, the results may not reflect ideal mineral equilibrium in waters. Calcite, Aragonite and dolomite are present in the reservoir.
The temperature range calculated with chalcedony geothermometer is from 65 to 75.5°C. Temperatures estimated by the quartz geothermometers are 95.4 to 102.7°C which are greater than results obtained by the chalcedony geothermometer and even than the measured temperatures of waters.
As stated by Fournier (1991), at temperatures of less than 180°C, the solubility of silica is controlled by chalcedony rather than quartz and by both minerals in some cases. The SiO2 content of fluids is relatively low in our results suggesting that thermal waters rise to the surface without equilibration due to their rapid circulation. These waters may also precipitate silica or mix with dilute cold waters returning to the surface. In this respect, the temperatures calculated by the chalcedony geothermometers may closely match the reservoir temperatures; in any case, they are more realistic than those obtained by the quartz geothermometers (Mutlu 1998).
Environmental isotopes
Oxygen-18 and deuterium contents were used for calculating the recharge elevations of waters, and tritium was used to determine the relative age and residence time of waters. Carbon-13 was used to investigate the source of carbon in water samples.
δ18O-δD compositions
The results of the δ18O-δD ratios of Kirşehir thermal waters are presented in Table 6. The δ18O ratio of thermal waters ranges from −11.12 to −10.45‰ and that of cold waters is between -8.39 and -10.02‰. Deuterium values for thermal and cold waters are −83.9 to −76.8‰ and −74.7 to −58‰, respectively.
In the δ18O-δD diagram (Fig. 6a), all of the Kirşehir waters indicate a common meteoric origin on the Global meteoric water line (δD = 8 δ18O + 10) of Craig (1961) and the Konya meteoric water line (δ D = 8δ18O + 16) of Şentürk (1970). These waters are percolated downwards through faults and fractures, and are heated by the geothermal gradient, later rising to the surface along permeable zones. The geothermal gradient in the area is found to be 100 m/12°C (Canik 1991). The presence of Tertiary granite intrusion in Kirşehir massif and Pliocene volcanism (Ercan 1985) in and around the study area caused the increase in temperature value of the geothermic gradient.
a δ18O-δD, b Tritium-δ18O diagram, c δ18O-TDS diagram, d δ18O-HCO3 diagram (Samples names are the same as Table 1)
In the study area, the δ18O-δD ratios of cold waters are slightly higher than those of thermal waters (Table 6). This may indicate that thermal waters are recharged from a different source probably from a higher elevation than cold waters. The isotope values of thermal waters are more negative than those of cold waters, indicating that thermal waters are recharged from continental precipitation falling onto higher elevations. Positive δ18O values were observed in the cold waters, although they have low TDS and HCO3 (Fig. 6c, d). These values indicate that cold waters recharge at low elevations with shallow circulation path.
Plotting of Karakurt cold water sample 2 (Şeyh Mustafa) below the Meteoric Line reveals that these waters have undergone some evaporation. In the region an average temperature of 30°C during the summer facilitates the occurrence of evaporation before filtration. The δ18O values of thermal waters are very close indicating that they are continuously fed from the same recharge area.
In the δ18O-3H graphic, sample no. 5 (Terme T5 well water) has the highest value while cold water samples 2 and 10 namely Karakurt Şeyh Mustafa and Terme Üçgöz Fountains have the lowest values in the recharge area of Kirşehir (Fig. 6b).
Tritium-EC and Tritium-Cl relations
Tritium (3H)-electrical conductivity (EC) and 3H-Cl relations for the Kirşehir geothermal area are given in Fig. 7. Low tritium but high EC values of thermal water wells indicate that these waters are deeply circulated. Regarding relative residence times, Kirşehir cold waters are represented by high tritium and low EC values while thermal waters are characterized by low tritium and high EC values. As the circulation path of waters of meteoric origin increases, their tritium values decrease due to radioactive decay of tritium. Therefore, Kirşehir cold waters represent young, but deeply circulated thermal waters having longer residence time in the aquifer and represent older groundwater.
a EC-Tritium, b Cl–Tritium relations for Kirşehir waters (samples names are the same as Table 1)
13C Composition
In order to investigate the origin of carbon in the waters, all Kirşehir thermal and cold waters were analyzed for δ13C contents (Pasvanoglu S Gültekin 2007). Analyses were carried out on dissolved inorganic carbon (DIC) for δ13C (Table 6). The major sources of carbon contributing to DIC in the waters are CO2 derived from the decay of organic matter in soils and from the dissolution of carbonate, while in general the contribution of atmospheric CO2 is negligibly small.
The carbon isotopic ratio of dissolved inorganic carbon (DIC) in the Kirşehir thermal and cold waters ranges from 0.20 to +5.61‰ and from −1.38 to −10.46‰, respectively (Table 6). The δ13C values of total dissolved inorganic carbon are plotted versus alkalinity (expressed as HCO3) in Fig. 8. As expected, there is a trend of increasing alkalinity with increasing δ13C values.
HCO3-δ13C relation for Kirşehir waters (samples names are the same as Table 1)
The δ13C values of Kirşehir waters have positive values, while the carbon isotope ratios of Hirla lake waters and cold springs are represented by negative values. The source of carbon in thermal water wells (TW) and hot spring (TS) (4) with δ13C values ranging from 0 to +5‰ might be a mixture of metamorphic CO2 and marine carbonates; the source of carbon in cold waters is organic (Clark and Fritz 1997).
The δ13C ratio of Kirşehir Terme travertine is 6.82‰ which corresponds to a thermogene type travertine (Pentecost 2005). Some processes such as separation/escape of gases in parallel with morphology, evaporation and deposition rate might have affected the geochemistry and isotopic composition of Kirşehir thermal waters and travertines. Thermogene travertine is rapidly precipitated from high-temperature waters during cooling. It shows less organic material content and massive structure (Pentecost 1995, 2005). Thermogene travertine is generally associated with tectonic and volcanic activities. This type of travertine has high inorganic carbon content and its δ13C value changes from −3 to +10‰ (Pentecost 2005). The source of carbon in cold waters of the Şeyh Mustafa and Terma Üçgöz fountains (δ13C = −10‰) is of freshwater carbonates. Carbon in these waters with low TDS contents might have been derived from dissolution of Pliocene lacustrine carbonate deposits in the region or from CO2 gas that accumulated in pores.
Conclusions
In this study area Eocene and younger units were exposed. Drilling data and field observations yielded that metamorphic rocks of the Kirşehir Massif, which are found to be reservoir rock of the geothermal system, comprise the basement. The thermal waters are enriched with Ca–Mg–HCO3, while the cold waters are Ca–HCO3 type. The cold waters of Şeyh Mustafa (2) and Terme Üçgöz (10) Fountains which discharge along the contact between metamorphic rocks and overlying sedimentary rocks have a chemical composition similar to that of Terme Kirşehir thermal waters. These shallow-circulated waters with low TDS contents and the Terme thermal waters have the same origin. Recent travertine depositions still continue around the thermal wells and springs. Kirşehir Terme and Karakurt water is generally carbonate supersaturated, which suggests a degassing effect. Saturation for carbonate mineral occurs at lower temperature. The cause is re-equilibration with this mineral in the up flow where the water cools. Findings indicate that thermal waters from Kirşehir Terme and Karakurt are not well equilibrated probably because the water feeding the springs is supersaturated with respect to calcite. This also implies that fluid is rapidly circulated along the fractures. Scaling of carbonate minerals could be expected for all the thermal waters. Major and trace element composition of travertines is similar to that of thermal waters. Terme thermal waters and cold waters springs show hydrogeochemically similar compositions, while Karakurt waters at the easternmost parts are represented by different chemical compositions. This may be attributed to recharge of these springs in different basins. Waters of T5 (5), T6 (6), T12 (7) and T1 (8) thermal wells in the Terme Kirşehir area show signs of ion exchange. There is cold water flux to the water of T10 (9) well (44°C) which was opened to a depth of 165 m. The Cl/HCO3 ratio is less than 1. The SO4/Cl ratio of Karakurt well water differs extremely from all other waters probably due to dissolution of SO4-bearing minerals (e.g. gypsum). The Na/Cl ratio of all the waters is about 1. Calculations assuming equilibrium with chalcedony prior to mixing indicate a deep aquifer temperature of possibly as much as 100°C. Environmental isotope results indicate that thermal waters have a meteoric origin and that rainwater is percolated downward through fracture and faults, gets heated with the geothermic gradient and rises to the surface along fault and effective fractures that act as hydrothermal conduits. Isotope values yield that thermal waters are recharged from higher elevations in comparison with cold waters. Based on the δ13CDIC values, carbon in waters has multiple sources. It is thought that carbon in high-temperature waters is derived from dissolution of marine carbonates while carbon in low-temperature waters is sourced from an organic material. This may indicate that fresh waters have interacted with shallow-seated carbonate rocks which are widely exposed in the area while thermal waters are in contact with deep-seated marine carbonates and metamorphic rocks that comprise the basement of the area.
References
Akiman O, Erker A, Göncüoğlu MC, Güleç N, Geven A, Türeli TK, Kadioğlu YK (1993) Geochemical characteristics of granitoids along the western margin of the Central Anatolian Crystalline Complex and their tectonic implications. Geol J 28:371–382
Arnōrsson S, Gunnlaugsson E, Svavarsson H (1983) The chemistry of geothermal waters In Iceland. III. Chemical geothermometry investigations. Geochim Cosmochim Acta 47:567–577
Birgili Ş Yoldaş R Ünalan G (1975) Çankırı-Çorum havzasının jeolojisi ve petrol olanakları, MTA rap. No: 5621 Ankara (yayımlanmamış)
Canik B (1982) Kirşehir-Çiçekdağı, Bulamaçlı kaplıcasının hidrojeoloji incelemesi, MTA, dergisi 93/94 Ankara
Canik B (1991) Kirşehir Terme kaplıcası hidrojeoloji incelemesi, Kirşehir Il Özel Idaresi
Canik B Pasvanoğlu S (1993) Hydrogeological investigation of the mineralized and thermal water of the karstic aquifers arround Kirşehir and the possibility of utilization in thermal spas. In: International symposium on water resources in Karst with special emphasis on arid and semiarid zones. Shiraz-Iran, vol 1, pp 153–167
Clark I, Fritz P (1997) Environmental ısotopes in hydrogeology. Lewis publishers, New York, p 328
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702–1703
Didik S Kalkan I Süzük H Tok Ç (1994) Kirşehir-Karakurt kaplıcasının jeoloji- jeofizik- toprak gazı ölçümleri ve hidrojeoloji etüd. MTA rap. No. 9794 Ankara
Ercan T (1985) Orta Anadolu’daki Senozoyik volkanizması, Maden Tetkik ve Arama Dergisi, Ankara, pp 119–140
Erkan Y (1981) Result of the studies on the metamorphism of the Central Anatolian Massif (in Turkish). In: Akkök R, Oygur V, Terlemez I (eds) Proceedings of symposium on geology of Central Anatolia. Türkiye Jeoloji Kurumu, Ankarapp 9–11
Fournier RO (1973) Silica in thermal waters:laboratory and field investigations. In: Proceedings of international symposium on hydrogeochemistry and biogeochemistry, Tokyo, pp 132–139
Fournier RO (1979) A revised equation for the Na-K geothermometer. Geothermal Res. Council Trans. 3:221–224
Fournier RO (1991) Water geothermometers applied to geothermal energy. In: D’Amore, Applications of geochemistry in geothermal reservoir development. UNITAR/UNDP publication, Roma, pp 37–69
Fournier RO, Truesdell AH (1973) An empirical Na-K-Ca geothermometer for natural waters. Geochim Cosmochim Acta 37:1255–1275
Giggenbach WF (1988) Geothermal solute equilibrium. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta, 52, 2749-765. NITAR/UNDP Publication, Rome, pp 119–142
Göncüoğlu CM, Toprak V, Kuşcu I, Erler A, Olgun E (1991a) Geology of the western part of the Central Anatolian Massif. Part I: Southern section (in Turkish). Türkiye Petrolleri Anonim Ortaklığı Rapor No: 2909, 140 p
Göncüoğlu CM, Toprak V, Kuşcu I, Erler A, Olgun E (1991b) Orta Anadolu Masifinin batı bölümünün jeolojisi Bölüm 1: Güney kesim: Türkiye Petrolleri Anonim Şirketi rapor No: 2909, 140s
Göncüoğlu CM, Erler A, Toprak V, Yalnız K, Olgun E, Rojay B (1992) Orta Anadolu Masifinin batı bölümünün jeolojisi, Bölüm 2: Orta kesim: Türkiye Petrolleri Anonim Şirketi Rapor No: 3155,76s
Göncüoğlu CM, Erler A, Toprak V, Olgun E, Yalnız K, Kuşçu I, Koksal S, Dirik K (1993) Orta Anadolu Masifinin orta bölümünün jeolojisi, Bölüm 3: Orta Kızılırmak Tersiyer baseninin jeolojik evrimi: Türkiye Petrolleri Anonim. Şirketi rapor No: 3313, 104s
Göncüoğlu CM, Dirik K, Erler A, Yalnız K (1994) Orta Anadolu Masifinin doğu bölümünün jeolojisi, Bölüm 4: Orta Anadolu masifinin Sivas baseni ile ilişkisi: Türkiye Petrolleri Anonim Şirketi rapor No: 3535, 135s
Gündüz M (1995) Kirşehir-Karakurt-1 sıcak su sondaj kuyu bitirme ve koruma alanları. MTA rap. No:9904, 18s, Ankara
Hem JD (1970) Study and interpretation of the chemical characteristics of natural water. United States Government Printing Office, Washington 363 p
Kara H (1991) 1:100.000 Ölçekli Kirşehir G-18 Paftası Jeoloji Haritası [Geological Map of the Kirşehir G-18 Quadrangle, Scale 1:100.000]. General Directorate of Mineral Research and Exploration (MTA) Publications, Ankara
Kara H (1997) 1:100.000 Ölçekli Kirşehir G-19 Paftası Jeoloji Haritası [Geological Map of the Kirşehir G-19 Quadrangle, Scale 1:100.000]. General Directorate of Mineral Research and Exploration (MTA) Publications, Ankara
Ketin I (1955) Yozgat bölgesinin jeolojisi ve Orta Anadolu Masifinin tektonik durumu: Türkiye Jeol. Kur Bült 6/1:1–28
Kuşçu I, Erler A (1998) Mineralizations in the Central Anatolian Crystalline Complex: metallogeny of a collision related setting. Int Geol Rev 40:552–565
Langmuir D (1997) Aqueus Environmental Geochemistry. Prentice Hall, New Jersey, p 600p
Lünel AT (1985) An approach to the naming, origin and age of Baranadağ monzonite of the Kirşehir intrusive suite. METU J Pure Appl Sci 18:385–404
Mutlu H (321) 1998. Chemical geotermometry and fluid-mineral equilibria for the Ömer-Gecek thermal waters, Afyon Area, Turkey J Volcanol Geothermal Res 80(3–4):303–321
Parkhurst DL Appelo CAJ (1999) User’s guide to PHREEQC (Version 2)–A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water Res Invest Rep 99–4259
Pasvanoğlu S, Gültekin F (2007) Hydrogeochemical and isotopic evaluation of thermal and mineralized waters of Terme-(Kirşehir) and Kozakli (Nevşehir), Areas, Turkey International Symposium on Advances in Isotope Hydrology and its Role in Sustainable Water Resources Management, Vienna, Abstracts, p 120
Pentecost A (1995) The Quaternary travertine deposits of Europe and Asia Minor. Q Sci Rev 14:1005–1028
Pentecost A (2005) Travertine. Springer, Berlin
Piper AM (1944) A graphic procedure in geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
Şahinci A (1991) Doğal suların jeokimyası (in Turkish). Reform Matbaası, Izmir, 548 p
Schoeller H (1934) Les échanges de bases dans les eaux souterraines; trois exemples es Tunisie. Bull Soc Géol Fr 4:389–420
Şengör AMC, Satır M, Akkök R (1984) Timing of tectonic events in the Menderes Massif, western Turkey: implications for tectonic evolution and evidence for Pan- African basement in Turkey. Tectonics 3:693–707
Şentürk F, Bursali S, Omay Y, Eran I, Güler S, Yalçin H, Önhon E (1970) Isotope techniques applied to groundwater movement in the Konya plain, Isotope Hydrology. Proc.Symp. Vienna, IAEA, Vienna, p 153
Seymen I (1981) Kaman (Kirşehir) dolayında Kirşehir Masifi’nin stratigrafisi ve metamorfizmasi. Türkiye Jeoloji Kurumu Bülteni 24(2):101–108
Seymen I (1982) Kaman dolayında Kirşehir Masifinin jeolojisi. Doç. Tezi, ITÜ, Maden Fak., 164 pp (In Turkish)
Seymen I (1984) Kirşehir Masifi metamorfitlerinin jeoloji evrimi. Ketin Sempozyumu, Special Paper, Geological Society of Turkey, Ankara, pp 133–148
Tonani F (1980) Some remarks on the application of geochemical techniques in geothermal exploration. In: Proceedings Adv. Eur. Geoth. Res. 2nd Symposium, Strasbourg, pp 428–443
Truesdell AH (1976) Summary of section III-geochemical techniques in exploration. In: Proceedings of the 2nd U.N. symposium on the development and use of geothermal resources. San Francisco. I, pp Iiii–Ixxix
Whitney DL, Dilek Y (2001) Metamorphic and structural geology of the Hirkadağ block, Central Anatolian Crystalline Complex. Turkish J Earth Sci 10:1–15
Whitney DL, Tkyssier C, Dilek Y, Fayon AK (2001) Metamorphism of the Central Anatolian Crystalline Complex, Turkey: influence of orogen-normal collision vs wrench dominated tectonics on P–T–t paths. J Metamorph Geol 19:411–432
Acknowledgements
This study was granted by The Scientific and Thecnical Research Council of Turkey (TÜBİTAK; Grant No: 104Y167). Prof. Dr. Serdar Bayarı and Dr. Nur Özyurt of the Hacettepe University are greatly acknowledged for performing of tritium and chemical analyses. We extend our special appreciation to Adriana Druma for the English editing of this article. Thanks to the editorial team of Environmental Earth Science for their critical review and comments of an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pasvanoğlu, S., Gültekin, F. Hydrogeochemical study of the Terme and Karakurt thermal and mineralized waters from Kirşehir Area, central Turkey. Environ Earth Sci 66, 169–182 (2012). https://doi.org/10.1007/s12665-011-1217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1217-3