Abstract
The suitability of groundwater quality for drinking and agricultural purposes was assessed in the rural areas of Delhi based on the various water quality parameters. A total of 50 ground water samples were collected randomly from different sources viz. hand pump, tube well, boring and analyzed for major ion chemistry to understand the operating mechanism of geochemical processes for ground water quality. The quality analysis is performed through the estimation of pH, EC, TDS, total hardness, total alkalinity, Na, K, Cl, NO3, SO4, DO, BOD, Cu, Cr, Cd, Ni, Zn and Pb. Hydrochemical facies were identified using Piper, Durov and Chadha diagram. Chemical data were also used for mathematical calculations (SAR, %Na, RSC, PI, KI, and chloroalkaline indices) for better understanding the suitability of ground water for irrigation purposes. The results of saturation index shows that all the water samples were supersaturated to undersaturated with respect to carbonate minerals and undersaturated with respect to sulphate and chloride minerals. According to USSL diagram, most of the samples fall in the field of C3S1, indicating medium salinity and low sodium water which can be used for almost all types of soil with little danger of exchangeable sodium. Assessment of water samples from various methods indicated that majority of the ground water in the study area is chemically suitable for drinking and agricultural uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ground water is one of the great natural resource in the biosphere. The groundwater is utilized in Delhi for both domestic and agricultural purposes, where we stressed its ability to provide farms and small rural communities with simple supplies relatively cheaply, in close proximity to the users and commonly without the need for complex treatment. In the rural areas, agriculture is the main source of livelihood of the population and ground water is the major source of irrigation. Heavy dependence of ground water resources for irrigation purposes is evident from the increase in number of tube wells in the region. Nearly 90% of the rural population in India is primarily dependent on untreated surface or ground water and about 30% of the people’s need in urban areas is met by groundwater (Rakesh et al. 2005). Due to the unavailability of surface water at many places, groundwater is the only alternate source of good quality water in rural areas. While access to drinking water in India has increased over the past decade, the tremendous adverse impact of unsafe water on health continues (WHO/UNICEF 2004). It is estimated that about 21% of communicable diseases in India is water related (Brandon and Homman 1995).
The availability of this important natural resource has been taken for granted increasing ground water use and pollution generation have crossed the sustainable limits in many parts, due to fast changing land use pattern. Land filling is still the most common way to dispose municipal and industrial wastes. Subsequent leaching of toxic contaminants through these landfills also leads to extensive contamination of ground water at many places.
During last decades, this is observed that the intensive use of natural resource and increased human activities are posing great threat to groundwater quality (Foster 1995; Mor et al. 2006). In Delhi, drinking water supply is not from single source. 68% of the population is getting supply from water of Yamuna River. Groundwater sources like tube wells, hand pumps and borings are the other sources. The routine monitoring of groundwater can assure the populace that the quality of their drinking water is adequate. It can also be beneficial in detecting deterioration in the quality of drinking water and facilitate appropriate timely corrective actions with minimal negative impacts on population health (Hook 2005; Hudak and Sanmanee 2003; Kruawal et al. 2005; Robins 2002).
For the purposes of ground water management, there is a requirement for improved understanding of the controlling processes and where possible, the natural geologically controlled baseline chemistry. This is especially important if the impacts of contaminants on groundwater are to be assessed. In order to understand the pollution trends and impacts on an aquifer, it is essential to have knowledge of the natural baseline quality so that imposed environmental change can be measured with an acceptable degree of confidence (Edmunds et al. 2003). The control of water quality must be effective. High clean up costs for effluent treatment and the low general public awareness are the main reason why an improvement in the situation is difficult to achieve (Mull et al. 1992).
Considering this, the present study investigates the quality of ground water for drinking as well as irrigation purpose in rural areas of Delhi (India). The data has been analyzed with reference to WHO and BIS standards. An attempt has also been made to discuss the major ion chemistry, trace metal chemistry, hydrochemical facies, and classify the ground water on the basis of different classification scheme.
Study area
National Capital Territory (NCT), Delhi, the capital of India is facing a water crisis and is even likely to face a water famine. Rapid urbanization coupled with population explosion is attributed as the major cause (Lorenzen et al. 2010). The situation becomes grimmer during dry seasons and large numbers of residents have to depend on ground water to augment the municipal water supply. The population of Delhi increases from 0.4 million in 1911 to 13.783 million in (Ministry of Finance 2008). Against a present requirement of about 3,324 million litres per day (MLD), the installed capacity is only 2,304 MLD and the average water consumption in Delhi is estimated at being 2,401 litres per capita per day (Central Ground Water Board 2006), the highest in the country. There has been a widespread drop in the ground water table in every district of Delhi. Lack of regulation related to private and individual extraction of ground water aggravates this situation.
The total area of NCT, Delhi in terms of rural and urban consumption during 1991 census is 1,483 Sq kms. Out of which 797.66 km2. Is designated as rural and 685.34 km2 as urban. Delhi is located in northern India between the latitudes of 28°-24′-17″ and 28°-53′-00″ North and longitudes of 76°-50′-24″ and 77°-20′-37″ East. The average annual rainfall in Delhi is 714 mm, three-fourths of which falls in July, August and September. Heavy rainfall in the catchment area of the Yamuna can result in a dangerous flood situation for the city. During the summer months of April, May and June, temperatures can rise to 40–45°C. Winters are typically cold with minimum temperatures during December and January falling to 4–5°C.
Hydrogeology
The National Capital Territory of Delhi is part of the Indo-Gangetic alluvial plains. The river Yamuna, a tributary of the Ganga, flows through the Eastern part of the territory, and a Quartzitic Ridge, rising between up to 91 m above the surrounding plains acts as a groundwater divide between the western and eastern parts of Delhi. The alluvial formations overlying the quartizitic bedrock have different nature on either side of the ridge. The nearly closed Chattarpur alluvial basin covering an area of about 48 km2 is occupied by alluvium derived from the adjacent quartzite ridge (Sett, 1964). Yamuna flood plains contain a distinct river deposit. Alluvial plains on eastern and western sides of the ridge are characterized by the occurrence of older alluviums. The geological units that influence and control the ground water occurrence and movement are (1) Alluvial plain on eastern and western sides of the ridge (2) Yamuna flood plain deposits (3) Isolated and nearly closed Chattarpur alluvial basin and (4) NNE–SSW trending Quartzitic Ridge. Chemical quality of ground water in NCT Delhi varies with depth and space. In alluvial formations, the quality of ground water deteriorates with depth, which is variable in different areas. During 1960, the ground water level was by and large within 4–5 m and even in some parts water logged conditions existed. During 1960–2001, water levels have been declined by 2–6 m in every part of the alluvial areas.
Materials and methods
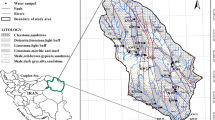
In our study, 50 groundwater samples were collected to assess the ground water quality from various rural areas as per census of India (Census of India 1991), covering seven districts of Delhi viz. south (S1–S8), south west (SW1–SW7), west (W1–W7), north west (NW1–NW10), east (E1–E6), north east (NE1–NE7) and north (N1–N5) in the month of April–June 2009. The sample locations in the study area are shown in Fig. 1. General procedure for ground water sampling, preservation and chemical analysis were carried out according to the standard procedure describe in standard methods for the examination of water and waste water (3). The analyzed parameter were electrical conductivity (EC), hydrogen ion activity (pH), total dissolved solid (TDS), calcium (Ca2+), magnesium (Mg2+), total hardness (TH), sodium (Na+), potassium (K+), chloride (Cl−), nitrate (NO3 −), total alkalinity (TA), sulphate (SO4 2−), dissolved oxygen (DO), biochemical oxygen demand (BOD) and trace metals. Further the SAR, percent sodium, RSC, Kelly’s index, permeability index, and indices of base exchange were calculated. Metal ion concentrations were determined by Atomic Absorption Spectrophotometer, Perkin Elmer (Model 3110) using air–acetylene flame.
Results and discussion
Understanding the ground water quality is important as it is the major factor determining its suitability for drinking, domestic, agricultural and industrial purposes. The data revealed that there were considerable variations in the examined sample from different sources with respect to their chemical characteristics. Physical and chemical parameters including statistical measures such as minimum, maximum, average, median and mode are summarized in Table 1. The values were compared with the World Health Organization (WHO 1993) and Bureau of Indian Standard (1991) standard (Table 2).
Major ions chemistry
pH is the term used universally to express the intensity of the acid or alkaline condition of a solution. The pH values of ground water samples of the study area were varying from 6.5 to 8.45 with an average value of 7.56. The pH values for samples are well within the limits prescribed by WHO and BIS.
The measurement of EC is directly related to the concentration of ionized substance in water and may also be related to problems of excessive hardness and other mineral contamination. EC of the groundwater is varying from 502 to 4,670 μS/cm at 30°C with an average value of 1,848 μS/cm. The classification of ground water on the basis of EC is given in Table 3. It is found that only 52% of the samples are with in the permissible limit, 30% of the samples fall in the not permissible limit but they are marginally poor in quality and 18% of the sample locations can be classified as hazardous according to the WHO standard.
In natural waters, dissolved solids consists mainly of inorganic salts such as carbonates, bicarbonates, chlorides, sulphates, phosphates and nitrates of calcium, magnesium, sodium, potassium, iron etc. and small amount of organic matter and dissolved gases. To ascertain the suitability of ground water of any purposes, it is essential to classify the ground water depending upon their hydrochemical properties based on their TDS values (Davis and De Wiest 1966; Freeze and Cherry 1979) which are presented in Tables 4 and 5, respectively. The TDS of the water samples ranges from 149 to 2,305 mg/l with an average value of 937.8 mg/l. According to the WHO and BIS specification, TDS up to 500 mg/l is desirable for drinking water. The study shows that only 16% of the sample is below desirable limit of TDS which can be used for drinking without any risk, 68% of the samples belong to maximum permissible category and remaining 16% of samples exceed the WHO and BIS specification. The ground water of the study area is fresh water for 72% of the sample locations and rest of the sample represents brackish water (Freeze and Cherry 1979).
The classification of groundwater (Table 6) based on TH shows that almost all the groundwater samples fall in the very hard water category. Ground water exceeding the limit of 300 mg/l is considered to be very hard (Sawyer and McCartly 1967). The desirable limit of TH for drinking purpose is 600 mg/l as per BIS standard (BIS 1991). The TH varies from 120 to 1,240 mg/l with an average value of 717.56 mg/l. About 56% groundwater samples exceed the acceptable limit of 600 mg/l (BIS 1991). The high amount of hardness in the study area samples is due to the presence of carbonate rocks i.e. dolomite.
The chloride concentration varies between 30 and 1,250 mg/l with an average value of 301.86 mg/l. The chloride ion concentration in ground water of the study area exceeds the desirable limit of 250 mg/l in 24 sample locations. The limits of chloride ion have been laid down primarily from taste consideration. A limit of 250 mg/l chloride has been recommended as desirable limit for drinking water supplies (BIS 1991; WHO 1993). However, no adverse health effects on human have been reported from intake of water containing an even higher content of chloride.
Nitrate contamination in groundwater is one of the major issues in water quality studies (Schilling and Wolter 2007; Raju et al. 2009). The occurrence of high levels of nitrate in ground water is a prominent problem in many parts of the country. The concentration of nitrogen in groundwater is derived from the biosphere (Saleh et al. 1999). Nitrogen is originally fixed from the atmosphere and mineralized by soil bacteria into ammonium. The concentration of nitrate in the study area varies from 3 to 124 mg/l with an average value of 29.3 mg/l. It is found that only 12 groundwater samples exceed the desirable limit of 45 mg/l as per WHO (1993) and BIS (1991) standard. The high concentration of nitrate in drinking water is toxic and causes blue baby disease/methemoglobinaemia in children and gastric carcinomas (Comly 1945; Gilly et al. 1984). Nitrate produces no color or odor in water and can cause cancer in humans when consumed over a long period of time (Jahed et al. 2008). The high concentration of nitrate is due to the intensive urbanization and industrialization (Raju et al. 2009).
Sulphate occurs naturally in water as a result of leaching from gypsum and other common minerals. The sulphate content changes significantly with time during infiltration of rainfall and ground water. The concentration of sulphate is likely to react with human organs if the value exceeds the maximum allowable limit of 400 mg/l and cause a laxative effect on human system with the excess magnesium in groundwater. However, the sulphate concentration varied between 15 and 251 mg/l with an average value of 83 mg/l and found within the maximum allowable limit in all sample locations as per WHO and BIS specification.
Alkalinity of water is its capacity to neutralize a strong acid and it is normally due to the presence of bicarbonate, carbonate and hydroxide compound of calcium sodium and potassium. BIS has recommended 300 mg/l as the desirable limits and 600 mg/l as the maximum permissible limit for drinking water (BIS 1991). It is evident from the result that 20% of the sample fall within the desirable limit of 200 mg/l, 72% of the sample exceed the desirable limit but are within the maximum permissible limit of 600 mg/l. Four samples of the study area even exceed the maximum permissible limit.
Dissolved oxygen is required to convert biodegradable organic matter from one form to another by living organisms mainly bacteria to maintain the metabolic process and produce energy for their growth and reproduction. It was found that the DO value of the groundwater samples varied from 2.7 to 9.7 mg/l. The DO concentration in 11 samples found low probably due to the pressure of materials of high organic content leading to oxygen depletion.BOD is the amount of oxygen utilized by microorganisms in stabilizing the organic matter. Higher BOD values may attribute to the stagnation of water body leading to the absence of self purification cycle. The BOD of the ground water sample ranged from 0.43 to 3.5 mg/l.
Sodium is the most abundant alkali metal. The concentration of sodium varies from 21 to 1,420 mg/l with an average value of 88.7 mg/l in this study. However, the concentration of sodium in almost all the samples (except two samples) was found well within the permissible limit of 200 mg/l. Such water should be treated before being used for domestic applications. Higher concentration of Na+ may pose risk to persons suffering from cardiac, renal and circulatory diseases. The concentration of potassium varies from 1.2 to 156 mg/l with significant fluctuation. K+ is an essential nutrient but if ingested in excess may behave as a laxative. 19 samples out of 50 have K+ concentration above the recommended value of 12 mg/l according to WHO (1993).
Trace metal chemistry
Ground water gets polluted with trace metals from a variety of sources, such as chemical weathering of rocks and soils, dead and decomposing vegetation and animal matter, wet and dry fallout of atmospheric particulate matter and humanity’s activities including the discharge of various domestic and industrial effluents. Though trace metal such as copper, cadmium, chromium, zinc etc. are extremely essential to humans but large quantities of them may cause physiological disorders. Cd, Cr and Pb are highly toxic to humans even in low concentration. The results of concentration of the trace metals for all the measured groundwater samples in the study area are summarized in Table 2.
Copper is essential to human life and health but, like all heavy metals, is potentially toxic as well. Continued inhalation of Cu containing spray is linked with an increase in Lung cancer among exposed worker. The BIS has recommended 50 μg/l as the desirable limit and 1,500 μg/l as the permissible limit in the absence of alternate source (BIS 1991). WHO has recommended 2,000 μg/l as the provisional guideline value for drinking water purposes (WHO 1993).The concentration of Cu in all the ground water samples from rural areas of Delhi are well within the permissible limit of drinking water. Beyond permissible limit, the water imparts astringent taste and cause discoloration and corrosion of pipes, fittings and utensils.
Chromium is a naturally occurring element found in rocks, animals, plants, soil and in volcanic dust and gases. Cr is strongly attached to soil and only a small amount can dissolve in water and move deeper in the soil to under groundwater. A concentration of 50 μg/l has been recommended as a desirable limit for drinking water (BIS 1991). WHO has also prescribed 50 μg/l as the guideline value for drinking water. The concentration of Cr in 88% of the groundwater samples were Below Detection Limit and rest were found within the prescribed limit of BIS and WHO.
Cadmium is a non essential non beneficial element known to have a high toxic potential. In most of the samples under investigation, the cadmium concentrations were below detection limit. 24% of the samples have much higher Cd concentration than the guideline value of 3 μg/l (WHO 1993). Cd above the permissible limit can potentially cause nausea, vomiting, diarrhea, muscle cramps, salivation, sensory disturbances, liver injury, shock and renal failure along with kidney, liver, bone and blood damage from a life time exposure.
Zinc is considered as an essential element for life and act as a micronutrient when present in trace amounts. But too much Zn can be harmful to health. Symptoms of Zn toxicity include irritability, muscular stiffness, loss of appetite and nausea. The World Health Organization (1993) has recommended 3,000 μg/l of Zn as the guideline value for drinking water. BIS has prescribed 5,000 μg/l zinc as the desirable limit and 15,000 μg/l as the permissible limit for drinking water (BIS 1991). Analysis of groundwater samples shows that all the samples are well within the desirable limit prescribed by BIS (1991) and WHO (1993).
Nickel at trace level is essential to human nutrition and no systemic poisoning from nickel is known in this range. The WHO has recommended 20 μg/l as the guideline value for drinking water (WHO 1993). The concentrations of Nickel in 70% groundwater samples were beyond the WHO limit. High concentration of Ni may cause dermatitis, dizziness, headache, nausea and carcinogenesis. In the study area, the Lead was present below detection limit except in three samples but the concentration was found well within the WHO limit. Therefore, the ground water of the study area do not pose any lead hazard. Lead has been known to be toxic to human. The effect of Lead on the mental development of children causes the most concern. It has been calculated that Lead can cause a reduction of between 5 and 15% of a child’s intelligence depending on the amount found in water.
Saturation index
The ground water chemistry exchanges matter with the various minerals and gases with in an aquifer resulting in a dissolution or precipitation of minerals. Equilibrium calculations are most commonly used to assess whether ground water is in equilibrium with respect to one or more minerals. The saturation state of minerals in the water can be expressed by the saturation index (SI). The SI is defined as (Chapell 1993).
where IAP is the ion activity product of the dissociated chemical species in solution, K sp is the equilibrium solubility product for the chemical involved at the sample temperature. An index (SI), less than zero (−ve), indicate that the groundwater is under saturated (dissolution) with respect to that particular mineral. Such a value could reflect the character of water from a formation with insufficient amount of the mineral for solution or short residence time. An index (SI), greater than zero (+ve), specifies that the groundwater being supersaturated (precipitation) with respect to the particular mineral phase and therefore incapable of dissolving more of the mineral (Cidu et al. 2009). Such an index value reflects groundwater discharging from an aquifer containing ample amount of the mineral with sufficient resident time to reach equilibrium. Nonetheless, super saturation can also be produced by other factors that include incongruent dissolution, common ion effect, and evaporation, rapid increase in temperature and CO2 exsolution (Appelo and Postma 1996; Langmuir 1997). The Table 2 gives the result of SI for anhydrite, aragonite, calcite, dolomite, gypsum and halite. Plots of SI against TDS for all the investigated groundwater samples are shown in Fig. 2. It is noticeable that the ground water samples are significantly supersaturated to undersaturated with respect to calcite, dolomite and aragonite (precipitation takes place) and all samples are under saturated with respect to anhydrite, gypsum and halite.
Hydrochemical facies
Hydrochemical concepts can help to elucidate the mechanisms of flow and transport in groundwater systems, and unlock an archive of paleoenvironmental information (Pierre et al. 2005; Ophori and Toth 1989; Hem 1992). Piper (1944) has developed a form of trilinear diagram, which is an effective tool in segregating analysis data with respect to sources of the dissolved constituents in ground water, modifications in the character of water as it passes through an area and related geochemical problems (Piper 1944). The ionic concentration of major cations and anions found in groundwater of the study area are plotted in Piper’s trilinear diagram (Fig. 3a, b) by the geochemical software AQUAChem.
The classification for cation and anion facies, in terms of major ion percentage and water types, is according to the domain in which they occur on the diagram segment (Back 1966). The district wise details of hydrochemical facies are given in Table 7. The main hydrochemical species in the study area are CaMgHCO3, CaClHCO3, and CaMgCl, respectively according to their order of dominance (Fig. 3a). From the plot it is observed that, majority of the groundwater samples exhibit that the alkaline earth metals (Ca + Mg) significantly exceed the alkalis (Na + K) and HCO3 − and Cl− exceeds the other ions. The hydrochemical facies of groundwater are summarized in Table 1. The Durov plot is an alternative to the Piper plot. This diagram was developed in 1948 and modified by (Chilingar 1956). The trilinear Durov diagram (Fig. 3b) is based on the percentage of major ion milliequivalents. The cation and anion values are plotted on two separate triangular plots and the data points are projected onto a square grid at the base of each triangle. The diagram indicates dominance of the major ions Ca2+, Mg2+, HCO3 −and Cl− while other ions, such as Na+, K+ and SO4 2−, are comparatively less represented, indicating anthropogenic input in the groundwater system. The diagram also shows that HCO3 − is dominating with Cl− and Na+ is replaced by Ca2+ and Mg2+ indicating some reverse ion exchange and recharge.
Chadha (1999) has proposed new diagram for geochemical data presentations. The proposed diagram is a modification of piper diagram with a view to extend its applicability in representing water analysis in the possible simplest way. In this proposed diagram, the difference in milliequivalent percentage between alkaline earths (Ca + Mg) and alkali metals (Na + K), expressed as percentage reacting values, is plotted on the X axis, and the difference in milliequivalent percentage between weak acidic anions (CO3 + HCO3) and strong acidic anions (Cl + SO4) is plotted on the Y axis. The resulting field of study is a square or rectangle, depending upon the size of the scales chosen for X and Y co-ordinates. The milliequivalent percentage differences between alkaline earths and alkali metals, and between weak acidic anions and strong acidic anions, would plot in one of the four possible sub-fields of the proposed diagram. The main advantage of the proposed diagram is that it can be made simply on most spreadsheet software packages. The square or rectangular field describes the overall character of the water. The proposed diagram has all the advantages of the diamond-shaped field of the Piper diagram and can be used to study various hydrochemical processes, such as base cation exchange, cement pollution, mixing of natural waters, sulphate reduction, saline water (end-product water), and other related hydrochemical problems. Results of analyses were plotted on the proposed diagram to test its applicability for geochemical classification of ground water and to study hydrochemical processes (Fig. 4). It is clearly evident from the results that majority of the ground water samples fall in groups 5 and 6 i.e. Ca–Mg-HCO3 and Ca–Mg-Cl water type, respectively.
Irrigation water quality
The concentration and composition of dissolved constituents in water determine its quality for irrigation use. Several chemical constituents affect water suitability for irrigation from which the total concentration of the soluble salts and the relative proportion of sodium to calcium and magnesium. The suitability of ground water for agricultural purposes depends on the effect of mineral constituent of water on both plants and soil (Wilcox 1955). Effects of salts on soil causing changes in soil structure, permeability and aeration in directly affect plant growth.
Percent sodium (%Na)
Irrigation water containing large amounts of sodium is of special concern due to sodium’s effects on soil and poses a sodium hazards. Excess sodium in water produces the undesirable effects of changing soil properties and reducing soil permeability (Subba Rao 2006). Hence, the assessment of sodium percentage is necessary while considering the suitability for irrigation.
where all the ion concentrations are expressed in meq/l. The classification of ground water on the basis of percentage sodium alone is given in Table 8 and found that 80% of the samples are excellent for irrigation. The Wilcox (1955) diagram (Fig. 5) relating percentage sodium and EC shows that 62% of the ground water sample fall in the category of excellent to good and good to permissible for irrigation purposes. 14% of the sample have doubtful irrigation water quality, 18% of the sample have unsuitable irrigation water quality and only three samples have fall on permissible to doubtful category (Table 9).
The quality of water in relation to salinity and sodium hazards (after US salinity Laboratory 1954)
Sodium adsorption ratio
Sodium hazard is also expressed in terms of the sodium adsorption ratio (SAR). SAR is calculated from the ratio of sodium to calcium and magnesium. The latter two ions are important since they are tending to counter the effect of sodium. Continued use of water having a high SAR leads to breakdown in the physical structure of the soil. Sodium is adsorbed and becomes attached to soil particles. The soil then becomes hard and compact when dry and impervious to water penetration. The degree to which irrigation water tends to enter into cation exchange reactions in soil can be indicated by the sodium adsorption ratio. Sodium replacing adsorb calcium and magnesium is a hazards as it causes damage to the soil structure. SAR is an important parameter for the determination of the suitability of irrigation water because it is responsible for the sodium Hazards (Nagarajah et al. 1988). SAR has become calculated as follows:
where all the concentrations are expressed in meq/l. The analytical data plotted on the US salinity diagram proposed by US salinity Laboratory (1954). Figure 6 illustrates that 60% of the ground water falls in the field of C3S1, indicating water of medium high salinity and low sodium, which can be used for irrigation in almost all types of soil with little danger of exchangeable sodium. 30% of the ground water sample falls in the field of C4S1, indicating very high salinity and low alkalinity hazards. This water will be suitable for plants having good salts tolerance and it restricts suitability for irrigation, especially in soils with restricted drainage. 8% of the ground waters fall in the field of C2S1, indicating medium salinity and low sodium content. This water will be suitable for all plants but natural drainage should be good. One sample comes under C4S2 classification (Table 10).
Electrical conductivity and percent sodium relationship for rating irrigation water (Wilcox 1955)
Residual sodium carbonate
When total carbonate levels exceed the total amount of calcium and magnesium, the water quality may be diminished. When the excess carbonate (residual) concentration becomes too high, the carbonate combines with calcium and magnesium to form a solid material (scale) which settles out of the water. The relative abundance of sodium with respect to alkaline earths and the quantity of bicarbonates and carbonate in excess of alkaline earths also influence the suitability of water for irrigation. RSC is given by the relation:
where all the concentrations are expressed in meq/l. According to the US salinity laboratory (1954), an RSC value less than (1.25 meq/l) is safe for irrigation, a value between 1.25 and 25 meq/l is of marginal quality and a value more than (25 meq/l) is unsuitable for irrigation. All the samples have RSC values much less than 1.25 meq/l which indicate that all samples are of safe quality categories for irrigation. Further the value of RSC is negative at all sampling sites, indicating that there is no complete precipitation of calcium and magnesium (Tiwari and Manzoor 1988).
Permeability index
The soil permeability is affected by long term use of irrigation water. Sodium, calcium, magnesium and bicarbonate content of the soil influence it. Permeability index is defined by the following equation (Raghunath 1987):
where all the ions are expressed in meq/l. The PI values >75% (Class I) indicate excellent quality of water for irrigation. If the PI values are between 25 and 75% (Class II), they indicate good quality of water for irrigation. However; if the PI values are less than 25% (Class III), they reflect unsuitable nature of water for irrigation. Permeability index of study area varies from 10.265 to 85 meq/l with an average value of 32.789 meq/l. According to PI values, 72% ground water in the study area can be designated as class II (25–75%) that shows majority of the ground water in the study area is good for irrigation purposes.
Kelly’s index
Based on Kelly’s index (KI) waters are classified for irrigation. Sodium measured against calcium and magnesium was considered by Kelly (1940) and Paliwal (1967) to calculate this parameter. A Kelly’s index of more than one indicates an excess level of sodium in waters. Therefore, water with a Kelly’s index less than one are suitable for irrigation, while those with a ratio more than one are unsuitable. Kelly’s index was calculated by using the following expression:
where all the concentrations are expressed in meq/l. It is observed from Table 2 that all the samples from the study area are good for irrigation regarding alkali hazards. However, the water samples of polluted station NW4 is unsuitable for irrigation as the KI value is more than one (4.21 meq/l).
Chloro alkaline indices
It is essential to know the changes in chemical composition of ground water during its travel in the subsurface (1), the ion exchange between the ground water and its host environment during residence or travel can be understood by studying the chloroalkaline indices (Schoeller 1977). The chloroalkaline indices are calculated by using the equations:
If CAI is negative then there is base exchange between sodium and potassium (Na + K) in water with calcium and magnesium (Ca + Mg) in rocks. If the ratio is positive, then there is no base exchange. The CAI 1 and 2 are calculated for the water of the study area is given in Table 1. The CAI calculation shows that 20% of the ground water samples are negative and 80% positive ratios.
Conclusions
The study provides significant information on the development of ground water quality in rural areas of Delhi. The major ion chemistry data revealed that the ground water in the study area is hard to very hard and fresh to brackish in nature. The sequence of the abundance of the major ions are in following order; Ca > Mg > Na > K and HCO3 > Cl > NO3 > SO4. The alkali earth (Ca + Mg) significantly exceed alkalis (Na + K) and HCO3 − and Cl− exceeds the other anions. The hydrochemical facies of ground water sample fall in CaMgHCO3, CaClHCO3 and CaMgCl. Distribution of the ground water samples in Chadha diagram reveals that the majority of the samples fall under CaMgCl and CaMgHCO3 category. The result of calculation of SI shows that nearly all of the water samples were supersaturated to undersaturated with respect to carbonate minerals (calcite, dolomite and aragonite) and undersaturated with respect to anhydrite, gypsum and halite. According to the WHO (1993) and BIS (1991) classification of water based on TDS, 16% samples exceeds the maximum allowable limit. A total 56% of the ground water samples in the study area exceeded the maximum permissible limit of TH. The concentration of Na at all the sampling location is in desirable limit except in two locations while 12 samples exceeded the maximum allowable limit for potassium. Nitrate concentrations were found beyond the maximum allowable limit for drinking water in 12 locations only. The trace metals in the ground water except cadmium and nickel have been found below the prescribed permissible limits in all samples. The cadmium and nickel exceeds the maximum allowable limit in 24 and 70% sample locations, respectively. Based on the Wilcox 1955; classification, 62% of the water samples belong to excellent to good and good to permissible category. Irrigation water quality based on percentage sodium alone indicates that 78% of the water samples belong to excellent category. The analytical data plotted on the US Salinity diagram illustrates that 30% of the ground water samples fall in the field of C4S1, indicating high salinity, low sodium and 60% samples fall in the field of C3S1 indicating medium salinity, low sodium. Based on the classification of irrigation water according to RSC values, all the sample locations belong to good category. According to PI values, 72% ground water samples are suitable for irrigation purposes. Kelly’s index shows that nearly all the ground waters are suitable for irrigation. Chloroalkaline indices 1, 2 calculation shows that 20% of the ground water sample is negative and 80% positive ratio. The positive values indicate absence of base exchange reaction.
References
Aastri JCV (1994) Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling. Bharathidasan University, Thiruchirapalli
APHA (1998) Standard Methods for the Examination of Water and Wastewater, 20th edn. Washington
Appelo CAJ, Postma D (1996) Geochemistry, groundwater and pollution. Balkema, Rotterdam, p 536
Back W (1966) Hydrochemical facies and ground-water flow patterns in northern part of Atlantic Coastal Plain, US Geol. Surv. Prof. Paper 498-A, p 42
BIS (1991) Specifications for Drinking Water. IS:10500:1991. Bureau of Indian standards, New Delhi
Brandon C, Homman K (1995) The cost of interaction: valuing the economy- wide cost of environmental degradation in India. Asia Environment Division, World Bank 7, October memo
Census of India (1991) District census Handbook, part XII A & B, Village & Town Director, Village & Town wise, primary Census Abstract, Delhi District, pp 288–294
Central Ground Water Board (2006) Ground water year book of national capital of territory. Delhi. Ministry of Water Resources, Government of India, New Delhi
Chadha DK (1999) A proposed new diagram for geochemical classification of natural water and interpretation of chemical data. Hydrogeol J 7:431–439
Chapell FH (1993) Ground water microbiology and geochemistry. Wiley, New York, p 424
Chilingar GV (1956) Durovs classification of natural water and chemical composition of atmospheric precipitation in U.S.S.R. Transactions - American Geophysical Union 37:193–196
Cidu R, Biddau R, Fanfani L (2009) Impact of past mining activity on the quality of groundwater in SW Sardinia (Italy). J Geochem Explor 100:125–132
Comly HH (1945) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 129(129):12–144
Davis SN, De Wiest RJM (1966) Hydrogeology, vol 463. Wiley, New York
Edmunds WM, Shand P, Hart P, Ward RS (2003) The natural baseline quality of ground water: a UK pilot study. Sci Total Environ 310:25–35
Foster SSD (1995) Groundwater for development- an overview of quality constraints. In: Nash H, McCall GJH (eds) Groundwater quality. 17th Special report. Chapman and Hall, London, pp 1–3
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, New Jersey
Gilly G, Carrao G, Favilli S (1984) Concentration of nitrates in drinking water and incidence of carcinomas. First descriptive study of the Piemonate Region, Italy. Sci Total Environ 34:35–37
Hem JD (1992) Study and interpretation of the chemical characteristics of natural water. U.S.Gov. Print. Office, Washington
Hook Z (2005) An assessment of the water quality of drinking water in rural districts in Zimbabwe. The case of Gokwe South, Nkayi Lupane, and Mwenezi districts. Phys Chem Earth 30:859–866
Hudak PF, Sanmanee S (2003) Spatial patterns of nitrate, chloride, sulfate, and fluoride concentration in the woodbine aquifer of North-Central Texas. Environ Monit Assess 82:311–320
Jahed KGR, Dehghani MH, Mahvi AH, Rafati L, Tavanafar E (2008) Concentration of nitrate and nitrite in groundwater resources of Hamdan Province, Iran. Res J Chem Environ 12(4):56–58
Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceeding of the ASCF 66, pp 607
Kruawal K, Sacher F, Werner K, Mqller J, Knepper TP (2005) Chemical water quality in Thailand and its impacts on the drinking water production in Thailand. Sci Total Environ 340:57–70
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, India, p 601
Lorenzen G, Sprenger C, Taute T, Pekdeger A, Massmann G (2010) Assessment of the potential for bank filteration in a water-stressed megacity (Delhi, India) 61:1419–1434
Ministry of Finance (2008) Economic survey. Government of India, New Delhi
Mor S, Ravindra K, Dahiya RP, Chandra A (2006) Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Monit Assess 118:435–456
Mull R, Harig F, Pielke M (1992) Ground water management in the urban area of Hanover Germany. J Inst Water Environ Manage 6(2):199–206
Nagarajah S, Emerson BN, Abeykoon V, Yogalingam S (1988) Water quality of some wells in Jaffna and killinochchi with special reference to nitrate pollution. Tropical Agriculture 44:61–73
Ophori DU, Toth J (1989) Patterns of groundwater chemistry, Ross Creek basin, Alberta, Canada. Ground Water 27:20–26
Paliwal KV (1967) Effect of gypsum application on the quality if irrigation waters. The madras agricultural journals 59:646–647
Pierre D, Glynn L, Plummer N (2005) Geochemistry and the understanding of ground-water systems. Hydrogeol J 13:263–287
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923
Raghunath HM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna River basin, Varanasi district, Uttar Pradesh, India. Journal of Geological Society of India 73:178–192
Rakesh K, Singh RD, Sharma KD (2005) Water resources in India. Curr Sci 89:794–811
Robins NS (2002) Groundwater quality in Scotland: Major ion chemistry of the key groundwater bodies. Sci Total Environ 294:41–56
Saleh A, Al-Ruwih F, Shehata M (1999) Hydrogeochemical process operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sawyer GN, McCartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York, p 518
Schilling KE, Wolter CF (2007) A GIS-based groundwater travel time model to evaluate stream nitrate concentration reductions from land use change. Env Geol 53:433–443
Schoeller H (1977) Geochemistry of groundwater. In: Ground water studies-An international Guide for Research & Practice. UNESCO, Paris, pp 1–18
Sett DN (1964) Ground water geology of the Delhi region, Bulletin. Geological survey of India, Series B 16:1–35
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49:413–429
Tiwari TN, Manzoor A (1988) Pollution of Subarnarekha river near Jamshedpur and the suitability of its water for irrigation. Indian journal of environmental protection 8(7):494–497
US Salinity Laboratory (1954) Diagnosis and improvement of saline and alkali soils. Agricultural Handbook, USDA, No 60, pp 160
WHO (1993) Guidelines for drinking water quality, vol.1, recommendations, 2nd edn. WHO, Geneva
WHO/UNICEF (2004) Meeting the MDG drinking water and sanitation target: A mid-term assessment of progress. WHO, Geneva
Wilcox LV (1955) Classification and use of irrigation water. USDA, Circular. Washington, pp 969
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, M., Rais, S. & Aslam, M. Hydrochemical investigation and quality assessment of ground water in rural areas of Delhi, India. Environ Earth Sci 66, 97–110 (2012). https://doi.org/10.1007/s12665-011-1210-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1210-x