Abstract
Field data from 25 karst springs were collected during the summer of 2008 near Verrettes, Haiti, to gain a better understanding of water quality in fresh water springs used as the primary source of potable water in rural Haiti. Two water samples were taken at each spring for bacterial analysis: one sample was submitted to a local hospital for analysis, while a duplicate sample was cultured using inexpensive and commercially available Coliscan® Easygel® Kits. Both capped and uncapped springs were sampled in order to evaluate whether capping improves water quality. Water quality parameters and flow volume estimates were recorded at each spring; however, no correlations between field water parameters and the presence of E. coli or total coliform bacteria were found. Spring flow rates varied from 0.4 to 268.5 L/min. Geologic contacts and regional structures trend in a northwest direction and appear to be exerting a primary control on spring locations. E. coli and total coliform colony counts from the local hospital and Coliscan® Easygel® Kits yielded different results for the same springs due to sample handling and analysis complications common in rural Haiti. Water sample analysis and storage problems encountered during this study likely resulted in underprediction, rather than overprediction, of bacterial contamination. Water from 71 to 100% of the springs was unsafe to drink based on the World Health Organization (WHO) drinking water standard. Both capped and uncapped springs had bacterial counts in excess of the WHO standard, suggesting that water treatment from all sources is necessary to ensure clean and safe drinking water. Negatively impacted subterranean microbial ecosystems, poor sanitation practices, shallow karst aquifers with open flow paths, and high spring water temperatures, averaging 26.5°C, may be contributing to the observed bacterial abundance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is often assumed that spring water emerges from the ground clean and free of contaminants, especially in rural areas where industrial contamination is not present. Many rural Haitians know that drinking untreated water from surface streams and rivers is not safe; however, they often assume that water emerging from the ground at a spring is clean and safe to drink. This paper presents original research evaluating the quality and quantity of spring water resources in rural Haiti that demonstrates this is a dangerous assumption. Many interventions to provide safe drinking water remain problematic.
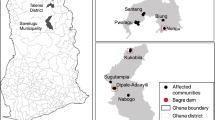
Haiti is a small island country located in the Caribbean, on the western one-third of the Island of Hispaniola (Fig. 1). More than half of Haiti’s roughly 8.3 million people are without access to clean water and sanitation; and water-borne illnesses account for the deaths of up to one-third of all children under the age of five (Knowles et al. 1999). Many rural Haitians are subsistence farmers tilling the land to grow corn, sorghum, beans, and rice for food, and harvesting trees to make charcoal for cooking. Subsistence farming and charcoal production from trees have resulted in dramatic deforestation and soil loss in much of the country.
Deforestation since the early 1900s has left only 2% of Haiti’s countryside forested, and resulted in severe soil erosion in the steep mountainous terrain (LOCFRD 2006). Deforestation reduces infiltration of precipitation, increases surface water runoff, and decreases groundwater recharge (LOCFRD 2006; Knowles et al. 1999). Changes to infiltration rates and groundwater flow are important since much of the rural population of Haiti uses shallow karst aquifers and springs as their primary source of potable water (Knowles et al. 1999). In many cases, Haitians living in rural mountainous regions collect water from muddy pools where groundwater discharges at springs. Spring locations are also popular watering holes for animals and quickly become contaminated with bacteria and other water-borne pathogens that cause illness and death. Non-Governmental Organizations (NGO) and government entities have undertaken spring capping and reservoir projects to isolate springs from surface water contamination. However, spring capping does not prevent contamination from entering groundwater systems due to poor sanitation. Rural dwellings do not have plumbing or outdoor latrines. As a result, most rural Haitians defecate on the ground near dwellings which is washed into shallow aquifers during rain events.
An NGO, Faith in Action International (FIAI), has been working in Verrettes, Haiti for nearly a decade helping Haitians obtain clean water, reforest hillsides, and become more literate through education. One aspect of the work done by FIAI is the capping of springs to isolate them from surface contamination (Figs. 2, 3). However, little is known about the distribution of springs and the level of contamination of the springs where they emerge. This study was conducted to (1) locate and identify springs in the study area; (2) estimate the amount of water flowing from each spring; and (3) analyze water samples from each spring to determine the presence and quantity of E. coli and other coliform bacteria.
Example of a typical capped spring structure at Ozys Spring. Accumulated flow is piped from this location to a reservoir ~50 m down the ravine. Inset is a typical spring cap design (UNESCO 1991)
Water quality and quantity data for this study were collected over a period of 15 days between 17 June and 2 July 2008. Many logistical difficulties were encountered during the study due to the somewhat unique conditions in rural Haiti. Sample transport was challenging due to a lack of roads in many mountain areas; sample storage was difficult without power for refrigeration or a consistent ice supply. Consequently, some sampling and analysis procedures did not conform to standards applied for water sampling in developed countries. The paucity of data from this region and the value of this study to provide an example for others attempting to undertake similar studies justify publication of this unique, but somewhat imperfect, dataset.
Study area and geologic setting
The study area, encompassing approximately 87 km2 near the town of Verrettes, Haiti, is located on the island of Hispaniola, approximately 120 km north of the capital city of Port au Prince. Verrettes is a city of roughly 10,000 people near the Artibonite River (Fig. 4). Many rural residents reside in the mountains above the town and travel to the town for food and supplies on market days. The study areas include four subcatchments within the roughly 9,000 km2 Artibonite River watershed.
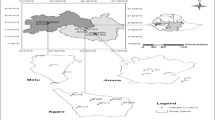
Simplified hydrogeologic map of the study area (modified from Ministère de l’Agriculture 1990). Inset is an example of pinnacle karren from the southwest corner of the map area
Limestone bedrock; geologic structures, such as faults and folds; and the karst geomorphology of Haiti have a profound effect on the quantity and quality of water, both from springs and surface water sources. Shallow karst aquifers, common in the mountains of Haiti, and deforestation reduce natural filtration of biological contaminants and exacerbate Haiti’s water quality problems.
The island of Hispaniola is mountainous and rugged as a result of its position between two large and active tectonic plates. The Puerto Rico Trench, which lies north and northeast of the island, marks the boundary between the eastward moving Caribbean Plate and the southwest moving North American Plate. This oblique collision results in wrench-fault tectonism characterized by shearing, folding, thrusting, and numerous large east–west to northwest–southeast trending faults (Maurrasse 1982).
The study area is part a northwest-striking limestone belt in the Sierra de Neiba physiographic province (Maurrasse 1982). Numerous northwest trending faults have been mapped in the region and these faults, combined with the stratigraphy of limestone beds, exert primary control on spring locations. Rock types are dominated by gently to complexly folded, faulted, recrystallized, non-fossiliferous limestone. Bedding planes generally strike in a northwesterly direction and dip toward the northeast (Fig. 4).
Dissolution of the limestone bedrock and the development of karst in the region have resulted in a complicated network of shallow limestone aquifers and springs that are characterized by fissures and open conduits that provide little resistance to flow and do not significantly filter groundwater (White 2002; Kacaroglu 1999). Although karst structures are seldom exposed at the surface, surface karst features such as several-meter-high pinnacle karren, sporadic dolines, and terra rossa (locally referred to as “te rouge” in Creole) were observed (Fig. 4, inset). Pinnacle karren were similar to those described as resulting from phytokarst and a lack significant soil cover (White 1988). This is consistent with a general lack of soil cover observed over much of the study area.
Springs generally emerged within stream valleys, either within the stream bed or from the banks of the stream. Vegetation near springs was typically lush compared to adjacent ridges and mountain slopes.
Methods
A Geographic Information System (GIS) was utilized to map spring locations, locate areas that may contain untapped springs using satellite imagery, count and determine the color of bacterial colonies on cultures; and produce maps of results. Spring flow and distribution were determined for 27 springs, 25 of which were analyzed for bacterial content. Field water quality parameters were collected to determine if easily measurable field parameters could be correlated with more time-consuming and expensive bacterial analyses. Water samples were collected and analyzed for bacteria using two different methods to evaluate the feasibility and accuracy of an economical water testing method which does not require a laboratory.
Water quality and flow estimates
Fieldwork at each spring consisted of estimating spring flow, collecting water samples for analysis, taking photos, drawing a sketch of the spring, obtaining Global Positioning System (GPS) coordinates of the spring, describing the type of rock surrounding the spring, and measuring the strike and dip of exposed bedrock. GPS coordinates from 27 springs were collected in the WGS84 latitude and longitude coordinate system with a hand-held Garmin GPSMAP 76Cx. GPS locations were imported into ArcMap as layers, including an attribute indicating flow volume, and whether the spring was capped, uncapped, or a spring reservoir.
A water sample was taken in a plastic beaker and a YSI model 85-25 water quality meter was used to measure conductivity, dissolved oxygen (DO), salinity, and water temperature. A Hannah pH Meter and Oakton T-100 Turbidity Meter were used to measure pH and turbidity, respectively.
In order to estimate spring flow, a variety of methods were used based on configuration of each spring and whether the spring was capped or uncapped. Some springs emerged at or near surface water streams while others appeared to be emerging from a water table higher than stream level. At some springs, the spring conduit was covered with colluvium and topsoil resulting in a diffuse flow. For these springs, a 1-m long, 2-in. PVC well screen pipe was used to concentrate water into a single defined flow for measurement and sampling purposes. In some cases, this meant capturing water within the hillside before it was discharged at the spring into a stream or pool. For some uncapped springs, estimates were made by approximating water velocity and flow area to estimate total flow. Capped spring flows were either measured by capturing the water as it was forced out an overflow pipe, or measured from the reservoir outlets. All flow measurements from capped springs were taken using a stopwatch and either a 1,000-mL graduated cylinder or 5-gallon bucket. Measurements were repeated five times and flow rates were calculated by averaging the five measurements.
Bacterial analysis
Two water samples were taken for bacterial analysis and water quality parameter tests. 35-mL samples were taken in sterile bottles for analysis using Coliscan® Easygel® Kits, and 250-mL water samples were taken in sterilized bottles for analysis at the local hospital, Hospital Albert Schweitzer (HAS). Samples were kept in a cooler, when possible, between sample collection and analysis. The logistics of accessing remote sites and the schedule of lab personnel at HAS resulted in some samples being delayed as much as 72 h between sample collection and analysis. It was not always possible to refrigerate samples or obtain ice to keep them cool due to the widespread lack of resources and basic infrastructure, such as power and water. This undoubtedly resulted in bacterial mortality in some spring samples due to lack of nutrients and high temperatures. For this reason, bacterial contamination results should be viewed as minimum values, likely under-representing the extent of bacterial contamination in rural Haiti.
Sample analysis at HAS was performed by HAS personnel. A Hach Company (HACH) hand operated vacuum pump was used to pump water samples through a 47-mm diameter, 0.45-μm filter membrane which was then placed in a petri dish with M-TEC dehydrated growth medium and incubated for 48 h in an incubator. After incubation, E. coli and total fecal coliform colony counts were done. Total chlorine, ferrous iron, and phosphate were measured using a HACH colorimeter. Turbidity and pH were also analyzed for each sample by HAS.
For bacterial analysis using Coliscan® Easygel® Kits, 5 mL of the 35-mL water sample was withdrawn using a sterile 1-mL dropper and added to the sterile container with Coliscan® Easygel® medium. The mixture was gently mixed and carefully poured into a covered, sterile, petri dish for incubation at room temperature (25–35°C). Temperatures likely varied within a 10°C range between mid-afternoon and early morning. In order to analyze samples under conditions that local Haitians could sustainably reproduce, an incubator was not used.
The Coliscan® Easygel® medium contains sugar linked to a dye, which, when acted upon by the enzyme β-galactosidase produced by coliform bacteria, turns the colony pink. A second sugar, linked to another dye, and acted upon by the enzyme, β-glucuronidase produced by E. coli colonies, grows blue-green colonies. Only E. coli releases both enzymes, reacting with both dyes, resulting in a purple or dark blue color (Micrology Laboratories 2005). Digital photos of each sample were taken at the start of incubation and at roughly 24–72 h, and in some cases after 72 h. Approximate counts of E. coli colonies, fecal coliform and non-coliform colonies were taken within 24–48 h. Visual inspection of the number of colonies suggests that the number did not change greatly after 48 h.
Because some colonies were difficult to differentiate visually by color, two methods were used to count bacterial colonies using the Coliscan® Easygel® Kits: a subjective visual count and a digital photo analysis using ArcMap GIS. Digital photos of each sample were imported into ArcMap 9.2. A point layer was created for each water sample culture. Automated colony classification and counting were accomplished by querying the digital images of each colony in the petri dish image and updating the attribute tables for each point with Red (R), Green (G), and Blue (B) values. Attribute tables from ArcMap were imported into Microsoft Excel® for the automated classification and analysis.
A series of tests were created in Excel using if–then statements to classify the colonies as E. coli, coliform, or non-coliform using the RGB values (Fig. 5). The first test was derived from the observation that purple colonies had greater B than G pixel values. Subtracting the B pixel value from the G pixel value yielded a value which, if greater than 0, classified the colony as purple. The second test separated pink colonies based on high R pixel values. Colonies with R pixel values greater than 146 were considered pink. The third test was based on the observation that purple colonies tended to have similar RGB pixel values. The standard deviation of good examples of purple colonies was used to establish a standard deviation value of less than 27 for a colony to be classified as purple. Standard deviations values greater than 27 were classified as pink or teal. If a colony was determined to be purple by all three tests, it was classified as E. coli. Pink colonies were classified as coliform bacteria. Teal, or non-coliform colonies, were not included in counts. Final visual, computer automated, and HAS E. coli and fecal coliform counts were compared in order to assess the accuracy of the three methods and to report the total bacteria presence in the springs.
Results
Water quality measurements revealed a high average temperature of 26.5°C (79.7°F). Field-measured water quality parameters did not correlate well with bacterial counts or each other. Bacterial analysis by different methods from the same springs provided different results, although contamination levels were high by all measures (Table 1).
Water quality and flow estimates
Spring water temperatures averaged 26.5°C and ranged from 23.7 to 29.5°C; salinity was almost constant at 0.2 ppt. Turbidity measurements ranged from 0.2 to 25.3 NTU. High turbidities measured in the field may have resulted from disturbance of the sediment during sampling. Turbidities greater than 5 NTU were recorded at four springs, all of which were uncapped. Spring flows ranged from approximately 0.4 to 258.5 L/min with a standard deviation of 53.5 L/min. Conductivity varied from 328 to 594 μs with an average value of 446 μs. DO averaged 2.4 mg/L; total chlorine was typically below detection limits; iron averaged 0.028 mg/L; and phosphate averaged 0.51 mg/L.
Bacterial analysis
Twenty-one spring bacterial analyses were conducted by HAS and 25 springs were analyzed using Coliscan® Easygel® Kits (Table 1). Ten coliform Colony Forming Units (CFU)/100 mL was the criteria used by HAS to determine whether water samples were deemed safe to drink. Based on this standard, 52% of the water samples tested by HAS were unsafe for consumption. However, based on the more stringent World Health Organization (WHO) standard of 1 CFU/100 mL for total coliform, 71% of the samples tested by HAS and 100% of the samples tested using Coliscan® Easygel® Kits were deemed unacceptable for consumption (Table 2; WHO 2008).
Discussion
Despite numerous sampling and sample transport difficulties, bacterial analysis results from the sampled springs indicate that most are unsafe to drink. Spring capping improves water quality, but is not a guarantee of safe water. This suggests that emphasis on point of use (POU) treatment methods, decontamination of capped and uncapped springs, and behavioral interventions to improve sanitation practices are needed. Variability between the results from HAS and the Coliscan® Easygel® Kits leaves some doubt about the reliability and reproducibility of the Coliscan® Kits; however, results by both methods indicate high levels of spring contamination. Coliform and thermophilic bacterial typically die off at an exponential rate after leaving the host (WHO 2008). The inability to refrigerate samples and delays in analysis would be expected to result in bacterial die-off and lower overall bacterial abundance.
Water quality and flow estimates
Measured groundwater temperatures are comparable to those found in other Caribbean aquifers and are roughly comparable to Haiti’s mean annual air temperature (Beddows et al. 2007). Warm groundwater temperatures and high survival rates in the groundwater may be contributing to high bacterial loads measured in spring water. Some studies have found that bacterial survival in stream substrates and soils is greater than in either groundwater or surface water (WHO 2008). However, other studies have found that bacterial die-off in groundwater is relatively slow (Bitton et al. 1983). High temperatures in Haitian springs may allow bacteria to survive longer than in cooler groundwater at higher latitudes. Spring capping provides a somewhat unique environment in which elevated temperatures and substrate presence may enhance bacterial survival. Water temperature for capped springs was, on average, 1°C warmer than uncapped springs, suggesting that spring capping may be warming spring water after it emerges from the ground. Studies in France suggest that survival of aerobic bacteria in karst environments may be affected by the presence of zooplankton and a complex microbial ecosystem which can reduce E. coli bacteria on substrates (Mahler et al. 2000; Personne et al. 2004). However, the subterranean microbial ecosystem in Haiti has likely been negatively impacted, as has the macro-scale ecosystem, by severe erosion and deforestation.
Anecdotal accounts by local residents suggested that some of the springs tasted “salty;” however, salinities were very low for all springs sampled. Salinity and pH values were consistent with limestone aquifers not located near salt water sources. Estimating flow from uncapped springs was problematic due to the diffuse nature of many springs, and the fact that many springs emerged at or near the banks of surface water streams. Some of the greatest spring flows were measured from springs within areas mapped by previous workers as aquifers with poor productivity (Ministère de l’Agriculture 1990). Many of these spring locations are likely controlled by faults, bedding planes and karst development within geologic units with otherwise low productivity. Iron, phosphate, chlorine, and other water quality parameters did not correlate with bacterial abundance.
Bacterial analysis
Measured coliform bacteria abundance in spring water may be related to high groundwater temperatures, averaging 26.5°C (79.8°F). Water temperature in springs, and especially in capped springs, is high enough to allow, and possibly even enhance, the survival of bacteria and other pathogens. Abundant sources of bacteria from inadequate human waste treatment and open conduits and fractures present in the shallow karst aquifers, result in a dangerous combination which both allow the proliferation of pathogens and prevent their removal by natural filtration processes. Spring capping, although effective at isolating springs from surface contamination, may be providing a warm, dark, environment that is conducive to bacterial growth and survival. More detailed sampling is needed to determine whether spring caps and associated substrates are contributing to bacterial growth and abundance. A prudent practice would be to disinfect all spring caps with bleach at least annually, or more frequently, to insure that bacteria are not able to colonize and reproduce in the spring cap structure.
Discrepancies between Coliscan® Easygel® Kit analyses and duplicate samples analyzed by HAS, although troubling, did not alter the overall conclusion that the majority of the springs sampled were not safe to drink by WHO standards. Possible causes for the difference in colony counts are: (1) limitations of Coliscan® Easygel® Kits, particularly in differentiating between pink, blue, and purple colonies; (2) the roughly 10°C variation in temperature during incubation using the Coliscan® Easygel® Kits; (3) variation in storage temperature and duration while samples were in transit from the field to HAS; (4) outside contamination of the samples; (5) sample size; and (6) differences in size criteria for determining whether to include colonies in colony counts.
Micrology Laboratories indicated that blue colonies should be regarded as distinct from teal (non-coliform) colonies, and should be included in E. coli counts (Dr. Jonathan Roth from Micrology Laboratories, personal communication). In practice, this determination was sometimes difficult. If the blue colonies were excluded from the counts, the number of E. coli determined using the Coliscan® Easygel® Kits would be reduced.
The lack of ready access to an incubator while in Haiti may have resulted in the growth of non-coliform species at rates comparable to the coliform species and may have inhibited E. coli growth. It sometimes took hours to collect water samples from multiple springs, and this resulted in variable times before samples could be put on ice. During this time, outside temperatures often heated the samples significantly and may have resulted in die-off of bacteria. Once on ice, it sometimes took up to 3 days to get samples tested by HAS due to staffing and logistical issues. This may have caused an increased die-off of bacteria, and low colony counts, for the samples processed at HAS (Pope et al. 2003).
Coliscan® Easygel® collection bottles and transfer pipits were sterile upon ordering; however, it is possible outside contamination may have occurred resulting in increased coliform growth. Coliscan® Easygel® instructions suggest that either 1- or 5-mL samples can be mixed with the medium depending on expected contamination levels. All samples were analyzed using Coliscan® Easygel® recommendations for drinking water using 5 mL (Micrology Laboratories 2005). Using a 1-mL sample could have facilitated colony counting in water samples with large numbers of colonies. However, a limited number of kits were available, so samples were typically not reanalyzed with a smaller sample size. Colony size varied considerably, making it difficult to determine which colonies to include in colony counts. Micrology Laboratories instructions indicate that small colonies should not be included in colony counts; however, no size criteria were specified.
Several samples with digital photos taken after variable incubation times were reanalyzed in order to determine if the differing results from the HAS bacterial analysis and the Coliscan® Easygel® Kit cultures were related to differing incubation times. Digital images of each Coliscan® Easygel® test at multiple incubation times were analyzed and compared in ArcMap to determine if the counts changed significantly after 48 h. Micrology Laboratories, Coliscan® Easygel® Kit instructions recommend incubation times of 48 or more hours at room temperature, for higher temperatures, no counts should be made after 48 h. Instructions also indicated that any new colonies forming after 48 h are likely not coliform bacteria and may throw off the accuracy of the data (Micrology Laboratories 2005).
A comparison of the same culture after 27–29 and 208–259 h indicated that E. coli counts remained unchanged; however, fecal coliform colonies grew more faint and difficult to count after 48 h. This suggests that E. coli count results are probably not significantly affected by the variability in incubation time. However, total coliform colony counts may have been low due to a decrease in size of these colonies prior to counting (Table 3).
Conclusions and recommendations
Water quality results did not show significant correlation between easily measurable parameters and bacteria presence, and thus cannot be used as a predictive tool of coliform bacteria presence. This leads to the conclusion, which has been reached by other researchers, that there is no reliable substitute for bacterial analyses for determining bacterial presence and abundance (Dussart-Baptista et al. 2003). The more economical Coliscan® Easygel® Kits provided a good measure of bacterial presence; however, they appear to yield problematic quantitative data when compared to more traditional lab-based analysis methods using an incubator.
Based on the WHO drinking water standard and the water quality of springs tested, the majority of the water that the Haitians in the study area are drinking is unsafe due to bacterial contamination. Spring capping has been a common practice to capture spring flow and protect springs from surface contamination in Haiti. However, this study shows both capped and uncapped springs can be unsafe to drink based on the WHO drinking water standard. In order to ensure safe water, additional focus is needed on: (1) improved water handling; (2) sanitation; (3) spring cap disinfection and maintenance; and (4) POU water treatment methods (Duke et al. 2006; UNICEF 2008; Sobsey et al. 2008). In some instances, improving spring cap design, preventing leaks, and annual or more frequent maintenance with bleach treatment of the cap could reduce fecal coliform bacteria contamination. Uncapped springs that are heavily used, but are highly contaminated, should be a priority for capping, monitoring for water quality, and targets for POU treatment methods.
Due to large discrepancies between HAS and Coliscan® Easygel® bacterial results, future tests using the Coliscan® Easygel® Kits should utilize an incubator to maintain a constant temperature. Also, maintaining a more stable and sterile environment for conducting the bacterial tests could reduce possible contamination. Refrigerating water samples before analysis and keeping them no longer than 24 h prior to analysis would improve the accuracy of total coliform determinations (Pope et al. 2003). In Haiti, where groundwater emerges warm, it is unclear whether refrigeration of samples while in transit would yield more accurate bacteria counts. Additional work is needed to verify that sample refrigeration in an environment with warm groundwater is needed.
Future work
Future research will focus on providing more controlled conditions for bacteria cultures using Coliscan® Easygel® Kits and use of more quantitative methods such as field-based bacterial analysis with a PROFILE® 1 Bioluminometer, and quantitative determination of E. coli and coliform bacteria using IDEXX quanti-tray methods. More research on groundwater sources and pathways using conductivity and saline tracer solution is also needed. The Coliscan® Easygel® Kits remain an economic and viable method for developing countries, but they should be considered a presence/absence measure. HAS has been collecting and analyzing water samples from wells and springs in the region for several decades. Much of this data resides in paper ledgers which have never been compiled or analyzed. These data represent a vast untapped resource for understanding the temporal and spatial variability of bacterial contamination in rural Haiti. More detailed sampling, both temporally and spatially, of capped springs and capping substrates will help determine whether capping practices are resulting in enhanced bacterial growth.
References
Beddows PA, Smart PL, Whitaker FF, Smith SL (2007) Decoupled fresh-saline groundwater circulation of a coastal carbonate aquifer: spatial patterns of temperature and specific electrical conductivity. J Hydrol 346:18–32
Bitton G, Farrah SR, Ruskin RH, Butner J, Chou YJ (1983) Survival of pathogenic and indicator organisms in ground water. Groundwater 21:405–409
Duke WF, Nordin RN, Baker D, Mazumder A (2006) The use and performance of biosand filters in the Artibonite Valley of Haiti: a field study of 107 households. Int Electron J Rural Remote Health Res Educ Pract Policy 6(3):19
Dussart-Baptista L, Massei N, Dupont J, Jouenne T (2003) Transfer of bacteria-contaminated particles in a karst aquifer: evolution of contaminated materials from a sinkhole to a spring. J Hydrol 284:285–295
Kacaroglu F (1999) Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut 113:337–356
Knowles RB, Markley B, Buckalew JO, Roebuck LW (1999) Water resources assessment of Haiti. US Army Corps of Engineers, p 93
Library of Congress Federal Research Division (2006) Country profile: Haiti, 2006. http://lcweb2.loc.gov/frd/cs/profiles/Haiti.pdf. Accessed 1 May 2008
Mahler BJ, Personné J, Lods GF, Drogue C (2000) Transport of free and particulate-associated bacteria in karst. J Hydrol 238:179–193
Maurrasse F (1982) Survey of the geology of Haiti, guide to field excursions in Haiti, p 103
Micrology Laboratories (2005) Detection of waterborne coliforms and E. coli with Coliscan Easygel. http://www.micrologylabs.com/Home/Our_Methods/Coliscan_Easygel/Coliscan_Easygel_Instructions.pdf. Accessed 1 May 2008
Ministère de l’Agriculture, des Ressources Naturelles, du Développement Rural Service National des Resources en Eau (1990) Carte Hydrogeologique Republique D’Haiti. 1:250,000
Personne J, Poty F, Mahler BJ, Drogue C (2004) Colonization by aerobic bacteria in karst: laboratory and in situ experiments. Groundwater 42:526–533
Pope M, Bussen M, Feige M, Shadix L, Gonder S, Rodgers C, Chambers Y, Pulz J, Miller K, Connell K, Standridge J (2003) Assessment of the effects of holding time and temperature on Escherichia coli densities in surface water samples. Appl Environ Microbiol 69:6201–6207
Sobsey MD, Stauber CE, Casanova LM, Brown JM, Elliot MA (2008) Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol 42:4261–4267
United Nations Education Scientific and Cultural Organization (UNESCO) (1991) Hydrology and water resources of small islands, a practical guide. Studies and Reports on Hydrology No. 49. UNESCO, p 435
United Nations International Children’s Emergency Fund (UNICEF) (2008) Promotion of household water treatment and safe storage in UNICEF wash programmes. UNICEF, p 10
White WB (1988) Geomorphology and hydrology of karst terrains. Oxford University Press, New York
White W (2002) Karst hydrology: recent developments and open questions. Eng Geol 65:85–105
World Health Organization (WHO) (2008) Guidelines for drinking-water quality. Third edition incorporating first and second addenda, vol 1: Recommendations. p 515
Acknowledgments
This report was supported by the Grand Valley State University Student Summer Scholars Program and FIAI. Special thanks to Tom Braak of FIAI, for his hospitality while in Haiti and for providing access to many of the springs, along with Jean Babtiste Rony, and many other FIAI employees. We also appreciate help received from Hospital Albert Schweizter (HAS) in conducting water sample analyses of the springs. Additional funding was provided by Grand Valley State University, Padnos International Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wampler, P.J., Sisson, A.J. Spring flow, bacterial contamination, and water resources in rural Haiti. Environ Earth Sci 62, 1619–1628 (2011). https://doi.org/10.1007/s12665-010-0645-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0645-9