Abstract
In this work we have studied the geochemistry of stream waters arising from waste dumps at the Peña de Hierro mine (Iberian Pyrite Belt, SW Spain), and we have correlated them with the mineralogical and geochemical characteristics of the wastes to asses the source and factors affecting the release of trace elements. The mineralogical composition and geochemistry of 58 borehole samples of waste dumps were studied in the <2 mm fraction. Twenty-eight water samples collected in winter and summer from streams emerging from the waste dumps were analysed for pH, Eh, conductivity, temperature, sulphates and major and trace elements. The leachates from pyrite-rich volcanic tuffs produced very acidic waters, usually with pHs below 2 and reaching values as low as 0.7. The partial dissolution of gossan, which is mainly composed of Fe oxy-hydroxides and is rich in trace elements, released high concentrations of Fetot (up to 33 g/L), As (up to 72), Mo (up to 11 mg/L). On the other hand Cd, Zn and Pb reached up to 0.85, 142 and 0.42 mg/L, respectively, in the stream arising from roasted pyrite ashes and other pyritic wastes. Several elements such as Al, Fe, As, Co, Cu and Mo were strongly correlated with the pH, but Cd and Zn were not correlated under such acidic conditions. The precipitation of jarosite seems to be an important factor in the retention of Pb. The mobility sequence of trace elements shows that Co, Zn and Cd were among the most mobile elements; Cu, As and Mo had intermediate mobility, and Pb was the most immobile. This work shows that uncontrolled waste dumping increases the pollution potential, and a selective management could reduce the release of trace elements into stream waters and mitigate the contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and aims
Acid mine drainage (AMD) is the major pollution problem associated with coal and metallic sulphide mining worldwide. The term “AMD” refers to acidic waters originating from the oxidation of pyrite and other sulphides. In the United States, AMD affects more than 19,000 km of rivers and streams (Kleinmann 1989). The main problems associated with AMD are its acidity and high concentration of trace elements (As, Cd, Cu, Co, Mo, Ni, Pb, Se, Zn, etc.) carried in solution.
The sources of AMD include tailings, mine adits, pit lakes or any material containing sulphides that is prone to oxidation. One of the most important sources of AMD is the waste rock piles, which usually are very heterogeneous in grain size, mineralogy and geochemistry. This makes very difficult to study the generation of AMD from them, since the water geochemistry is affected by all these waste characteristics (Pantelis and Ritchie 1991; Ritchie 1994; Nordstrom and Alpers 1999a; Lefebvre et al. 2001).
Extreme acid waters, with a pH below 1, have occurred in several mines such as Genna Luas (Sardinia, Italy), Iron Duke (Zimbabwe) and San Telmo (Spain) (Frau 2000; Williams and Smith 2000; Sánchez España et al. 2008, respectively). Trace element concentrations were also high in all these waters. For instance, up to 60 mg/L of Cd, 73 mg/L of As and 10,700 mg/L of Zn in Genna Luas, Italy (Frau 2000); 72 mg/L of As in Iron Duke, Zimbawe (Williams and Smith 2000); and 13.76 mg/L of Cd and 1.1 g/L of Zn in San Telmo, Spain (Sánchez España et al. 2008). But all these values were far from those found in Iron Mountain, California (Nordstrom and Alpers 1999b; Nordstrom et al. 2000), with pH values as low as −3.6, As as high as 850 mg/L, Cd up to 370 mg/L, and Zn up to 49 g/L.
In Spain, the contamination associated with AMD has been documented at several sites such as Galicia or Asturias (e.g. Monterroso and Macías 1998a; Loredo et al. 2005), but it largely occurs in the Iberian Pyrite Belt (IPB), which is one of the most important metallogenic provinces in the world. Most of the mines in this area have been abandoned and are discharging huge quantities of acidic waters and heavy metals into the Tinto and Odiel Rivers (González et al. 2004; Sánchez España et al. 2005; Cánovas et al. 2007). As a result, these rivers are among the most polluted in the world and have gained the attention of many researchers and studies (Fernández-Caliani et al. 1997; van Geen et al. 1997; Elbaz-Poulichet and Dupuy 1999; Hudson-Edwards et al. 1999; Braungardt et al. 2003; Galán et al. 2003). However, there is not enough information about the contribution that the different abandoned mine spoils have on those rivers. This is reasonable if we consider that the headwaters of both rivers receive leachates from huge quantities of mine spoils of the Riotinto mining district. The volume of these wastes has not been assessed, but it is known that at least 6.6 million tons of slags are from the Roman and pre-Roma period (Rothenberg et al. 1990). The intense exploitation of the Riontinto Mines, especially during the nineteenth century, has caused about 1,800 ha of land to be covered with dumps, tailings and open pits, making it difficult to characterize and remediate.
Here, we have investigated the geochemistry of stream waters in the Peña de Hierro abandoned mine site, located in the catchment area of the Tinto River and being the first source of pollution to this river (Fig. 1). The deposit consists of a copper-bearing pyrite ore with variable concentrations of copper, usually comprised between 1 and 4 wt%, and lower concentrations of Zn and Pb (Pinedo Vara 1963; IGME 1982; Tornos 2008). The mine was working between the middle of the nineteenth century and 1966, and it produced about 3 million tons of waste rock and other residues that were dumped around the pit, covering 25 hectares. The characterization of the wastes and the mapping of the dumps have been carried out by Romero et al. (2006a). There are several streams that arise from these waste dumps, and thus, the geochemistry of the waters can be directly linked to the different waste rocks. The aim of this work was to correlate the trace element concentration in stream waters with the different mine-waste dumps, in order to determine the source and factors affecting the genesis of AMD. To achieve these objectives, we studied the geochemistry of the stream waters and the results were compared with the chemical and mineralogical characteristics of the waste dumps.
Materials and methods
Waste dumps
Waste rock samples were collected from boreholes drilled into the dumps by the company RIOTINTO S.A.L., at depths between 1.5 and 14.0 m. A representative sample was collected from each borehole, up to a total of 58 samples. Since the dumps consist of different wastes, the mineralogy and chemistry were analysed in the <2 mm fraction. The mineralogical characterization was carried out by X-ray diffraction (XRD) using a Bruker D8 Advance equipment with slit fixed at 12 mm and monochromatic Cu Kα radiation. Random powders were scanned at 40 kV and 30 mA from 3° to 70° 2θ at a speed of 0.3° 2θ/min.
Chemical analyses of major, minor and trace elements were made in the <2 mm fraction of selected samples. Nineteen elements were analysed at Activation Laboratories Ltd (1428 Sandhill Drive; Ancaster, Ontario, Canada) from 3 g of sample ground in agate mortar and sieved at 50 μ. Analysis methods included Instrumental Neutron Activation Analysis (INAA), Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and Infra-Red Spectroscopy (IR). The detection limits were 1 mg/kg for As, Co, Cu, Hg, Mn, Mo, Ni and Zn, 0.3 mg/kg for Cd, 2 mg/kg for Cr, 3 mg/kg for Pb, 50 mg/kg for Ba and 0.01% for major elements and S. The results were contrasted against certified materials such as DMMAS-14, G-2, SDC-1, DNC-1, SCO-1, GXR-1, GXR-2, GXR-4 and GXR-6. Errors were always below 10%, except for Cd, Al and Mg, which could be higher.
Stream water
A total of 28 water samples were collected in PVC bottles from the streams arising from the waste dumps during two field trips (Fig. 2): the first one was carried out in winter (March 2002, 12 samples), 2 weeks after a period of continuous rain. The second was carried out in summer (July 2002, 16 samples), after more than 2 months of high temperatures and no precipitation. Water samples were collected from the headwaters of each stream next to the waste dumps, and several metres downstream from the confluence of different watercourses. After collection and filtration to 0.45 μ, samples were acidified below pH 2 when needed and kept at 4°C until chemically analysed.
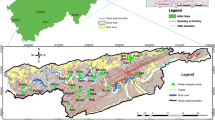
Distribution of mine-waste dumps in Peña de Hierro and location of streams and sampling stations. Blue circles denote sampling in winter, and red triangles are for summer. Modified after Romero et al. 2006a
The pH was measured in the field using a CRISON 507 portable pH meter equipped with a 52-00 electrode and automatic temperature compensation. Conductivity was measured using a CRISON 524 portable conductimeter equipped with a 52-90 cell and automatic temperature compensation. Temperature and Eh were measured (in July 2002) using a CRISON 52-61 platinum electrode coupled with the above-mentioned pH meter. All instruments were calibrated in the field before measurements were taken.
In the laboratory, the sulphate concentration was determined by turbidimetry on a Zuzi 4200/50 UV–vis spectrophotometer. Samples collected from the headwaters and some from the confluence of watercourses were selected to analyse the concentration of major, minor and trace elements (21 samples). All analyses were carried out at Activation Laboratories (http://www.actlabs.com) by Inductively Coupled Plasma Mass Spectroscopy (ICP-MS). The detection limits for the analysed elements were 2 μg/L for Al, 50 μg/L for Ca and Si, 5 μg/L for Fe and Na, 10 μg/L for K, 1 μg/L for Mg, 0.03 μg/L for As, 0.01 μg/L for Cd, 0.005 μg/L for Co, 0.5 μg/L for Cr and Zn, 0.2 μg/L for Cu and 0.1 μg/L for Mn, Mo and Pb. The accuracy and precision of the analytical method was verified against the certified reference waters SLRS-4 for major elements and NIST 1640 for trace elements. Errors were always below 5% except for As and Pb (7.5%) and Ni (10%).
Results and discussion
Waste dumps
Waste dumps consist of a mixture of different materials such as volcanic rocks, gossan, shales, and mining residues such as roasted pyrite ashes or floated pyrite. A detailed description of the wastes is presented by Romero et al. (2006a). According to which of these materials predominates in the dumps, a mapping of the waste has been carried out, with the following being the most important units (Fig. 2, Romero et al. 2006a):
-
1.
Tuff dumps are mainly made up of acid volcanic tuffs, but usually gossan and shales are mixed in the dumps. The mineralogy is mainly composed of quartz, muscovite, pyrite and jarosite (Table 1). Pyrite is common in blocks of volcanic rocks, and jarosite precipitates in the <2 mm fraction after the oxidation of pyrite. When gossan fragments are present, hematite and amorphous Fe oxy-hydroxides occur in the fine fraction of the dumps. The concentration of trace elements in the <2 mm fraction of this unit is low when compared with other residues. For example, As is below 263 mg/kg and Pb is below 628 mg/kg (Table 2). By contrast, the concentration of Co (64 mg/kg) was higher than that found in other materials.
Table 1 Mineralogy of fraction <2 mm in different types of mine-waste dumps (in %) Table 2 Chemical analysis of <2 mm fraction of borehole samples (Romero et al. 2006a) -
2.
Gossan dumps may also contain remains of tuffs and shales. They have a high content of hematite, goethite and X-ray amorphous iron oxy-hydroxides. The <2 mm fraction of these dumps displays high concentration in several trace elements such as As (538–1,710 mg/kg), Mo (143–331 mg/kg), Pb (318–989 mg/kg) or Cu (90–895 mg/kg). These concentrations can be higher in rock fragments of gossan. For example, Romero et al. (2006a) determined up to 2,330 mg/kg of As, 4,560 mg/kg of Pb, and 749 mg/kg of Mo.
-
3.
Tuff–gossan dumps consist of a mixture of tuff, gossan and shales. Their mineralogical and geochemical characteristics are intermediate between the two units mentioned above.
-
4.
Roasted pyrite ash dumps are characterized by a high content of hematite and X-ray amorphous iron oxides. They are also rich in trace elements such as Pb (2,000–3,460 mg/kg), As (303–971 mg/kg), Mo (81–351 mg/kg), Cu (419–527 mg/kg), Cd (2–3 mg/kg) and Zn (283–536 mg/kg).
Other dumps such as floated pyrite and mix dumps are rich in fine pyrite and may have high concentration of trace elements, but they are less important because their volume is relatively low (Romero et al. 2006a, Fig. 2).
Stream waters
Physical–chemical features
The pH was very acidic in most of the streams, ranging between 0.7 (site C, July) and 3.5 (site Z1, March) (Table 3). Streams Y, B, C and D arising from the tuff dumps displayed the lowest pH values (always <2). Site G, located outside the mining area, showed neutral natural water. Stream Z was not affected by leachates from the waste dumps, but pH ranged between 3.1 and 3.5. The pH values recorded in summer were lower than those of winter in most of the streams.
Conductivity ranged between 1,000 and 47,000 μS/cm in the acidic streams, being of 130 μS/cm in the neutral stream G. The most acidic streams (Y, B, C, D) recorded the highest conductivity values, which were over or about 20,000 μS/cm. The values recorded in the summer field trip were higher than those of winter in most of the streams. The sulphate concentration, which correlates with the conductivity, ranged between 363 and 33,202 mg/L in the acidic samples, and it was about 6–7 mg/L in the neutral stream.
The Eh in the acidic streams ranged between 333 (site Z0) and 563 mV (site Y), and it was of 202 mV for the neutral water. The Eh increased as water moved downstream.
According to the temperature values, stream Z displayed values of about 24°C, streams Y, A and B were about 28–30°C and the other streams ranged between 20 and 22°C. The temperature values recorded at the confluence of different streams, as well as pH, conductivity and sulphate concentration, showed intermediate values with upstream waters.
Stream water geochemistry
The chemical analyses of major and trace elements are shown in Table 4. Among the major elements, Fe displayed the highest concentration, up to 30 g/L at stream C, and many times it was over 10 g/L, especially in streams arising from the tuff dumps. Aluminium and Mg reached 3,730 and 1,680 mg/L, respectively. Calcium ranged between 21 and 470 mg/L. Sodium and Si were lower than 100 mg/L. Potassium displayed the lowest concentration, being in most of the samples below 3 mg/L.
Among the minor and trace elements, Mn, Cu and Zn displayed values over 100 mg/L in several samples (Table 4). Arsenic and Mo reached up to 72 and 11.3 mg/L, respectively (site C, July). Cadmium was usually over 0.1 mg/L and reached up to 0.85 mg/L in site F (July). Cobalt usually displayed a few mg/L, and it was over 10 mg/L in the most acidic streams. Among the most immobile elements was Pb, which was always below 0.5 mg/L.
Figure 3 shows the relative concentration of elements in the headwaters of every stream, corresponding to summer sampling. The element concentrations of every sample were normalized with the maximum value found for each element. Thus, a value of 1 for a particular element points out the sample with the maximum concentration for this element. So, Fig. 3 shows that stream C had the lowest pH and the maximum concentration in sulphate, Fe, As, Co, Cu and Mo. High concentrations of these elements were also found in other streams arising from the tuff dumps (B, D, Y), and they correlated negatively (r < −0.7) with pH (Table 5). The maximum concentration of K was recorded in stream C, but the concentrations in the other streams were very low.
Other elements such as Ca and Mn showed low values in the most acidic streams, and the highest values occurred in streams Z, A and F. These streams flow over the bedrock constituted of shales that are rich in Ca and Mn (Fernández-Caliani and Galán 1991), suggesting that these elements can be leached from them into the stream water.
Cadmium and Zn displayed high values in stream F where pH was also high, and in streams B, C and D. The concentrations of Pb were very low in most of the streams, and the highest values were found in streams C and F.
These results show that the streams in Peña de Hierro are among the most polluted in the world (Table 6). For example, the minimum pH and maximum concentration of As (72 mg/L) were similar to those found in other mines such as Genna Luas, Italy, or Iron Duke, Zimbabwe (Frau 2000; Williams and Smith 2000). The concentration of Mo recorded in Peña de Hierro (up to 11.3 mg/L) was extremely high. This value is even higher than the maximum found in Iron Mountain (4.2 mg/L, Nordstrom and Alpers 1999b). A similar value of Mo (10.41 mg/L) has been measured in San Telmo, another mine of the IPB (Sánchez España et al. 2008).
Source of AMD and trace elements
One possible source of acidic waters in Peña de Hierro is the natural acid rock drainage produced by the bedrock, as other authors have suggested (González-Toril et al. 2003; Fernández-Remolar et al. 2004). In this sense, stream Z, which is located in the eastern part of the mine, does not seem to be affected by mine waters (Fig. 2), and its geochemistry is highly affected by elements leached from the bedrock, such as Ca, Na and Mn. Therefore, these waters can represent the natural acid rock drainage in the headwaters of the Tinto River. In any case, and in comparison with the geochemistry of streams arising from these waste dumps, the low content of trace elements in stream Z shows that the mismanagement of mine wastes and their subsequent abandonment have a very negative impact on the environment, since it causes the load of acid and trace elements in stream water to rise by several orders of magnitude.
The high acidity of the streams arising from the tuff dumps (Y, B, C, D) shows that these wastes, which contain pyrite and have no neutralizing minerals, are a very important source of AMD in Peña de Hierro. Although there are other pyrite-containing wastes that may generate AMD, the coarse grain size of the tuff dumps (Romero et al. 2006a) allows the air to enter easily into the dumps, causing the oxidation of pyrite through the following reaction 1 (Ritchie 1994; Lefebvre et al. 2001; Nordstrom and Alpers 1999a):
The tuff dumps are also an important source of trace elements to streams Y, B, C and D (Fig. 3). Some elements such as Co seem to come from pyrite oxidation, because this element is mainly present in the tuff dumps (Table 2), probably associated to pyrite. By contrast, other elements such as Fe, As and Mo displayed high concentration in gossan wastes, and their occurrence in the water may be influenced by the dissolution of this material. In fact, the Fe/SO 24 ratio is up to more than three times greater than that expected from pyrite dissolution alone (Fig. 4), which suggests that the dissolution of gossan is being accomplished. This process can occur easily if we consider that the gossan is mixed in the tuff dumps and acidic waters with pH < 2 can partially dissolve the Fe oxy-hydroxides and release Fe3+ through the following reaction 2 (Nordstrom and Alpers 1999a). Although we have not measured the Fe2+/Fe3+ ratio, this hypothesis is consistent with the wide occurrence of Fe3+ rich efflorescent sulphates (coquimbite, rhomboclase, magnesiocopiapite) found in the banks of the streams (Romero et al. 2006b). The releasing of Fe3+ may also contribute to lower the pH to values down to 0.7, because it can react with pyrite and oxidize it rapidly through the following reaction 3 (Singer and Stumm 1970; Nordstrom and Alpers 1999a).
The dissolution of gossan does not only release iron but also trace elements, particularly As and Mo, which occur widely in these wastes. This explains their very high concentration in the stream water (Table 4). The strong correlation Fe–As–Mo in stream water samples (r ≥ 0.86, Table 5) and wastes with variable concentrations of gossan (r ≥ 0.84, Romero et al. 2006a) support this idea. These results have an important environmental impact because the gossan is stable in an oxidant environment, and a low mobility of trace elements is expected under these conditions. By contrast, when gossan wastes are mixed with high acid potential materials, the acidic leachates can dissolve them and release huge contents of trace elements into the water.
Other elements as K, Na and Pb were not correlated with pH, but they displayed high concentrations in stream C (Fig. 3b, d). The low values displayed by K in most of the water samples probably indicate that it is largely retained by the precipitation of secondary jarosite. When the physical–chemical conditions are unfavourable for the precipitation of jarosite (e.g. pH < 1, Dutrizac and Jambor 2000) K is leached into the water. Similarly, other authors have also measured high concentrations of K in waters with pH < 1 in other areas (e.g. Williams and Smith 2000; Frau 2000). In the same way, the release of Na and Pb into stream C suggests that they can be also retained by jarosite when this phase precipitates. Indeed, Na and Pb interexchange with K in the jarosite structure as other authors have previously reported (Dutrizac and Jambor 2000; Acero et al. 2006; Graupner et al. 2007).
Besides the tuff dumps and gossan wastes, the mixed dumps, which are rich in pyrite and the roasted pyrite ashes can be important sources of Cd, Zn and Pb to the water. For example, stream F, which receives leachates from both materials, showed high concentrations of Cd, Zn and Pb (Table 2) despite the fact that the pH was about 2–3, which is higher than the values recorded in other streams. With respect to Cd and Zn, there is a strong correlation between them (r = 0.89), but not with the pH (Table 5). These elements can be easily released into the water due to their high mobility and low pH dependence in very acidic environments (Nordstrom and Alpers 1999a; Plumlee et al. 1999). By contrast, Pb is a very immobile element, and its high concentration in stream F seems to be related to the low Eh values (375 mV), which are unfavourable for the precipitation of secondary phases such as jarosite (Bigham et al. 1996) or any other oxidized phase.
Element mobility
According to the above-discussed considerations, it is plain to see that the release of major and trace elements into the water depends on several factors such as their concentration in the wastes, the pH–Eh environment or the dissolution/precipitation of mineral phases. So, depending on these factors, the mobility of an element may change in different environments.
Considering the waste dumps as the source of elements and the stream water as the environment into which they are released, it is possible to asses their mobility by determining the ratio between their concentration in water versus wastes. So we have calculated the average concentration of major and trace elements in water and divided it by their average concentration in wastes (Table 7). The mobility of the major elements shows the following sequence:
The minor and trace elements show the following mobility sequence:
Thus, Na and K are among the most immobile elements, because after their release from primary minerals, they can be largely retained by the precipitation of jarosite. With regard to trace elements, Cd and Zn are highly mobile, while As and Mo are only moderately mobile and Pb is relatively immobile. Similar results were obtained in other AMD-affected areas by McGregor et al. (1998) and Monterroso and Macías (1998b).
Conclusions
Mining operations in Peña de Hierro have produced extremely acidic waters containing high concentrations of trace elements such as As, Cd, Cu, Mo, Pb, and Zn. Dumps composed of volcanic tuffs are the main source of AMD, and the partial dissolution of gossan mixed in these dumps is an important source of As and Mo. Roasted pyrite ashes and pyrite wastes are sources of Cd, Pb and Zn, and the bedrock leaches Ca, Na and Mn. Several elements such as Fe, As, Co and Mo are greatly affected by the pH, but others as Pb and Cd–Zn are conditioned by their low or high mobility, respectively.
This study has shown that the geochemistry of stream waters strongly depends on the characteristics of the materials drained. On one hand, the dumping of wastes increased the trace element concentration in streams compared with natural waters, both neutral and acid. On the other hand, the mismanagement of waste rocks when mixing pyrite-rich spoils with others rich in trace elements (e.g. gossan) involved their release through dissolution of iron oxy-hydroxides, increasing the contamination of stream waters.
A selective management of these different mine spoils could be an easy way to control and mitigate the pollution, as it would avoid the leaching of potentially toxic wastes (e.g. gossan) practically without any corrective action, and it would reduce the volume of acid generating wastes. These results can be also applied to the management of waste dumps in new or reopened mines of the IPB.
Characterization studies of waste rocks are necessary for planning accurate remediation actions. Classification/selection of different wastes according to their acid/basic generating potential, concentration in potentially toxic elements and factors affecting their mobility or their harmlessness are necessary to control them. According to the waste potential hazard, specific management criteria can be established, such as insulation, covering, mixing, supervision and temporary control, abandonment, revegetating, etc.
References
Acero P, Ayora C, Torrentó C, Nieto JM (2006) The behaviour of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim Cosmochim Acta 70:4130–4139
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M (1996) Schwertmannite and the chemical modelling of iron in acid sulfate waters. Geochim Cosmochim Acta 60:2111–2121
Braungardt CB, Achterberg EP, Elbaz-Poulichet F, Morley NH (2003) Metal geochemistry in a mine-polluted estuarine system in Spain. Appl Geochem 18:1757–1771
Cánovas CR, Olías M, Nieto JM, Sarmiento AM, Cerón JC (2007) Hidrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain). Factors controlling metal contents. Sci Total Environ 373:363–382
Davis A, Ashenberg D (1989) The aqueous geochemistry of the Berkeley Pit, Butte, Montanta. USA Appl Geochem 4:23–26
Dutrizac JE, Jambor JL (2000) Jarosites and their application in hidrometalurgies. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals: crystallography, geochemistry and environmental significance. Rev mineral 40. The Mineralogical Society of America, Washington, DC, pp 405–452
Elbaz-Poulichet F, Dupuy C (1999) Behaviour of rare earth elements at the freshwater-seawater interface of two acid mine rivers: the Tinto and Odiel (Andalucia, Spain). Appl Geochem 14:1063–1072
Fernández-Caliani JC, Galán E (1991) Las pizarras de la Faja Pirítica Ibérica (Zona Sur-Portuguesa): geología, mineralogía y aplicaciones industriales. Estudios Geológicos 47:295–303
Fernández-Caliani JC, Ruiz Muñoz F, Galán E (1997) Clay mineral and heavy metal distributions in the lower estuary of Huelva and adjacent Atlantic shelf, SW Spain. Sci Total Environ 198:181–200
Fernández-Remolar D, Gómez-Elvira J, Gómez F, Sebastian E, Martín J, Manfredi JA, Torres J, González Kesler C, Amils R (2004) The Tinto river, an extreme acidic environment under control of iron, as an analog of the Terra Meridiani hematite site of Mars. Planet Space Sci 52:239–248
Frau F (2000) The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardania, Italy: environmental implications. Mineral Mag 64:995–1006
Galán E, Gómez-Ariza JL, González I, Fernández-Caliani JC, Morales E, Giráldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18:409–421
González I, Romero A, Galán E (2004) Environmental aspects of waste dumps at the Peña del Hierro Mine (Iberian Pyrite Belt SW Spain). In: Pecchio M, Andrade FRD, D’agostino LZ, Kahn H, Sant’agostino LM, Tassinari MMML (eds) Applied mineralogy: developments in science and technology. ICAM, Sao Paulo, pp 419–422
González-Toril E, Llobe-Brossa E, Casamayor EO, Amann R, Amils R (2003) Microbial ecology of an extreme acidic environment, the Tinto River. Appl Environ Microbiol 69:4853–4865
Graupner T, Kassahun A, Rammlmair D, Meima JA, Kock D, Furche M, Fiege A, Schippers A, Melcher F (2007) Formation of sequences of cemented layers and hardpans within sulfide-bearing mine tailings (mine district Freiberg, Germany). Appl Geochem 22:2486–2508
Hudson-Edwards KA, Schell C, Macklin MG (1999) Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, Southwest Spain. Appl Geochem 14:1015–1030
IGME (1982) Mapa geológico de España, 1:50.000, Nerva, 938-10-38. Servicio de Publicaciones del Ministerio de Energía, Madrid
Kleinmann RPL (1989) Acid mine drainage in the United States. Controlling the impact on streams and rivers. 4th World Congress on the Conservation of the Built and Natural Environments, Toronto, pp 1–10
Lefebvre R, Hockley D, Smolensky J, Gélinas P (2001) Multiphase transfer processes in waste rock piles producing acid mine drainage. 1: conceptual model and system characterization. J Contam Hydrol 52:137–164
Loredo J, Álvarez R, Ordóñez A (2005) Release of toxic metals and metalloids from Los Rueldos mercury mine (Asturias, Spain). Sci Total Environ 340:247–260
McGregor RG, Blowes DW, Jambor JL, Robertson WD (1998) The solid-phase controls on the mobility of heavy metals at the Copper Cliff tailings area, Sudbury, Ontario, Canada. J Contam Hydrol 33:247–271
Monterroso C, Macías F (1998a) Drainage waters affected by pyrite oxidation in a coal mine in Galicia (NW Spain): composition and mineral stability. Sci Total Environ 216:121–132
Monterroso C, Macías F (1998b) Procesos de inmovilización de elementos traza en aguas ácidas de mina. Edafología 5:59–70
Nordstrom DK, Alpers CN (1999a) Geochemistry of acid mine wastes. In: Plumlee GS, Logsdon MJ (eds) The environmental geochemistry of ore deposits. Part A: processes, techniques, and health issues. Reviews in Economic Geology, vol 6A. Society of Economic Geologists, Littleton, pp 133–160
Nordstrom DK, Alpers CN (1999b) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci USA 96:3455–3462
Nordstrom DK, Alpers CN, Ptacek CJ, Blowes DW (2000) Negative pH and extremely acidic mine waters from iron mountain, California. Environ Sci Technol 34:254–258
Olias M, Nieto JM, Sarmiento AM, Cerón JC, Cánovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain). Sci Total Environ 333:267–281
Pantelis G, Ritchie AIM (1991) Macroscopic transport mechanisms as rate-limiting factor in dump leaching of pyretic ores. Appl Math Model 15:136–143
Pinedo Vara I (1963) Piritas de Huelva. Su Historia, minería y aprovechamiento. Editorial Summa, Madrid 1003 pp
Plumlee GS, Smith KS, Montour MR, Ficklin WH, Mosier EL (1999) Geologic controls on the composition of natural waters and mine waters draining diverse mineral-deposit types. In: Plumlee GS, Logsdon MJ (eds) The environmental geochemistry of ore deposits. Part B: case studies and research topics. Reviews in Economic Geology, vol 6B. Society of Economic Geologists, Littleton, pp 373–432
Ritchie AIM (1994) Sulfide oxidation mechanisms: controls and rates of oxygen transport. In: Jambor JL, Blowes DW (eds) The environmental geochemistry of sulfide mine-wastes. Short course handbook, vol 22. Mineralogical Association of Canada, Waterloo, pp 201–270
Romero A, González I, Galán E (2006a) Estimation of potential pollution of waste mining dumps at Peña del Hierro (Pyrite Belt, SW Spain) as a base for future mitigation actions. Appl Geochem 21:1093–1108
Romero A, González I, Galán E (2006b) The role of efflorescent sulfates in the storage of trace elements in stream waters polluted by acid mine-drainage: the case of Peña del Hierro, Southwestern Spain. Can Mineral 44:1431–1446
Rothenberg B, García-Palomero F, Bachman HG, Goethe JW (1990) La Minería y la Metalurgia en las Antiguas Civilizaciones Mediterráneas y Europeas. Tomo I, Madrid
Sánchez España J, López Pamo E, Santofimia E, Aduvire O, Reyes J, Barettino D (2005) Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): geochemistry, mineralogy and environmental implications. Appl Geochem 20:1320–1356
Sánchez España J, González-Toril E, López Pamo E, Amils R, Diez Ercilla M, Santofimia Pastor E, San Martín-Úriz P (2008) Biogeochemistry of a Hyperacidic and Ultraconcentrated Pyrite Leachate in San Telmo mine (Iberian Pyrite Belt, Spain). Water Air Soil Pollut 194:243–257
Singer P, Stumm W (1970) Acidic mine drainage: the rate-determining step. Science 167:1121–1123
Smuda J, Dold B, Friese K, Morgenstern P, Glaesser W (2007) Mineralogical and geochemical study of element mobility at the sulfide-rich Excelsior waste rock dump from the polymetallic Zn–Pb–(Ag–Bi–Cu) deposit, Cerro de Pasco, Peru. J Geochem Explor 92:97–110
Tornos F (2008) La geología y metalogenia de la Faja Pirítica Ibérica. Macla 10:13–23
van Geen A, Adkins JF, Boyle EA, Nelson CH, Palanques A (1997) A 120-yr record of widespread contamination from mining of the Iberian Pyrite Belt. Geology 25:291–294
Williams TM, Smith B (2000) Hydrochemical characterization of acute acid mine drainage at Iron Duke mine, Mazowe, Zimbabwe. Environ Geol 39:272–278
Acknowledgments
This work was partially supported by the Junta de Andalucía through the Research Group RNM 135.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, A., González, I. & Galán, E. Stream water geochemistry from mine wastes in Peña de Hierro, Riotinto area, SW Spain: a case of extreme acid mine drainage. Environ Earth Sci 62, 645–656 (2011). https://doi.org/10.1007/s12665-010-0554-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0554-y