Abstract
Saline land rehabilitation plays a crucial role in expanding arable land resources and ensuring food security. To achieve green and sustainable improvement of saline and alkaline land, the present study utilizes vinegar residue waste composted with inter-root bacteria PGPR (phosphorus solubilizing, potassium solubilizing, and nitrogen fixing bacteria) to produce organic fertilizers that are beneficial to plants, thus rehabilitating saline and alkaline land. The total nutrient content (Σ (N + P2O5 + K2O) of the heap at the end of composting increases by 49.85%. PKN-VR (vinegar residue compost with added phosphorus solubilizing, potassium solubilizing, and nitrogen-fixing bacteria) treatment group significantly increases the stem length (55.99%), root length (54.29%), fresh weight (71.4%), and dry weight (57.9%) of wheat seedlings in the saline soil. In addition, the compost products increase the content of chlorophyll (62.2%), proline (94%), and soluble sugar (62.7%), and decrease the content of MAD (malondialdehyde) by 24.05%, thus enhancing the resilience of wheat seedlings. The contents of total nitrogen, total potassium, and total phosphorus, as well as quick-acting potassium, quick-acting phosphorus, and alkaline dissolved nitrogen in the soils of the treatment groups are significantly increased by the addition of microorganisms. The soil fertility enhancement also increases the enzyme activities of the soil. The results show that PKN-VR has considerable potential in saline soil remediation, realizes the resource utilization of vinegar residue waste, and provides a new management method for the green development of agriculture.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of novelty
-
Vinegar residue is used to produce biofertilizer.
-
The biofertilizer greatly improved the land quality of saline and alkaline land.
-
The biofertilizer improved wheat growth.
Introduction
The rehabilitation of saline and alkaline land is crucial in China as it plays a vital role in expanding the area of arable land, improving crop productivity, and ensuring food security [1]. The total area of saline and alkaline land in China is about 99.13 million hectares, ranking the third in the world and the first in Asia, accounting for about 13% of the national land area [2]. Moreover, higher salinity levels can cause osmotic stress and physiological drought in plants, restricting their growth and development [3]. The rational development and utilization of saline land is crucial for the sustainable development of modern agriculture in China. Researchers adopted several physical, chemical, and hydrological measures for the improvement of saline soils [4,5,6]. However, these methods have defects such as high cost, large workload and easy to produce secondary pollution. To successfully implement the principles of green, low-carbon, and sustainable development, it is crucial to explore cost-effective, efficient, and environmentally sustainable methods for enhancing the capacity of saline land. Biofertilizer amendment is widely recognized as a highly effective strategy for enhancing saline soils, owing to its numerous advantages including cost-effectiveness, high efficiency, and environmental sustainability [7,8,9]. Numerous studies demonstrated that the utilization of biowastes, their derivatives, and soil microorganisms can effectively mitigate soil salinity and promote sustainable agricultural practices [10,11,12]. This approach was aimed at optimizing agricultural productivity and enhancing soil health.
Vinegar residue (VR) is a noteworthy by-product of the vinegar industry in China, boasting an annual production that exceeds 3 million tons [13]. It is derived from rice bran, bran, and sorghum husk, which are abundant sources of crude protein, cellulose, semi-fibre, and other valuable nutrients [14]. Although researchers have directly applied vinegar residue waste to soil improvement in saline soils and achieved certain resource utilization and improvement effects, the disadvantages of these treatments are low degradation efficiency, limited treatment capacity, insufficient economic value, and over-application can cause acidification, pathogen contamination, mold, etc., which can result in serious secondary contamination of the soil [15]. Composting is a cost-effective, efficient, and secure approach to agricultural waste management [16]. It enables the transformation of high moisture, low pH vinegar residue waste into valuable organic fertilizer. This process not only facilitates prompt disposal but also guarantees the productive utilization of vinegar residue [17, 18]. However, composting usually takes a long time and produces low quality compost. Therefore, by adding some additives (microorganisms and organic or inorganic substances) it is possible to reduce the composting time and improve the quality of the product [19]. Numerous studies have presented compelling evidence regarding the significant influence of vinegar residue introduction into soil-on-soil properties and microbial communities [15, 20]. The application of vinegar residue waste has several benefits, because it helps regulate soil pH, improve soil structure and nutrients, and also has a significant impact on the composition of the microbial community [21, 22]. In addition, soil microorganisms, as key drivers of plant productivity and ecosystem micro-processes, are effective in maintaining soil quality and plant health [23]. Certain salinity-tolerant inter-root bacteria (PGPR) can adapt to different soil salt levels [24]. These bacteria dissolve phosphorus, potassium, fix nitrogen, and assist plants in dealing with high salt levels. They improve nutrient cycling, making phosphate and potassium more accessible to plants. Additionally, these bacteria release substances that enhance plant growth, resulting in strong crops and sustainable agriculture [25]. However, the effects of compost products resulting from the application of inter-root bacteria (PGPR) in combination with vinasse waste on saline soil properties and crop growth have not been adequately studied. In this study, two experiments were designed to elucidate the changes in the properties of compost products and to evaluate their effects on saline soils and crop quality: (1) by a composting experiment carried out to assess the changes in compost properties and to evaluate the quality of the compost; and 2) a wheat seedling potting experiment conducted to study the effect of the compost products on the wheat seedlings growth and on the soil physicochemical properties of saline soils.
Materials and Methods
Raw Materials and Composting Design
The vinegar residue in this study was obtained from Guo Junqi Sauce and Vinegar Factory in Nanxiaozhang Village, Chang'an District, Xi'an City, Shaanxi Province, and the basic physicochemical properties of the vinegar residue were as follows: moisture of 65.3%, organic matter content of 658 g/kg, total nitrogen of 14.2 g/kg, pH 4.7, and C/N 26.9. Three bacteria were isolated from the independent isolation and screening of the saline alkaline land, with the the strains of PSB-1, K-2, N-5 showed significant activity in solubilizing phosphorus and potassium and fixing nitrogen, respectively. The PSB-1, K-2, N-5 were provisionally identified as Priestia Bacillus, Paenibacillus mucilaginosus, Paenibacillus sp., respectively. Before inoculation, each strain was inoculated into the Luria–Bertani liquid medium, and incubated for 2 days on a shaker at 28 ± 2 °C until the OD600 value reached 1.0 [26].
The study on natural composting was conducted outdoors at Northwestern University. The composting reactor was a tightly manufactured portable cylindrical composting bag with a diameter of 35 cm and a height of 60 cm. The composting experiments were carried out as follows: 15 kg of fresh vinegar residue was mixed with the test bacterial strains at a ratio of 100:1 (m:v). A total of six different sets of treatments were set up, and the total amount of bacterial agent added to each treatment was 150 mL, including the composting treatments without inoculation of strains (CK) and inoculated with Bacillus sp.: PK-VR (P 75 mL + K 75 mL), PN-VR (P 75 mL + N 75 mL), NK-VR (N 75 mL + K 75 mL), PKN-VR (P 50 mL + K 50 mL + N 50 mL), and PKN-Car-VR (P 50 mL + K 50 mL + N 50 mL + 0.75 kg miscellaneous salt). The miscellaneous salt was obtained by using traditional concentrated crystallization methods from the treatment of high-salt waste water in Shaanxi Huamu Qingchuan Biotechnology Co., LTD. The purpose of adding the miscellaneous salt was to verify the effect of salinity on the compost to explore its suitability to be applied to saline soils. The composting test was conducted for 56 days with the moisture content controlled at around 60%-65%, reactor aeration was done by manual turning of the pile once every 3 days, and the ambient temperature was recorded at 9 a.m. every day by inserting a thermometer into the centre of the compost pile 15 cm deep from the surface of the compost heap and taking the average of the three measurements as the temperature of the compost heap. Samples were collected at 0, 3, 7, 14, 28, 35, 42, 48 and 56 d by quartile method and mixed thoroughly. The collected samples were divided into two parts. One portion was immediately stored at 4 °C until analysed, while the remaining portion was air-dried, sieved through a 0.15 mm sieve, and stored for further analysis.
Determination of Basic Physical and Chemical Indexes During Composting
Fresh compost samples were oven dried at 105 °C for 12 h until to a constant weight to measure moisture content. Aliquot of 2 g of fresh compost sample was mixed with distilled water in the ratio of 1: 10 (w/v) and shaken at 200 rpm for 1 h. pH and EC were measured with a pH meter and EC meter, respectively (soil/water = 1:5) [27]. Fresh compost samples (5 g) and 50 mL of deionised water were shaken at 160 rpm for 30 min, then filtered through 0.45 μm filter paper and the filtrate was collected. The E4/ E6 ratio was measured from the above filtrate at wavelengths of 465 nm and 665 nm by an ultraviolet spectrophotometer. Cucumber seeds were chosen based on the criteria to be exposed to the filtrate for seed germination studies, with deionized water serving as a blank control [28], and the seed germination index (GI) was calculated using the following formula [29].
Organic matter was burnt by the scorching method, i.e. burnt in a muffle furnace at 550 °C for 4 h until constant weight, the ash mass was measured and then the percentage was subtracted from the ash mass fraction [30]. Total phosphorus was measured spectrophotometrically [31], total potassium was measured flame photometrically, total nitrogen was measured Kjeldahl, nitrate nitrogen was measured ultraviolet spectrophotometrically, and ammonium nitrogen was measured by direct titration after distillation [32].
Raw Materials and Pot Experiment
The soil utilized in this study was sourced from a saline farmland located in the salt-affected region of Lubotan (109°33′26″ E, 34°49′34″ N), Shaanxi, China. Post-collection, the soil was subjected to air-drying, homogenization, and passage through a 5 mm sieve to ensure uniformity. The selected test crop for this investigation was "Sinon 511" wheat, with the seeds procured from a seed, pesticide, and fertilizer factory in Xi'an, China. The physico-chemical characteristics of the soil were as follows: pH 8.64, EC 0.936 dS/m, organic matter 12.45 g/kg, fast-acting phosphorus 12.53 mg/kg, fast-acting potassium 138.23 mg/g, and total nitrogen 0.624 g/kg.
A series of potting experiments was conducted at outdoors in Northwestern University's Chang'an Campus to investigate the impact of metabolites produced by composting on plant growth in saline soil. The experiments were carried out during the autumn season. Standardized plastic pots were used, measuring 29.6 cm in diameter, 20 cm in bottom diameter, and 19.7 cm in height. Each pot contained 3 kg of soil, 30 wheat seeds, and 250 g of compost. The experiment consisted of seven groups: CK (untreated soil), S1 (VR), S2 (PK-VR), S3 (PN-VR), S4 (NK-VR), S5 (PKN-VR), and S6: in which compost product was added to Phosphate solubilizing bacteria, potassium solubilizing bacteria, nitrogen-fixing bacterium and 0.75 kg miscellaneous salt (PKN-Car-VR). Each treatment was replicated three times. Throughout the experiment, the soil moisture content was carefully maintained at approximately 60%.
Plant Biomass and Stress Resistance Indexes
Plant samples were collected after 30 d of potting trials to determine plant growth parameters. Wheat seedlings were washed with distilled water and then oven dried to a constant weight at 60 °C. A portion of the wheat seedlings was naturally air dried for nutrient analyses, and the other portion of the fresh samples was stored in a refrigerator at 4 °C for subsequent determinations. Fresh weight, dry weight, root length and stem length of wheat seedlings were determined [33]. Chlorophyll content was determined by ethanol-acetone extraction followed by spectrophotometric determination of chlorophyll a (Chl b), chlorophyll b (Chl b), and total chlorophyll (Chl a + b) content [34]. Proline content was determined by the method of Bates et al. [35] and malondialdehyde (MDA) content was determined by the thiobarbituric acid (TBA) reaction method [36]. Nitrogen, phosphorus and potassium contents in plants were determined by digesting naturally air-dried wheat seedling samples with H2SO4–H2O2. Total nitrogen content in wheat seedlings was determined by semi-micro Kjeldahl nitrogen determination [37], total phosphorus content by molybdenum-antimony colourimetry, and total potassium by flame atomic absorption spectrophotometry.
Soil Physicochemical Properties
In the potting experiment, three replicate samples of soil (the weight of each sample was 50 g) were collected for each treatment. Some of the soil samples were air-dried after grinding and sieving (< 2 mm) to determine soil pH and nutrient indices. Soil pH was determined using an acidimeter (P901, water/soil, 2.5:1), and electrical conductivity (EC) values were measured using a conductivity meter (DDS-307, Shanghai Jingkrei Magnetics, China), water/soil, 5:1. Soil samples were analysed for their basic chemical properties using standard soil agrochemical analysis methods [38]. Potassium dichromate oxidation method was used for the determination of soil organic matter.TN (total nitrogen), TP (total phosphorus) and TK (total potassium) were determined by semi-micro Kjeldahl nitrogen fixation, molybdenum-antimony colorimetry and flame photometric method [39]. Soil AN (alkaline nitrogen decomposition), AP (fast-acting phosphorus) and AK (fast-acting potassium) were determined by alkali diffusion, sodium bicarbonate extraction colourimetry and flame photometric method. Sodium hypochlorite colourimetric method, 3,5-dinitrosalicylic acid colourimetric method and disodium phenyl phosphate colourimetric method were used respectively [40].
Statistical Analysis
Soil and plant parameters under different treatments were analysed using one-way analysis of variance (ANOVA). Multiple comparisons (LSD test) were used to test the level of significance between the different treatments at P < for 0.05. Correlation between wheat parameters and soil quality was analysed and plotted using Origin (2022).
Results
Composting Process and Physicochemical Properties of Compost
The temperature variations in the study are depicted in Fig. 1a. The ambient temperature ranged during the composting process was between 16.1 and 36.1 °C. In all composting treatments, the temperature of the compost material passes through three typical phases: mesophilic, thermophilic, and curing. At the beginning of composting, the temperature of the compost increases dramatically due to intense microbial degradation of organic matter and rapid microbial multiplication, and all treatments enter the thermophilic phase (50 °C) on days 5 and 6, with the PN-VR treatment reaching a peak temperature of 57.9 °C on day 9. The thermophilic phase should be maintained for at least 3 days to ensure that the compost product is sterilized, and in this phase, the PKN-VR treatment lasted for a maximum of 6 days and the CK treatment lasted for a minimum of 4 days. This indicated that microbial metabolic activity varies in response to changes in temperature. However, the temperatures of the treatments begin to decrease after reaching the peak and reached the ambient level, entering the stabilization stage at 35 days. This indicated that the degradable organic matter is almost exhausted. At the end of composting, the cumulative temperature of the PKN-VR treatment (2061.3 °C) was 5.82% higher than that of the CK group (2458.1 °C). Additionally, the temperature of the PKN-VR treatment is significantly higher than that of the other treatments in the curing stage (Fig. 1b). As shown in Fig. 1c, which illustrates the change in moisture content during the composting process, the moisture content followed a similar pattern in all treatments. Initially, during the first 0–3 days of composting, there is a rapid decrease in moisture content. This decrease helped to speed up the compost maturation time. Then, from days 3–7, the moisture content decreased quickly due to the significant growth of microorganisms and the thermophilic stage of composting. After the 14th day, the rate of moisture content declined slows down as microbial activity decreases. By the end of the composting process, the moisture content stabilized at around 30% in all treatments. Figure 1d shows the color change during composting of vinegar residue waste, which generally turned darker due to the evolution of humic substances from the decomposition of organic matter. It can be seen that the vinegar residue composting starts with yellow color, on the 3rd day of composting the vinegar residue shows the appearance of white mycelium, which indicated that the microorganisms are multiplying in large quantities, and then it gradually changed to brown, and then to dark brown after 28 days, which indicated that the organic matter was decomposed in large quantities, and there was no significant change thereafter.
Throughout the composting process, the pH of the six treatments changed drastically with an increasing trend in pH, which gradually stabilized after 28 days (Fig. 2a). Upon completion of composting, the final pH of the six composts ranged from 7.40 to 7.70. At the completion of composting, the final pH ranged 7.40–7.70. Figure 2b shows the electrical conductivity (EC) during composting. The EC values of the six treatments show a similar trend. A partial decrease in EC values is shown on days 0–3, which is mainly attributed to the volatilization of ammonia as well as to mineral salts and may be partly attributed to the production of leachate. Finally, the EC values (0.65–1.89 dS/m) of the compost products of all six treatments are below the recommended threshold (4 dS/m), indicating that there is no phytotoxicity in the final product, and all of them meet the compost maturity criteria.
As shown in Fig. 2c, the changes in GI were similar in different treatments, and all of them showed an increasing trend. According to the newly revised Chinese organic fertilizer standard (NY525-2021), which stipulates the requirement of GI ≥ 70%, the phytotoxicity of the compost material was relatively low when the GI value is > 50%, and when the GI value is > 90%, it indicates that the compost is mature and almost non-phytotoxic (Yang et al. 2021). At the end of the composting process, GI values in all treatments ranged from 103.5 to 115.0%. The addition of microorganisms to the treatments resulted in higher GI values compared to the CK treatment. This may be due to the accelerated biodegradation of toxic substances in the composting material by microorganisms. The E4/E6 ratios of all treatments decreased at the beginning of composting due to the production of organic acids, tended to increase when entering the thermophilic phase, and then gradually decreased until the compost matures (Fig. 2d). Throughout the process, the highest E4/E6 ratio is observed in the NK-VR treatment, indicating a low degree of humification in this compost. The lowest E4/E6 ratio is observed in the PKN-Car-VR treatment, which indicates a high degree of condensation of humic aromatic nuclei. The E4/E6 ratios of all treatments were in the range between 3.81 and 4.53 at the beginning of the composting but decreased to 2.92–3.47 at the end of the composting (an average decrease of 23.4%), indicating that the composted products have a certain degree of humification or putrefaction.
As shown in Fig. 2, the changes in NH4+-N and NO3−-N content followed a similar trend in all treatments. At the beginning of composting, the concentrations of NH4+-N and NO3−-N gradually increased. The volatilization of ammonia at high temperatures as composting progresses leaded to a gradual decrease in NH4+-N and NO3−-N content, which stabilized at the end of composting. At the end of composting. In which, NH4+-N and NO3−-N contents decreased to 0.005–0.035 g/kg and 0.0398–0.0521 g/kg, respectively. The decrease in nitrogen content indicates that several treatments showed signs of good composting and maturation process. In this process, the NH4+-N content of PKN-VR treatment was higher than other treatments in the pre-composting stage, and the lowest in CK group. At the end of composting, the highest NH4+-N content was found in the CK treatment and the lowest in the NK-VR treatment, which indicates that microbial activities are favorable to promote the decomposition of organic matter and accelerate the volatilization of ammonia.
As shown in Fig. 3a, the organic matter content of each treatment gradually decreased during the composting process, especially the loss of organic matter was higher in the PKN-VR and PKN-Car-VR treatments, while it was the lowest in the CK treatment. As composting progresses, most of the small organic molecules were decomposed so that the rate of organic matter degradation gradually tended to stabilize, while the difficult-to-degrade substances were further decomposed and utilized by microorganisms during the cooling and decaying process. At the end of composting, the content of organic matter remaining in each treatment group was stable at 56.55–63.54%, with a reduction of 26.26–35.25%, and the NK-VR group has the highest degradation rate of 35.25%, followed by PKN-VR at 34.852%. Total nutrient content was a key parameter in assessing the fertility and application value of compost (Fig. 3b). At the end of composting, the concentrations of total nitrogen (TN), phosphorus (Meena et al.), and potassium (TK) reached 4.06–6.30%, 0.275–0.552%, and 1.693–2.301%, respectively, in each group. Although nitrogen loss and inorganic nitrogen production during the composting process reduced the absolute content of TN, the N, P, and K nutrient contents of the compost products in all groups are significantly higher compared to those before composting. Compared to CK, the concentrations of TN, TP, and TK were increased by 24.1–55.2%, 22.1–100.99%, and 7.46–35.93%, and the total nutrients [Σ (N + P2O5 + K2O)] increased by 21.35–49.85% in all groups of compost, respectively.
Effect of Vinegar Residue Biofertilizer on Wheat Seedlings
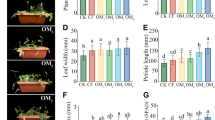
The present study verified the effect of vinegar residue-based biofertilizer on growth traits of wheat seedlings (Fig. 4). The mean stem length of wheat seedlings in all treatments ranged from 16.54 to 25.99 cm, with the S4 treatment having the largest mean stem length, followed by the S3 and S5 treatments (25.56, 25.8 cm). Compared with the CK group (6.65 cm), the S6 treatment showed longer root performance (11.54 cm), which is followed by S5 (10.26 cm) and S2 (9.24 cm) (Fig. 4b). The stem and root lengths of wheat seedlings were remarkably increased by 37.4–57.2% and 8.52–73.5%, respectively. In addition, wheat treated with VRBF also significantly increased in fresh and dry weights compared to the control, with the highest fresh weight of wheat seedlings in the S5 treatment (0.4239 g/plant), followed by the S1 treatment (0.4022 g/plant), with the treatments increasing by 47.1–71.4%, respectively (Fig. 4c). The highest dry weight of wheat seedlings is in the S5 treatment at 0.0593 g/plant, which increased significantly by 25.6% (S1), 41.3% (S2), 54.3% (S3), 50.5% (S4), 57.9% (S5), and 49.8% (S6) in S1-6 treatments, respectively, compared to CK (Fig. 4d).
Different VRBFs have a significant effect on chlorophyll content in wheat seedlings (Fig. 5). Compared with the control (0.827 mg/g), S5 treatment has the maximum chlorophyll a content (1.431 mg/g), followed by S3 (1.270 mg/g), S4 (1.090 mg/g), and the lowest performer is S1 (0.954 mg/g). Finally, the chlorophyll a content of the treatments is significantly increased by 15.4–73.0%, respectively (Fig. 5a). The highest chlorophyll b contents were found in S2 (0.316 mg/g) and S6 (0.288 mg/g), which increased their chlorophyll b contents in wheat seedlings by 65.8 and 51.3%, respectively, compared with the control group (0.191 mg/g). The lowest one was S3, which is only 0.125 mg/g (Fig. 5b), even though the chlorophyll b content of S3 treatments was slightly lower than that of the CK group. Its total chloroplast content was greater than that of the CK group. Figure 5c reflects the total chlorophyll content of wheat seedlings in each treatment. Compared with the CK group (1.017 mg/g), the average total chlorophyll contents under the S1-6 treatments were 1.185, 1.31, 1.395, 1.359, 1.649, and 1.258 mg/g, respectively, which were increased by 16.6% to 62.2%. The best performance of total chlorophyll content is under the S5 treatment. Soluble sugars played a key role in dehydration tolerance during plant seedling development. The highest soluble sugar content in the leaves of wheat seedlings treated with VRBF was found in the S4 treatment (3.517 mg/g), followed by S5 (3.479 mg/g) and S6 (3.420 mg/g). Compared to the control, the soluble sugar content of the S1-6 treatments significantly increased by 14.9, 37.5, 49.6, 64.5, 62.7, and 59.9% (Fig. 5d). Similarly, a significant increase in proline content was observed under different VRBF treatments, ranging from 26.0 to 94.0% in the S1-6 treatments, respectively, compared to the control. The highest proline contents are found in S5 (11.982 µg/g) and S4 (11.794 µg/g), and the lowest is in S1 treatment with 7.780 µg/g. In the present study, there was a reduction in MDA content in wheat seedlings under different VRBF treatments (Fig. 5f). The MDA content in the CK group is 17.350 µg/g. S5 treatment has the lowest MDA content (13.176 µg/g), followed by S6 (14.961 µg/g), S1 (14.970 µg/g). S5, S6, and S1 treatments have the MDA content decreases by 24.05, 13.83, and 13.77%, respectively.
Figure 6 shows the variation of nutrient content in wheat seedlings after the application of different VRBFs. The nitrogen (N) content of wheat seedlings in the CK group and VRBF treatments was 4.32, 5.37, 5.02, 6.18, 5.95, 6.88, and 6.18%, respectively (Fig. 6a). The S5 treatment had the highest N content, while there was no significant difference (P < 0.05) in the S3 and S6 treatments compared to the CK group. The S5, S3, and S6 treatments improved wheat N content by 59.2, 43.1, and 43.1% respectively. When VRBF is added to the soil, N-containing compounds decomposed slowly and provided a stable supply of N to wheat seedlings. Additionally, nitrogen-fixing bacteria increased N utilization in the soil and improved uptake by the wheat root system. This leaded to the accumulation of more N in wheat seedlings. The highest levels of phosphorus (P) in wheat seedlings were found in S5 (0.585%), S2 (0.545%), and S6 (0.532%), while the CK group only had 0.36569% of P (Fig. 6b). Compared with the CK group, the P content of wheat in the S2 and S5 treatments was 48.9 and 59.8% higher than that of CK, respectively (P < 0.05). From Fig. 6c, the highest potassium concentration in wheat seedlings was found in S5 treatment (1.883%), followed by S4 (1.715%). Wheat K concentration was significantly (P < 0.05) higher in S5 and S4 treatments by 59.7, 45.5% compared to CK group (1.179%).
Effect of Vinegar Residue Biofertilizer on the Physicochemical Properties of Potting Saline Soil
Soil pH and EC are direct indicators of soil salinity and can affect soil acidity. The application of VRBDF group treatments (S1-6) significantly reduced soil pH and EC compared to the CK group (Fig. 7a, b). In the CK group, soil pH was 8.52 and EC was 0.861 dS/m. The treatments that showed the largest decreases in soil pH and EC were S5 (7.93) and S4 (0.320 dS/m), respectively, which were 6.9 and 62.8% lower. The treatment with the highest soil organic matter content was S5 (22.1 g/kg), followed by S4 (21.88 g/kg), S6 (20.6 g/kg), and S2 (20.5 g/kg). The lowest organic matter content was found in S1 (17.92 g/kg) compared to the control group (13.24 g/kg). The S4, S6, and S2 treatments were 66.9, 65.3, and 54.8% higher, respectively, than the CK group (Fig. 7c). The nutrient (N, P, and K) levels of the potting soil are displayed in Fig. 7d. In the CK group, the effective phosphorus content was 12.5 mg/kg. The S5 group of treatments had the highest soil AP content, with values of 26.77 mg/kg for S5, 23.81 mg/kg for S2, and 22.90 mg/kg for S6. These values were 114.1, 90.4, and 83.2% higher, respectively, compared to the CK group (Fig. 7d). In the CK group, the soil AK content was 146.6 mg/kg. The S5 group had the highest soil AK content at 175.6 mg/kg, followed by S4 with 174.53 mg/kg. These values were 19.8 and 19.1% higher, respectively, than the CK group (Fig. 7e). The soil AN content of the treatment’s ranged from 41.4 to 67.67 mg/kg. The highest AN content was observed in the soil of the S4 treatment, which is 63.5% higher compared to the CK group. However, there was no significant difference in AN content between the S2 and S6 treatments (P < 0.05). The total soil phosphorus content in the S1 (0.57 g/kg), S2 (0.39 g/kg), S3 (0.55 g/kg), S4 (0.54 g/kg), S5 (0.58 g/kg), and S6 (0.53 g/kg) groups increased by a total of 25.8–87.1% compared to the CK group (0.31 g/kg). In terms of soil TK content, the treatment of group S4 had the highest level at 15.54 g/kg. This represents a significant increase of 102.8% compared to the control group (7.66 g/kg). On the other hand, group S6 has the lowest TK content at 10.98 g/kg, due to the salt content inhibiting the activity of potassium-solubilizing bacteria to some extent (Fig. 7h). Figure 7i shows the TN content of soil in potted plants. The soil TN content for each treatment group (CK, S1, S2, S3, S4, S5, and S6) significantly increased by 40–112% compared to the control group of CK. Notably, the TN content was higher in the S4, S2, and S6 treatments, indicating that the application of VRBF effectively increased the nitrogen content in the soil.
Effect of Vinegar Residue Biofertilizer on Enzyme Activity in Potted Saline Soil
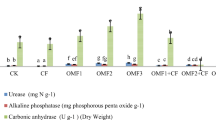
The changes in enzyme activities in the potting soil are depicted in Fig. 8. The S1-6 group exhibited significantly higher soil enzyme activities compared to the CK group (1.96 mL/g h). However, no significant differences in catalase activities were observed between the treatment groups (P < 0.05). The soil catalase activity reached its peak in the S5 and S3 treatments, both recording 2.27 mL/g h, while the lowest activity is seen in S2 with 2.13 mL/g-h. In comparison to the CK group, the catalase activity in the S1-6 treatments showed an increase of 8.67–18.88%. Additionally, the highest sucrase activity was recorded in S4 (18.84 mg/g/d), closely followed by S3 (18.61 mg/g/d). The sucrase activity in the S1-6 group exhibited a significant increase of 24.26–41.9% compared to the CK group (13.27 mg/g d) (Fig. 8b). The CK group had an alkaline phosphatase activity of 0.74 mg/g–24 h. In the S1-6 group, the ALPase activity was 0.89, 1.31, 1.20, 1.08, 0.95, and 1.02 mg/g·24 h, which represents increases of 20.3, 77.02, 62.2, 45.9, 28.4, and 37.8% respectively. Figure 8d demonstrates that all the S1-6 groups have higher urease activities compared to the CK group (0.94 mg/g·24 h), with S3 exhibiting the highest urease activity (1.27 mg/g·24 h), followed by S6 (1.26 mg/g·24 h). The groups S1-6 showed an increase in soil urease enzymes ranging from 18.08 to 35.1% when compared to the control soil.
Relationships and Correlations Between the Parameters
Principal Coordinate Analysis (PCA) showed the effect of VRBF on the relationship between physicochemical properties and enzyme activities of saline soils. Table 1 shows the results of analysis of variance (ANOVA). The cumulative variance explained by the first (75.161%), second (9.955%) and third (6.832%) axes accounted for 91.948% of the total variance. The significant differences in nutrient content and enzyme activities in the soil after application of VRBF further confirmed the great changes in soil fertility. Except for pH and EC, the loadings of the first principal component were all positive, indicating that the other soil nutrient indicators had strong positive correlations with PC1. However, pH and EC values were negatively correlated with PC1. Soil chemical variables such as P, K, N and OM contributed the most to the first principal component, which was mainly attributed to the increase in SOM content and nutrient levels of N, P and K by VRBF. This indicated that PC1 represents soil nutrient content. The second component mainly reflected the changes in soil enzyme activities after VRBF application. The third component, PC3, was mainly influenced by soil pH. PCA analysis (Fig. 9a) showed that soil nutrient content was the first driver of soil quality in saline soils, followed by soil enzyme activity, while pH and salinity were the third drivers. The correlation study (Fig. 9b) examined the relationship between wheat seedling growth and soil health under different VRBF treatments in a pot experiment. The results showed that there were significant positive correlations between soil pH and electrical conductivity and MAD. In addition, negative correlations (P < 0.05) were found with OM, AN, TK, AK, AP, S-CAT, S-UE and other wheat parameters except Chlb and MAD. This suggested that lowering soil pH in saline soils can effectively improve soil quality and promote wheat growth. In addition, soil electrical conductivity was significantly (P < 0.05) and negatively correlated with other soil physicochemical properties, enzyme activities and wheat growth parameters excluding Chlb and MAD. Wheat root length (RL) was negatively correlated with soil pH and EC, but significantly correlated with soil OM, AN, AK, AP and S-UE. This highlighted the close relationship between wheat root-soil environment and soluble N, P, K and urease contents. Except for wheat MAD, all other growth parameters were significantly and positively correlated with soil nutrient content and enzyme activities. This suggested that promotion of wheat growth depends on the increase in soil nutrient content and enzyme activity.
Discussion
Composting Process and Physicochemical Properties of Compost
As an important sign of microbial life activity during fermentation, the compost temperature can directly reflect the composting process and microbial activity, and also significantly affect the decomposition of organic matter and microbial community succession during fermentation [41]. During the 10th–13th day of composting, the temperature gradually decreases, which may be due to the insufficient carbon content of the microorganisms and the evaporation of water that carries away a large amount of heat, resulting in the presence of an anaerobic environment and limitation of the activity of aerobic microorganisms. Afterwards, after turning the piles, the six treatments enter the thermophilic stage again after the 14th day, and the temperatures of all the treatments increase slightly after each turn of the piles. This finding is consistent with the study conducted by Li et al. [42]. Moisture content is critical for soluble nutrients required for microbial metabolic activities during composting [43].
The pH of the treatments shows a decreasing trend at day 3, which is related to the production of small molecule organic acids from the microbial decomposition of carbohydrates in the materials during the fermentation process, This finding aligns with the outcomes of a previous investigation [44]. Afterwards, the mineralization and humification of organic matter within the vinegar residue during composting produced NH3, which neutralized the organic acids, leading to a gradual increase in pH [45]. The decrease in EC value at the beginning of composting is mainly attributed to volatilization of ammonia, as well as mineral salts and precipitation, and may be partially attributed to leachate production [46]. Subsequently, there is a gradual increase in EC values due to lower volatilization rates of ammonia, weaker degradation of organic matter, and nitrification or denitrification processes in the maturation phase. After the 21st day, the EC decreases again due to the conversion of small molecules and ions into large humus molecules [47]. The EC is able to reflect the salinity level and phytotoxicity of the compost, and some studies have reported that the addition of salt inhibits the microbial activity affecting the biodegradation process [48]. The reason for the initially low GI values during composting may be that toxicity from the accumulation of organic acids inhibits the growth of the seeds, which is in agreement with a previous study [49]. Subsequently, during the thermophilic phase (0–28 days), the GI values of all treatments increase significantly. This increase can be attributed to the biodegradation of toxic substances (e.g. NH4+, organic acids, and phenolic acids) in the composted material [50]. The E4/E6 ratio characterizes the quality and degree of aromatization of humic acids [51]. NH4+-N and NO3−-N as the two most abundant forms of nitrogen in the composting process, NH4+-N is converted to NH3 and released to the environment, and the change of NO3−-N content is the result of the combined effect of nitrification and denitrification [52]. At the early stage of composting, NH3 is produced due to the decomposition of amino acids and some nitrogenous compounds by microorganisms, leading to a NH4+-N content decreasing. The NH4+-N content increased and then decreased several times during the composting process, which may be due to the increase in temperature and the increased ammonification triggered by the degradation of many nitrogenous substances [53].The NH4+-N content of PKN-Car-VR treatment was higher than that of the other added microbial treatments is relatively low, and some researchers believe that the salt incorporation will have an inhibitory effect on organic matter humification, thus reducing the NH4+-N content [54]. During the early decomposition stage, microbial proliferation promotes the decomposition rate of organic matter and the moisture content of the compost decreases rapidly, which helps to shorten the maturation time of the compost. After that, the rate of decrease in moisture content slows down due to the thermophilic stage of composting, where microbial activity is reduced and less water is evaporated to the surrounding environment [55].
According to previous study [56], compost organic matter conversion mainly consists of three stages: degradation, CO2 release, and carbon sequestration, in which microorganisms play a dominant role. During the warming period, microorganisms use the soluble organic matter in the pile to rapidly multiply and mineralize to make the organic matter degradation rate faster. During the high temperature period, microorganisms decompose large molecules of cellulose, hemicellulose and lignin, resulting in a reduction of organic matter, but the degradation rate slows down relative to the warming period, due to the fact that the composting microorganisms consume amino acids and low-molecular-weight organic acids at the beginning, but the degradation of organic compounds produces many smaller soluble substances, resulting in a smaller change of organic matter content, which is similar to the results of Zhang et al. [57]. The mineralization of organic compounds in the composting process produces a “concentration effect”, leading to an increase in nutrient concentration [58]. According to the Chinese industry standard (NY/T 525-2021), the total nutrient content (Σ (N + P2O5 + K2O)) is total nitrogen (TN), total phosphorus (Meena et al.), and total potassium (TK), and the limit value is (Σ (N + P2O5 + K2O) ≥ 4.0%) [59]. Overall, the nutrient composition of the final product is quite impressive, and all of them meet the requirements of the Chinese Organic Fertilizer Quality Standard (NY/T 525-2021).
Effect of Vinegar Residue Biofertilizer on Wheat Seedling
The treatment with added microbiome promoted the growth of wheat seedlings better compared to CK. The related study shows that Bacillus sp. can improve plant growth through the production of IAA and ACC deaminases to improve plant growth, and these enzymes are mainly involved in improving the plant root system [60]. On the other hand, the microbial degradation of organic matter in the vinegar residue improved the soil microenvironment of the root system of wheat seedlings.
soil microenvironment, enhanced the nutrient content in the soil, and increased soil fertility. The results of this study are similar to those of Robas Morae et al. [61]. Chloroplast content is an important photosynthetic pigment in energy conversion and carbon assimilation in plants, and it reflects changes in leaf physiological activity [62]. The positive effect of the addition of VRBF treatment on photosynthetic pigment metabolism is further evident, which is attributed to the presence of nitrogen-fixing bacteria that fix atmospheric nitrogen and maintain the continuous use of nitrogen for the synthesis of chlorophyll molecules in seedlings [34]. Soluble sugars play a key role in dehydration tolerance during plant seedling development. This increase is attributed to VRBF enhancing nutrient uptake and promoting photosynthesis, resulting in increased water holding capacity. Consequently, the sugar content in wheat seedlings is effectively improved. Therefore, with the application of VRBF, the reduction of salinity stress in the soil led to an increase in the rate of proline accumulation in wheat seedlings, which makes the S5 and S6 treatments more resistant than the other treatments and more water stress resistant. This result is similar to the findings of Qayyum et al. [63]. Malondialdehyde (MDA) reflects the degree of oxidative damage in plants, with higher MDA levels indicating weaker damage capacity and resistance [64]. It has also been shown that an increase in proline in plants also promotes a higher concentration of reactive oxygen species in the soil, which reduces oxidative damage in plants [50]. The increase in phosphorus concentration in wheat seedlings may be due to the degradation of some difficult-to-use phosphates in saline soil by phosphate-solubilizing bacteria [65], which increases the concentration of phosphorus in the rhizosphere soil. The incorporation of VRBF into saline soils significantly increased the N, P, and K contents of wheat seedlings. Some of the nutrients in VRBF can be rapidly released into the soil solution and absorbed by wheat [66].
Effect of Vinegar Residue Biofertilizer on the Physicochemical Properties of Potting Saline Soil
The decrease in soil EC may be attributed to improved porosity, aeration, and hydraulic conductivity, which promotes leaching of salts from the surface to the lower layers. Decrease in soil pH, which may be due to the release of reactive organic acids and carbon dioxide from the decomposition of bio-organic additives [67]. It may also be attributed to the further decomposition of organic matter in the vinegar residue, leading to the production of organic acids [68, 69]. These findings indicate that the treatment groups that received added microorganisms (S2-6) have higher organic matter content compared to those with vinegar residue application alone (S1). This is likely because microorganisms promote the decomposition of soil organic matter, leading to the production of more organic matter and an improvement in soil quality. Previous studies have demonstrated that phosphorus in the soil primarily exists in the form of insoluble mineral complexes. However, the effectiveness of soil phosphorus can be enhanced by introducing phosphate-solubilizing bacteria which can release mineral-soluble substances like organic acid anions and iron carriers, and by decomposing organic compounds in humus [70]. Several studies have shown that phosphorus and potassium in soil exist mainly in the form of insoluble mineral complexes, and by adding VRBF, Bacillus sp. may help to improve soil nutrient effectiveness by releasing mineral-soluble substances such as organic acid anions and iron carriers, secretion of extracellular enzymes and substrate degradation as well as decomposition of humic substances in organic compounds [71]. In addition, the high content of nitrogen compounds in the raw material of VRBF compost can effectively enhance the accumulation of nitrogen in the soil.
Effect of Vinegar Residue biofertilizer on Enzyme Activity in Potted Saline Soil
Soil enzymes play a key role in various nutrient cycles and their activity is considered a key indicator of soil fertility [72]. Among these groups, the S2 group had the highest alkaline phosphatase activity, possibly due to a higher presence of phosphorus solubilizing bacteria that can produce phosphate through their phosphorus solubilizing capacity [72]. Other soil enzyme activities also showed different levels of increase. These results indicate that the application of VRBF can effectively enhance and modify enzyme activities in the soil, promoting soil nutrient cycling and providing ample energy for soil microorganisms, thus improving the soil environment [38].
Relationships and Correlations Between the Parameters
According to previous studies, enzyme activities in soil are closely related to soil physicochemical properties [20, 73]. PCA shows that soil organic matter, quick nutrients and enzyme activities were the main drivers limiting the fertility deficit of saline soils. Further analysis of hotspot diagrams showed that, except for soil pH, EC and MDA, there was a significant positive correlations. This result was similar to that of Zhang et al. [74]. However, some studies have shown that the changes in soil enzyme activity by fungal fertilizer application are indirect, and that it directly affects the microbial community mainly through the soil physicochemical properties, which indirectly affects the soil enzyme activity soil [32]. The results of this study confirmed that by improving saline soil pH, organic matter, nitrogen, phosphorus, potassium and other nutrient indexes at the same time, it is also conducive to the improvement of soil enzyme activity, which can further improve the soil fertility and promote the growth of wheat.
Conclusion
In this study, phosphorus solubilizing bacteria, potassium solubilizing bacteria and nitrogen fixing bacteria were screened from saline soil, and then the strains and vinegar residue were mixed according to the ratio of 1:100 (v:m) to prepare biological fertilizer. The results of potting experiments by saline soil amended with vinegar residue-based compost showed that PKN-VR could effectively reduce soil salinity and increase soil nutrient content and enzyme activity. The improvement of saline soil environment promoted the growth and development of wheat seedlings and the absorption of nitrogen, phosphorus and potassium. The results showed that PKN-VR has considerable potential in saline soil remediation, realizes the resource utilization of vinegar residue waste for the green development of agriculture. It is observed that the treatment with addition of such PGPR promotes better growth of wheat seedlings due to the presence of phosphorus, potassium and nitrogen fixing bacteria which increases the photosynthetic activity, chlorophyll formation, nitrogen metabolism and growth hormone content of wheat seedlings, which ultimately improves the root growth and fresh dry weight of the plant.
Data Availability
All relevant data are within the manuscript.
References
Yao, R., Gao, Q., Liu, Y., Li, H., Yang, J., Bai, Y., Zhu, H., Wang, X., Xie, W., Zhang, X.: Deep vertical rotary tillage mitigates salinization hazards and shifts microbial community structure in salt-affected anthropogenic-alluvial soil. Soil Tillage Res. 227, 105627 (2023)
Shi, Y., Liu, X., Zhang, Q., Li, G., Wang, P.: Biochar rather than organic fertilizer mitigated the global warming potential in a saline-alkali farmland. Soil Tillage Res. 219, 105337 (2022)
Singh, A.: Soil salinity: a global threat to sustainable development. Soil Use Manag. 38(1), 39–67 (2022)
Chen, Z., Li, Y., Hu, M., Xiong, Y., Huang, Q., Jin, S., Huang, G.: Lignite bioorganic fertilizer enhanced microbial co-occurrence network stability and plant–microbe interactions in saline-sodic soil. Sci. Total. Environ. 879, 163113 (2023)
Mukhopadhyay, R., Fagodiya, R.K., Narjary, B., Barman, A., Prajapat, K., Kumar, S., Bundela, D.S., Sharma, P.C.: Restoring soil quality and carbon sequestration potential of waterlogged saline land using subsurface drainage technology to achieve land degradation neutrality in India. Sci. Total. Environ. 885, 163959 (2023)
Zhou, Z., Li, Z., Zhang, Z., You, L., Xu, L., Huang, H., Wang, X., Gao, Y., Cui, X.: Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western Songnen Plain. Sci. Total. Environ. 797, 149190 (2021)
Heng, T., He, X., Yang, L., Xu, X., Feng, Y.: Mechanism of Saline-Alkali land improvement using subsurface pipe and vertical well drainage measures and its response to agricultural soil ecosystem. Environ. Pollut. (Barking, Essex: 1987) 293, 118583 (2021)
Sun, Z., Ge, J., Li, C., Wang, Y., Zhang, F., Lei, X.: Enhanced improvement of soda saline-alkali soil by in-situ formation of super-stable mineralization structure based on CaFe layered double hydroxide and its large-scale application. Chemosphere 300, 134543 (2022)
Xu, X., Wang, J., Tang, Y., Cui, X., Hou, D., Jia, H., Wang, S., Guo, L., Wang, J., Lin, A.: Mitigating soil salinity stress with titanium gypsum and biochar composite materials: improvement effects and mechanism. Chemosphere 321, 138127 (2023)
Hou, J., Wan, H., Liang, K., Cui, B., Ma, Y., Chen, Y., Liu, J., Wang, Y., Liu, X., Zhang, J., Wei, Z., Liu, F.: Biochar amendment combined with partial root-zone drying irrigation alleviates salinity stress and improves root morphology and water use efficiency in cotton plant. Sci. Total. Environ. 904, 166978 (2023)
Liu, D., Ding, Z., Ali, E.F., Kheir, A.M.S., Eissa, M.A., Ibrahim, O.H.M.: Biochar and compost enhance soil quality and growth of roselle (Hibiscus sabdariffa L.) under saline conditions. Sci. Rep. 11(1), 8739 (2021)
Wu, L., Zhang, S., Wang, J., Ding, X.: Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: adsorption, column and field tests. Environ. Pollut. 261, 114223 (2020)
Liu, Z., Wang, Y., Guo, S., Liu, J., Zhu, P.: Preparation and characterization of bacterial cellulose synthesized by Kombucha from vinegar residue. Int. J. Biol. Macromol. 258, 128939 (2024)
Chen, L., Meng, X., Zhou, G., Zhou, Z., Zheng, T., Bai, Y., Yuan, H., Huhe, T.: Effects of organic loading rates on the anaerobic co-digestion of fresh vinegar residue and pig manure: Focus on the performance and microbial communities. Biochem. Eng. J. 183, 108441 (2022)
Mei, Y., Yang, Z., Sun, P.-F., Zhou, S., Guo, H., Peng, L.E., Yao, Z., Yang, W., Tang, C.Y.: Polyelectrolyte-assisted interfacial polymerization for polyamide nanofiltration membrane with enhanced separation and anti-biofouling properties in groundwater treatment. Desalination 555, 116546 (2023)
De Corato, U.: Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: a review under the perspective of a circular economy. Sci. Total. Environ. 738, 139840 (2020)
Farid, I.M., Siam, H.S., Abbas, M.H.H., Mohamed, I., Mahmoud, S.A., Tolba, M., Abbas, H.H., Yang, X., Antoniadis, V., Rinklebe, J., Shaheen, S.M.: Co-composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: a trial for enhancing the health of low fertile arid soils. Chemosphere 292, 133389 (2022)
Liu, H., Li, D., Huang, Y., Lin, Q., Huang, L., Cheng, S., Sun, S., Zhu, Z.: Addition of bacterial consortium produced high-quality sugarcane bagasse compost as an environmental-friendly fertilizer: optimizing arecanut (Areca catechu L.) production, soil fertility and microbial community structure. Appl. Soil Ecol. 188, 104920 (2023)
Shang, X.C., Zhang, M., Zhang, Y., Hou, X., Yang, L.: Waste seaweed compost and rhizosphere bacteria Pseudomonas koreensis promote tomato seedlings growth by benefiting properties, enzyme activities and rhizosphere bacterial community in coastal saline soil of Yellow River Delta, China. Waste Manag. 172, 33–42 (2023)
Zhou, R., Wang, Y., Tian, M., Jahan, M.S., Guo, S.: Mixing of biochar, vinegar and mushroom residues regulates soil microbial community and increases cucumber yield under continuous cropping regime. Appl. Soil Ecol. 161(2021), 103883 (2021)
Ding, K., Zhou, X., Hadiatullah, H., Lu, Y., Zhao, G., Jia, S., Zhang, R., Yao, Y.: Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater. 420, 126551 (2021)
Li, Y., Pei, G., Zhu, Y., Liu, W., Li, H.: Vinegar residue biochar: A possible conditioner for the safe remediation of alkaline Pb-contaminated soil. Chemosphere 293, 133555 (2022)
Chamkhi, I., Sbabou, L., Aurag, J.: Improved growth and quality of saffron (Crocus sativus L.) in the field conditions through inoculation with selected native plant growth-promoting rhizobacteria (PGPR). Ind. Crops Prod. 197, 116606 (2023)
Li, H., La, S., Zhang, X., Gao, L., Tian, Y.: Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 15(10), 2865–2882 (2021)
Marghoob, M.U., Nawaz, A., Ahmad, M., Waheed, M.Q., Khan, M.H., Imtiaz, M., Islam, E.U., Imran, A., Mubeen, F.: Assessment of halotolerant bacterial and fungal consortia for augmentation of wheat in saline soils. Front. Microbiol. 14, 1207784 (2023)
Yu, S.Y., Peng, W., Si, W., Yin, L., Liu, S.G., Liu, H.F., Zhao, H.L., Wang, C.L., Chang, Y.H., Lin, Y.Z.: Enhancement of bacteriolysis of Shuffled phage PhiX174 gene E. Virol. J. 8, 1–6 (2011)
Wei, Z., Ahmed Mohamed, T., Zhao, L., Zhu, Z., Zhao, Y., Wu, J.: Microhabitat drive microbial anabolism to promote carbon sequestration during composting. Bioresour. Technol. 346, 126577 (2022)
Kong, Y., Zhang, J., Yang, Y., Liu, Y., Zhang, L., Wang, G., Liu, G., Dang, R., Li, G., Yuan, J.: Determining the extraction conditions and phytotoxicity threshold for compost maturity evaluation using the seed germination index method. Waste Manage. 171, 502–511 (2023)
Yang, Y., Wang, G., Li, G., Ma, R., Kong, Y., Yuan, J.: Selection of sensitive seeds for evaluation of compost maturity with the seed germination index. Waste Manag. 136, 238–243 (2021)
Zhang, L., Sun, X.: Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 171, 274–284 (2014)
Bremner, J.M.: Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55(1), 11–33 (1960)
Wu, L., Jiang, Y., Zhao, F., He, X., Liu, H., Yu, K.: Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 10(1), 9568 (2020)
Jiao, Y., Jia, R., Sun, Y., Yang, G., Yuan, L.: In situ aerobic composting eliminates the toxicity of Ageratina adenophora to maize and converts it into a plant- and soil-friendly organic fertilizer. J. Hazard. Mater. 410, 124554 (2020)
Ahmed, H.G.M., Zeng, Y., Yang, X., Anwaar, H.A., Mansha, M.Z., Hanif, C.M.S., Ikram, K., Ullah, A., Alghanem, S.M.S.: Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J Biol Sci 27(8), 2116–2123 (2020)
Bates, L.S., Waldren, R.P., Teare, I.D.: Rapid determination of free proline for water-stress studies. Plant Soil 39(1), 205–207 (1973)
Ates, O., Kivanc, M.: Effects of Arthrobacter arilaitensis and Pseudomonas putida on salt stress tolerance in wheat. Environ. Eng. Manag. J. 20(12), 2025-2032 (2021)
Stuart, N.W.: Adaptation of the micro-kjeldahl method for the determination of nitrogen in plant tissues1. Plant Physiol. 11(1), 173–179 (1936)
Chen, R., Wang, X.: Analytical methods for soil and agro-chemistry. Eur. J. Soil Sci. 73(4), e13280 (2022)
Walker, T.W., Adams, A.F.R.: Studies on soil organic matter: I. influence of phosphorus content of parent materials on accumulations of carbon, nitrogen, sulfur, and organic phosphorus in grassland soils. Soil Sci. 85, 307–318 (1958)
Tabatabai, M.A., Bremner, J.M.: Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1(4), 301–307 (1969)
Li, Y., Zhou, M., Li, C., Pan, X., Lv, N., Ye, Z., Zhu, G., Zhao, Q., Cai, G.: Inoculating indoleacetic acid bacteria promotes the enrichment of halotolerant bacteria during secondary fermentation of composting. J. Environ. Manag. 322, 116021 (2022)
Li, D., Wang, H., Ding, J., Zhou, Y., Jia, Y., Fan, S., Zhang, A., Shen, Y.: Comparative study on aerobic compost performance, microbial communities and metabolic functions between human feces and cattle manure composting. Environ. Technol. Innov. 31, 103230 (2023)
Xie, D., Gao, M., Yang, M., Xu, M., Meng, J., Wu, C., Wang, Q., Liu, S.: Re-using ammonium-rich wastewater as a moisture conditioning agent during composting thermophilic period improves composting performance. Bioresour. Technol. 332, 125084 (2021)
Wang, Y., Tang, Y., Li, M., Yuan, Z.: Aeration rate improves the compost quality of food waste and promotes the decomposition of toxic materials in leachate by changing the bacterial community. Biores. Technol. 340, 125716 (2021)
Guo, X.-X., Liu, H.-T., Wu, S.-B.: Humic substances developed during organic waste composting: formation mechanisms, structural properties, and agronomic functions. Sci. Total. Environ. 662, 501–510 (2019)
Li, M.-X., He, X.-S., Tang, J., Li, X., Zhao, R., Tao, Y.-Q., Wang, C., Qiu, Z.-P.: Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 264, 128549 (2021)
Wang, Y., Tang, Y., Yuan, Z.: Improving food waste composting efficiency with mature compost addition. Biores. Technol. 349, 126830 (2022)
Wang, S.P., Wang, L., Sun, Z.Y., Wang, S.T., Kida, K.: Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Biores. Technol. 337, 125492 (2021)
Liu, G., Yang, Y., Ma, R., Jiang, J., Li, G., Wang, J., Wuyun, D., Yuan, J.: Thermophilic compost inoculating promoted the maturity and mature compost inoculating reduced the gaseous emissions during co-composting of kitchen waste and pig manure. Environ. Technol. Innov. 32, 103427 (2023)
Talaat, M., Mohamed, A.K.S.H., Nassar, M.Z.A., Aboeldahb, N.: Response of some metabolic activities of wheat plants to different treatments of marine macroalgae from the Red Sea, Egypt. Egypt. J. Phycol. (2021)
Kong, Y., Wang, G., Chen, W., Yang, Y., Ma, R., Li, D., Shen, Y., Li, G., Yuan, J.: Phytotoxicity of farm livestock manures in facultative heap composting using the seed germination index as indicator. Ecotoxicol. Environ. Saf. 247, 114251 (2022)
Yan, R., Wu, H., Yang, X., Yang, C., Lyu, H., Zhang, H., Li, S., Liu, T., Li, R., Yao, Y.: Soil decreases N2O emission and increases TN content during combined composting of wheat straw and cow manure by inhibiting denitrification. Chem. Eng. J. 477, 147306 (2023)
Hussain, A., Huang, W.-Y., Lin, C.-Y., Ahsan, W.A., Lin, C.: Mitigation of ammonia emissions during food waste composting through acetic acid addition: a promising strategy for sustainable waste management. Sustain. Chem. Pharm. 36, 101324 (2023)
Mahmud, A.A., Sultana, M., Jahiruddin, M., Abedin, M.: Nitrogen, phosphorus and sulphur mineralization in soil treated with amended municipal solid waste compost under aerobic and anaerobic conditions. Islamic Azad University-Isfahan (Khorasgan) Branch (2021)
Yu, X., Li, X., Ren, C., Wang, J., Wang, C., Zou, Y., Wang, X., Li, G., Li, Q.: Co-composting with cow dung and subsequent vermicomposting improve compost quality of spent mushroom. Bioresour. Technol. 358, 127386 (2022)
Wei, Z., Mohamed, T.A., Zhao, L., Zhu, Z., Zhao, Y., Wu, J.: Microhabitat drive microbial anabolism to promote carbon sequestration during composting. Bioresour. Technol. 346, 126577 (2022)
Zhang, T., Li, H., Yan, T., Shaheen, S.M., Niu, Y., Xie, S., Zhang, Y., Abdelrahman, H., Ali, E.F., Bolan, N.S., Rinklebe, J.: Organic matter stabilization and phosphorus activation during vegetable waste composting: multivariate and multiscale investigation. Sci. Total. Environ. 891, 164608 (2023)
Zhang, J., Wu, Z., Huang, Y., Zhan, X., Zhang, Y., Cai, C.: Industrial-scale composting of swine manure with a novel additive-yellow phosphorus slag: variation in maturity indicators, compost quality and phosphorus speciation. Biores. Technol. 384, 129356 (2023)
Zhang, J., Wu, Z., Huang, Y., Zhan, X., Zhang, Y., Cai, C.: Industrial-scale composting of swine manure with a novel additive-yellow phosphorus slag: variation in maturity indicators, compost quality and phosphorus speciation. Bioresour. Technol. 384, 129356 (2023)
Rezakhani, L., Motesharezadeh, B., Tehrani, M.M., Etesami, H., Hosseini, H.M.: The effect of silicon fertilization and phosphate-solubilizing bacteria on chemical forms of silicon and phosphorus uptake by wheat plant in a calcareous soil. Plant Soil 477(1–2), 259–280 (2022)
Robas Mora, M., Fernández Pastrana, V.M., Probanza Lobo, A., Jiménez Gómez, P.A.: Valorization as a biofertilizer of an agricultural residue leachate: Metagenomic characterization and growth promotion test by PGPB in the forage plant Medicago sativa (alfalfa). Front. Microbiol. 13, 1048154 (2022)
Alamri, S.A., Siddiqui, M.H., Mukherjee, S., Kumar, R., Kalaji, H.M., Irfan, M., Minkina, T.M., Rajput, V.D.: Molybdenum-induced endogenous nitric oxide (NO) signaling coordinately enhances resilience through chlorophyll metabolism, osmolyte accumulation and antioxidant system in arsenate stressed-wheat (Triticum aestivum L.) seedlings. Environ. Pollut. 292, 118268 (2021)
Qayyum, A., Razzaq, A., Ahmad, M.Z., Jenks, M.A.: Water stress causes differential effects on germination indices, total soluble sugar and proline content in wheat (Triticum aestivum L.) genotypes. Afr. J. Biotech. 10, 14038–14045 (2011)
Shalaby, O.A.E.-S., Farag, R., Ibrahim, M.F.M.: Effect of hydrogen sulfide and hydrogen peroxide on growth, yield and nutrient content of broccoli plants grown under saline conditions. Sci. Horticult. 316, 112035 (2023)
Kodaolu, B., Mohammed, I., Gillespie, A.W., Audette, Y., Longstaffe, J.G.: Phosphorus availability and corn (Zea mays L.) response to application of P‐based commercial organic fertilizers to a calcareous soil. Soil Sci. Soc. Am. J. 87(6), 1386-1397 (2023)
Parton, W.J., Kelly, R.H., Hartman, M.D., Revallier, A., de Faria, A.B.B., Naves-Maschietto, G., Orvain, M., Houot, S., Albuquerque, M., Kech, S.: Agricultural and municipal organic waste amendments to increase soil organic carbon: How much, how often, and to what end? Soil Sci. Soc. Am. J. 87(4), 885–901 (2023)
Kim, Y.-J., Choo, B.-K., Cho, J.-Y.: Effect of gypsum and rice straw compost application on improvements of soil quality during desalination of reclaimed coastal tideland soils: ten years of long-term experiments. CATENA 156, 131–138 (2017)
Alghamdi, S.A., Alharby, H.F., Abdelfattah, M.A., Mohamed, I.A.A., Hakeem, K.R., Rady, M.M., Shaaban, A.: Spirulina platensis-inoculated humified compost boosts rhizosphere soil hydro-physico-chemical properties and Atriplex nummularia forage yield and quality in an arid saline calcareous soil. J. Soil Sci. Plant Nutr. 23(2), 2215–2236 (2023)
Meena, M.D., Narjary, B., Sheoran, P., Jat, H.S., Joshi, P.K., Chinchmalatpure, A.R.: Changes in physical and chemical properties of saline soil amended with municipal solid waste compost and chemical fertilizers in a mustard–pearl millet cropping system. Land Degrad. Dev. 33(10), 1677–1688 (2022)
Rahman, M.S., Schefe, C., Weatherley, A.: The combined addition of citric and aromatic organic acids to an acid soil prolongs phosphorus availability. Soil Sci. Soc. Am. J. 87(4), 797–807 (2023)
Ansari, M.F., Tipre, D.R., Dave, S.R.: Efficiency evaluation of commercial liquid biofertilizers for growth of Cicer aeritinum (chickpea) in pot and field study. Biocatal. Agric. Biotechnol. 4(1), 17–24 (2015)
Geng, C., Zhao, F., Niu, H., Zhang, J., Dong, H., Li, Z., Chen, H.: Enhancing the permeability, anti-biofouling performance and long-term stability of TFC nanofiltration membrane by imidazole-modified carboxylated graphene oxide/polyethersulfone substrate. J. Membr. Sci. 664, 121099 (2022)
Jie, C., Wenxiao, W., Jahan, M.S., Guo, S., Sun, J., Shu, S.: Improvement effects of conditioners on properties of acidified-salinized soils and lettuce growth. J. Plant Nutr. 45(6), 937–950 (2022)
Zhang, W.-W., Wang, C., Xue, R., Wang, L.-J.: Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 18(6), 1360–1368 (2019)
Funding
This work was financially supported by the Key Technology Research and Development Program of Shaanxi province (CN) (No. 2021KW-24) and National Natural Science Foundation of China (No. 21902127).
Author information
Authors and Affiliations
Contributions
Fang Feng: Writing-original draft, Formal analysis, Data curation, Validation, Investigation. Bin Jiang: Conceptualization, Writing-review & editing, Funding acquisition, Project administration. Banrui Yan: Writing-original draft, Formal analysis, Data curation, Validation, Investigation. Jiaxin Li: Writing-original draft, Validation, Data curation, Visualization. Shaik Firdoz: Writing – review & editing, Formal analysis.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, F., Jiang, B., Yan, B. et al. Optimization of Biofertilizers Derived from Vinegar Residues to Improve Soil Quality and Alleviate Salinization of the Land under Wheat Cultivation. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02712-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02712-z