Abstract
Plants have long been utilized as the primary source of medicines, providing conventional and effective treatment for a variety of ailments. The purpose of this study is to investigate the antidiabetic, antioxidant and antimicrobial properties from Pistacia lentiscus L. black fruits (PBF) and Clematis flammula L. leaves (CFL) phytochemicals extracted by choline chloride-acetic acid (ChCl-Acet) deep eutectic solvent (DES) and characterized using LC-ESI/MS. According to our findings, PBF and CFL both provide antioxidant activity in the TBARS assay (72.80± 9.67% and 81.32± 1.27% respectively). Moreover, β-Carotene Bleaching Assay showed that CFL had a better antioxidant potential (54.49± 0.91%) than PBF (60.99 ± 1.34%) (P = 0.0086). The data for the albumin denaturation activity were reversed, with CFL showing stronger anti-inflammatory activity (81.46± 1.87%) than PBF (69.68± 0.54%) (p < 0.05). Furthermore, PBF outperformed CFL in terms of antidiabetic effect (64.03± 1.21%) against α-amylase (p < 0.05). CFL and PBF samples demonstrated a good inhibition zone against pathogenic bacteria as well, although CFL showed larger inhibition zones than of PBF against both Staphylococcus aureus and Salmonella enterica. In addition, LC-ESI/MS analysis confirmed the antioxidant content of phenolic-based bioactive molecules for both extracts and identifying 17 and 12 compounds for BPF and CFL respectively. In conclusion, this study emphasis the significance of DES as an efficient and environmentally friendly approach for the extraction and the valorization of natural biomass waste for pharmaceutical and medical applications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
This paper is the first to study the utilization of combined DES-UAE green approach for in-vitro antidiabetic activity investigation using CFL and PBF.
Introduction
Plants form the basis of sophisticated traditional medicine which have existed for thousands of years and continue till nowadays, to provide new treatments for the man-kind. On the testimony of this, natural plant-based products and their derivatives are in clinical use world widely [1, 2]. Plants rich antioxidant content which is mainly consist of phenolic compounds, is characterized by the presence of one or many aromatic rings [2]. These molecules are not only indispensable elements for plants growth, in anatomical and morphological terms, but also possess high pharmaceutical value [3]. Phenolic compounds including phenolic acids and flavonoids are plants antioxidants that promote health benefits conferring anti-aging, anti-inflammatory and anti-proliferative protection [4]. Also, polyphenol-rich products can modulate lipid metabolism, attenuate dyslipidemia and insulin resistance by counteracting oxidative stress [5].
On the other hand, oxidative stress seems to be the core in many diseases such as cardiovascular diseases (CVD), cancer, neurodegenerative diseases and diabetes (type 1,2) [6]. Furthermore, it has been recently reported that the development of diseases complications may result to the insufficient effectiveness of pharmacotherapy of synthetic drugs [7]. For instance, in diabetes treatment α-amylase inhibitors are most warranted since they increase post-prandial hyperglycemic conditions. However, previous studies have shown that the chemical constituents extracted from plants can block the absorption of amylase in the treatment of diabetes [8]. In addition, infectious diseases caused by pathogenic bacteria have important impact on public health due to their high mortality rates [9]. Besides bacterial infection and intoxication can become more sever with more complex treatment for the health recuperation [9]. Yet, previous reports, have highlighted the effectiveness of phenolic compounds extracted from medicinal plants against various types of bacteria [5]. On this ground, there is an increasing attention towards revisiting plants for drug discovery, proving their role as popular remedies to diseases.
Pistacia lentiscus L. (PL) is a medicinal plant belonging to the Anacardiaceae family also known as ‘mastic tree’ or ‘lentisk’. It is widely distributed in the Mediterranean basin to central Asia [10]. PL is known for its aromatic resin which has traditionally been used as food [11]. The medicinal value of PL wood and leaves covers a wide range of diseases mainly including inflammatory processes and infections [10]. Also, PL fruits were used in folk medicine as anti-oxidant, antimicrobial, antidiabetic and antitumor agent [11]. Clematis flammula L. (CF) belongs to the Rununculaceae family widely used in folk medicine, clematis species are particularly used to treat wounds and ulcers [12]. It is distributed in the temperate region of Europe and Asia as well as the high tropics [12]. Deep eutectic solvents (DES) offer the advantage to be tailored in physicochemical properties, such as viscosities, pH, and polarities, by varying hydrogen bond donors. Their application in the extraction of phenolic compounds proved to improve extraction yields. The aim of this study is to explore in vitro, the anti-diabetic, anti-oxidant and anti-microbial potential of phytochemicals extracted from Clematis flammula L leaves and Pistacia lentiscus L. fruits using ChCl-Acet deep eutectic solvent for the very first time. In addition, this study has been designed test the antimicrobial activity toward pathogenic bacteria. An array of biochemical assays was used to prove their antioxidant, anti-diabetic and antimicrobial activities while an assay based on LC-(ESI)/MS confirmed their rich antioxidant phenolic-based content.
Materials and Methods
Apparatus
UV-Visible spectrophotometer (Ultrospec 2100 PRO), refrigerated centrifuge (SIGMA 2–16 PK), Ultrasound (Tierratech LT-100 PRO, 100 W), Balance (AS220/CI2), water bath (Mammert GmbH), Microplate reader (Synergy-HTX, Biotek).
Chemical Products
Choline chloride (C5H14CINO) (> 98.0%), Acetic acid (C2H3O2) (> 98.0%) β-Carotene (C40H56) (> 93%), Chloroform (CHCl3) (> 99%), Linoleic acid (C18H32O2), Iron (II) sulfate (FeSO4) (> 99%), Trichloroacetic acid (TCA) (C2HCl3O2) (> 99%), Thiobarbituric acid (TBA) (C4H4N2O2S) (99%) were purchased from Sigma Aldrich. α-amylase bacterial was purchased from Fisher chemical, 3-5-Dinitrosalicylic acid C7H4N2O7 (97%) was purchased from Thermo Scientific, Monopotassium phosphate (KH2PO4) (> 99%), Potassium dihydrogen phosphate (K2HPO4) (> 99%) were purchased from Chembiotin. Bovine Serum Albumin (BSA) (> 96%) was purchased from Biochem Chemopharma. (USA). Hydrochloric Acid (HCl) (37%), Sodium Hydroxide (NaOH) (96%), Tris(hydroxymethyl)aminomethane (Tris-HCl) (99%) were purchased from Chimoza (France). Muller Hinton Agar (M173-500G) was Purchased from HIMEDIA. Muller Hinton Broth (70192-500G) was purchased from FLUKA Analytical.
Plant Material
Clematis flammula L. leaves (CFL) and Pistacia lentiscus L. black fruits (PBF) were harvested from the forest of tizi Neftah and Azru’n bechar province of Amizour, Bejaia, Algeria for CF and PBF respectively. The botanical identification was confirmed according to a voucher herbarium specimen deposited in the National Institute of Agronomy Algiers, Algeria (N° 114 − 13) and (N° 970,704) for CF and PL respectively. The fruits of PL and the leaves of CF were air-dried at 30 °C in the dark, then crushed with an electric grinder and sieved to get a fine powder of (< 63 μm).
Preparation of the Deep Eutectic Solvent (DES)
DES was synthetized by the addition of choline chloride as hydrogen bound acceptor (HBA) and Acetic acid as hydrogen bound donor (HBD) at a ratio of 1:2 (Mole/Mole). The mixture was kept under constant stirring at 80 °C until a homogenous transparent liquid formed [13].
Extraction of Target Compound from CFL and PBF
Briefly, 500 mg of PBF and CFL dried powders were introduced into a flask, and 10 mL DES. The flask was placed in an ultrasonic bath (Tierratech LT-100 PRO, 100 W). The optimization of extraction parameters using Box Bhenken Design (BBD) and Response Surface Methodology (RSM) was previously investigated [14], [15].
The Assessment of the Biochemical Activities
Antioxidant Activity
Lipid Peroxidation Inhibition Assay
The thiobarbituric acid test is a quantitative assay for the detection of MDA, and is the most widely used technique to determine lipid peroxidation products [16]. In this assay, egg yolk homogenate (0.25ul, 10% in distilled water, (v/v) and 0.05 ml of extract and its dilutions were mixed in test tube, the volume was made up to 0.5 ml with distilled water.
0.025 ml FeSO4 (0.07 M) was added to the mixture and incubated for about 30 min in order to induce lipid peroxidation. After that, 0.75 ml of 20% acetic acid (PH = 3.4 adjusted with NaOH) and 0.75 ml of 0.8% TBA (w/v) (prepared in SDS 1.1%) and 0.025 ml of 20% TCA were added, the mixture was vortexed and heated in boiling water for 1 h. After cooling, 3ml of 1-butanol to each tube was added and then centrifuged at 3000 rpm for 10 min. the absorbance of the organic layer was measured at 532 nm.
where A0 was the absorbance of the blank control and A1 was the absorbance of the extracts sample.
β-Carotene Bleaching Assay
This assay was carried out with minor modifications as described by [17]. 1.0 mg of β-Carotene was dissolved in 2 ml of chloroform, followed by 25 ml of linoleic acid and 200 mg of tween. The chloroform was evaporated under vacuum at 40 °C, then an appropriate amount of distilled water was added in order to achieve an absorbance of 0,8 at 460 nm.
The extract and its dilutions were mixed with 100 µl of β-Carotene/ linoleic acid emulsions in each of the 96-well microplate. The absorbance was measured at 460 nm after incubation at 55 °C. All samples were read immediately (t = 0 min) and after 120 min of incubation.
Antioxidant activity was calculated as percentage of inhibition as follow:
where: At0: the absorbance of the sample at T = 0 min, At120: absorbance of the sample at T = 120 min, Acto: absorbance of the control at T = 0, Act120: absorbance of the control at T = 120 min).
In-vitro Anti-Inflammatory Activity
Albumin Denaturation Inhibitory Activity
Briefly, 2 ml of 1% aqueous BSA solutions containing the extract and its dilutions were incubated at 37 °C for 15 min before being heat treated for 5 min at 70 °C [18]. Turbidity was measured at 660 nm to express the percentage of protein denaturation using the following equation:
where At is the absorbance of test sample A0 is the absorbance in the absence of plant extract.
The In-Vitro Antidiabetic Effect
α-Amylase Inhibition Activity
The ability of plants extracts on α-amylase was also evaluated according to [19]. Briefly, 100 µL of the extracts, the same volume of sodium phosphate (0.02 M, pH: 6.9) and 10 µL of amylase enzyme solution (0.5 mg/mL) were incubated in a test tube at room temperature for 10 min. After that, 100 µL of 1% starch in sodium phosphate buffer (0.02 M, pH: 6.9) was added. The reaction mixtures were also incubated for another 10 min. the mixture was quenched by the addition of 100 µL dinitrosalicylic acid color reagent and boiled in a water bath until a yellowish orange color was developed. After cooling, the mixture was diluted with 5 mL of distilled water [19]. The absorbance at 540 nm was measured using a spectrophotometer.Where: Ac: is the control (acarbose), A0: is the blank solution (solvent alone) and As: the sample. The inhibition percentage (%) was measured as follow:
The Antimicrobial Effect
PBF and CFL extracts were tested for antimicrobial activity against Staphylococcus aureus, Bacillus subtilis (gram+), Klebsiella pneumoniae and Salmonella enterica (gram−). Using a platinum ance, the bacteria strains were transferred to the surface of nutritive gelose plates. They were left for 24 h at 37 °C to grow. The colonies were suspended in physiological water and monitored according to McFarland norms. After wells were created, the strains were transferred onto a Muller Hinton agar plate using a swab. About 50µL of CFL and PBF (50 mg/mL) extract were deposed in the wells and incubated for 1 h at 4 °C to allow time for diffusion and then incubated overnight at 37 °C. The control well contained only DES solvent, while tetracycline and ciprofloxacin were used as positive controls. After incubation, the inhibition zones around the wells were measured using a caliper. Furthermore, the minimum inhibitory concentration (MIC) was determined as the lowest concentration that ended with no growth.
Identification of Bioactive Molecules Using LC-ESI/MS
The analysis was based on LC-ESI single quadrupole MS (Shimadzu, Kyoto, Japan) using a modified method [20] to assess the profile of the bioactive metabolites in plant extracts. Briefly, the LC was performed on the reversed phase SyncronisTM C-18 column 150 × 4.6 mm; particle size: 5 μm (Thermo Fisher ScientificTM, Waltham, Massachusetts USA).
With a flow rate of 0.8 mL/min. The system was operated in gradient mode at a temperature of 45 °C. The mobile phase consisted of 50% acetonitrile (ACN) with 50% ddH2O (solvent A) and 95% dH2O, 5% MeOH and 0.2% acetic acid (solvent B). A linear gradient according to: 0-20 min 20% B, 20–40 min 55% B,40-55 min 100%B,55-65 min 100%B, 66-75 min 10%B.
The method was employed in both positive and negative detection mode to be able to detect metabolites such as phenolic acids, flavonoids, tannins and other compounds. The identification was based on the retention times and overlay curves. Phenolic compounds were identified by comparison with retention time of the standards of phenolic compounds [20]. The results were expresses as the mean value ± SD of three measurements.
Statistical Analysis
Data are presented as means ± S.D. of three independent experiments. The two-tailed unpaired t-Test at a 95% level of significance was used to evaluate for statistical differences. Statistical analysis was performed using GraphPad Prism version 8.0 for MacOSX (GraphPad Software, La Jolla California USA, www.graphpad.com.
Results and Discussion
In this study, we used eutectic solvent and the whole process was based on green chemistry principles.
The Antioxidant Activity
Antioxidants compete to reduce free radicals, no single method is considered as sufficient to take into account the various modes of action of antioxidants. Therefore, more than one type of antioxidant capacity measurement was performed in this study.
Lipid Peroxidation Measurement Using TBARS Method
This method is a widely used assay to estimate lipid peroxidation in membranes as well as in biological systems. As it is shown in Fig. 1 CFL and PBF showed a good but not statistically significant inhibition of lipid peroxidation (72.80± 9.67%) and (81.32± 1.27%) (P = 0.4169) respectively. Lipid peroxidation is an oxidative chain reaction begun by free radicals in which one lipid molecule after another is oxidized to the highest extent possible, resulting in the formation of lipid peroxide. This chain reaction is normally ended when the substrate is depleted. Other conditions include the reaction of two radicals to generate a non-radical product or the reaction of two radicals with antioxidants, which provides easily donatable hydrogen for abstraction by peroxyl radicals. [21].
Hydroxyl radicals are generated when Fe2+ reacts with H2O2 which as a result, can participate in lipid peroxidation [22]. This process is mainly caused by ROS, the free radical chain reaction occurs in the biological membrane phosphatide and initiates propagation reactions leading to the damage of erythrocytes and therefore inducing hemolysis [23]. MDA is the most important indicator of lipid peroxidation that decreases the membrane fluidity by biding to proteins and creating cytotoxic aldehydes [23]. In this report, there was a dose-dependent relationship between the concentration of CFL and PBF extracts and the degree of inhibition. Also, a strong positive correlation exists between the total phenolic content and the antioxidant activity [24]. Durand et al. [25] evaluated the ROS (reactive oxygen species) inhibitory power of different antioxidants dissolved in DES to their activity when dissolved in organic solvents (either DMSO or ethanol). They concluded that the antioxidant formulation in DES could greatly improve their activity for ROS inhibition. Although, previously, many reports described the ability of DES to extract many classes of molecules in addition to phenolic compounds [26], [27], [28], which contribute to the overall antioxidant activity of both plants extracts.
β-Carotene Bleaching Assay
The β-carotene bleaching assay based on the reduction of the orange color intensity of β-carotene due to its reaction with peroxyl radical (LOO •) generated by linoleic acid in the presence of oxygen during incubation at 50 °C [29]. In the presence of an antioxidant compound, this degradation process is prevented which reflects the ability to inhibit the lipid peroxidation in-vitro. This antioxidant assay is widely used, because β-carotene is extremely susceptible to free radical-mediated oxidation. In this study, both CFL and PBF showed a good and statistically significant inhibition of linoleic acid oxidation (54.49± 0.91% and 60.99 ± 1.34%) (P = 0.0086) (see Fig. 2). If β-carotene is decomposed before its intake, their biological functions won’t be observed in the body since it shows a strong biological activity. Also, if it is used as a coloring agent for food, its discoloration would markedly reduce their quality. As a result, CFL and PBF DES-based extracts could be useful in preventing the discoloring of β-carotene and therefore, maintaining its biological activity.
Anti-Inflammatory Activity
Albumin Denaturation Inhibitory Activity
Protein denaturation is a process in which proteins loss their structure by physical or chemical factors, which leads to the loss of their biological function. One of the major causes of inflammatory arthritic diseases is the denaturation of proteins tissue resulting in the production of auto-antigens [30]). The development of anti-inflammatory drug that prevents protein denaturation is therefore necessary. Here we studied the ability of CFL and PBF to prevent protein denaturation. As the Fig. 3 shows, both plants extract showed a good and statistically significant inhibition of proteins denaturation 69.68± 0.54% and 81.46± 1.87% for PBF and CFL respectively (P = 0.0005). The adoption of the BSA denaturation assay for the in-vitro anti-inflammatory effect of DES extracts circumvented the ethical issues associated with the use of animals, since the DES is composed of choline chloride and acetic acid and it is an early stage of screening for DES-phytochemicals potential from CFL and PBF.
The In-vitro Antidiabetic Effect
Pancreatic α-amylase is a key enzyme in digestive system that breaks down starch into oligosaccharides, and is ultimately responsible for the release of glucose which will be absorbed in the bloodstream [31]. The inhibition of α-amylase will reduce starch breakdown and therefore, reduces postprandial hyperglycemia [31].
We studied the anti-diabetic potential of PL and CF phytochemicals against bacterial α-amylase. Various concentrations of PBF and CFL extracts were tested and as a result, PBF (64.03 ±1.21%) showed a statistically significant inhibitory effect on α-amylase activity (P < 0.05) compared to CFL which was proven to have a moderate activity toward α-amylase (44.08± 2.61%) (See Fig. 4). These results suggest that the hypoglycemic effect of PBF plant is due, at least in part, to the inhibition of the α-amylase according to previous reported results [32] that showed that PBF curbs diabetes by inhibiting the conversion of starch to glucose [32]. The search for natural bioactive compounds with low toxicity and presenting antioxidant capacities has gained prominence in the control and treatment of diabetes mellitus and its complications [6]. In this study, we reported an in-vitro antioxidant and antidiabetic potential of both CFL and PBF. The DES-based extract of both plants showed a good activity toward ROS and α-amylase, making them promising agents with interesting properties for the management of diabetes mellitus and its complications.
Antimicrobial Effect
The in-vitro antimicrobial activities of CFL and PBF phytochemicals against pathogenic bacteria were qualitatively assessed by the inhibition zones. The data obtained showed that DES-extract obtained from CFL and PBF inhibited the growth of Staphylococcus aureus, Bacillus subtilis (gram+), Klebsiella pneumoniae and Salmonella enterica (gram−) as it is shown in Fig. 5. The inhibition zone of CFL (49.33± 2.01 mm) was higher than that obtained by PBF (44.3± 1.10 mm) (P = 0.0285) and the controls used to include Tetra-cycline and Ciprofloxacine (10± 1.23 mm and 30± 3.05 mm respectively) (P < 0.0001) against Salmonella enterica. However, for Klebsiella pneumoniae and Bacillus subtilis, no significant difference was recorded for PBF (34.3± 0.9 mm, 39.6±0.7 mm respectively) and CFL (31± 2.06 mM, 46.3±3.12 mm) (P = 0.2488). However, both extracts demonstrated a higher activity compared to that obtained by Tetracycline (9± 1.1 mm) (P < 0.0001). Moreover, CFL showed a better antimicrobial activity against Staphylococcus aureus strains with an inhibition zones of 46± 1.10 compared to PBF (35.6± 1.24 mm) (P = 0.0434).
Choline chloride-acetic acid DES (Blank) was placed in wells to see if the antimicrobial action was attributable solely to the DES solvent, which contained acetic acid as an HBD. In comparison to the extract, the results revealed a narrower inhibition zones which proves that the phytochemicals extracted by ChCl-Acet had an anti-microbial activity. Furthermore, the minimum inhibitory concentrations (MCI) by microdilution method were also determined and as a result, both extracts showed a good MCI values against all strains used in this study (390 µg/mL). Phytochemicals and plant extracts have a promising role in therapeutic applications against multi-resistant bacteria. For instance, phyto-extracts obtained by DES-based extracts exhibited strong activities in this study. This is probably due to the use of UAE for phenolic compounds since it has been previously reported to improve the extraction efficiency and biological activity of plants extracts [33]. Also, according to available data, secondary metabolites flavonoids, tannins, and other phenolic compounds are responsible for antibacterial activity in higher plants [34]. Flavonoids are hydroxylated phenolic compounds that plants produce in response to microbial illness. Their antibacterial activity is most likely due to their ability to form complexes with extracellular and soluble proteins, as well as complexes with bacterial cell walls, resulting in membrane rupture [34].
LC-(ESI)/MS Analysis for Plant Extracts
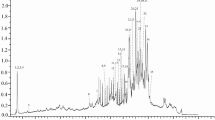
To understand further the bioactivity of the extracts we proceed on its phenolic content characterization. To identify the bioactive compounds of our samples LC-ESI/MS was carried out, in both negative and positive mode. The major antioxidant compounds of CFL and PBF were tentatively identified under analytical conditions as shown in Table 1. Our study is the first to detect phenolic compounds extracted from CFL and PBF by ChCl-Acet solvent.
As it is mentioned in Table 1 and Fig. 6, both CFL and PBF have shown richness in phenolic compounds including phenolic acids, flavonoids and conjugated tannins. The results of LC-MS demonstrated the presence of Caffeic acid (15.56 min, M/Z: 179.15−) Cinnamic acid (2.77 min, M/Z: 149.06−) ascorbic acid (2.52 min, M/Z: 175−) and citric acid (2.6 min, M/Z: 191−), Silymarin (47.62 min, M/Z: 481−), catechol (31.95, M/Z: 109−) and catechin (12.44, M/Z: 289.0) only in PBF. However, Chrysin (56.24 min, M/Z: 254.80+) and Coumaric acid (24.01 min, M/Z: 163−) were present in CFL and absent in PBF sample. Both DES extracts did not show the presence of tannic acid (M/Z: 1060.80) in their composition (Table 1). Phenolic compounds exhibit strong antioxidant properties due to their ability to scavenge free radicals, donation of free electrons and metal chelation. The antioxidant assays performed in this study demonstrated the efficiency of DES-PBF extract compared to CFL, which is explained by the richness of PBF in phenolic compounds compared to CFL. We can also say that the antioxidant activities of PBF is mainly due to the flavonoid groups, and phenolic acids which, some of them were absent in CFL extract.
For CFL, this is the first report to identify bioactive molecules using LC-MS extracted by DES. DES proved to extract a wider range of bioactive molecules compared to conventional solvents [35]. This is explained by its polarity and the hydrogen bond complexes between the solvent and the target molecules which is more important than that of ethanol [36]. The qualities of DES in general, as ionic liquids (ILs) equivalents in green chemistry, are particularly essential; they share characteristics such as very low vapor pressure, low cost of production, and ease of synthesis. Their prospective applications are determined by their physiochemical properties, which are influenced by intermolecular interactions between the components. Previously the physiochemical properties of ChCl-Acet DES were studied and optimized [37], [14], [15]). As a result, the extraction performances of the solvent were improved and the recovery rate for the low concentrations was more than 80% while for higher concentration was between 82 and 92% [38]. In addition, previously published results regarding the use of DES for the extraction of foue bioactive flavonoids from Pollen Typhae using UAE showed that the extration efficiency of choline chloride-Propan-diol (1:4) was significantly higher that of 75% ethanol for extraction of isorhamnetin (P < 0.05) [39]. Similar results were obtained with choline chloride based-DESs compared to 80% ethanol for the extraction of linarin from Chrysanthemum inducum L. flowers [40]. DES extraction approach used in this study is considered as simple, low-cost and efficient method that can be applied to the extraction and the isolation of natural products from biomaterials.
Conclusion
The results present in this work proved the importance of using DES combined to modern extraction techniques like UAE as an effective approach for the isolation of bioactive compounds from both Pistacia lenstiscus L. and Clematis flammula L. phyto-extracts. ChCl-Acet DES extracts of both samples showed good biological activities including antioxidant, antidiabetic and antimicrobial potential against pathogenic bacteria, Moreover, LC-ESI/MS analysis revealed the presence of important bioactive compounds that could be used in the cosmetics, medical and pharmaceutical industries. Further research will be needed to evaluate the bioactivity of specific phytochemicals in both plants extracts and explore the mechanism of antioxidant capacity as well as the in-vivo antidiabetic potentials.
Data Availability
All original data related to this study will be provided on demand.
Abbreviations
- BSA:
-
Bovin serum albumin
- CHCl-Acet:
-
Choline chloride-acetic acid
- CFL:
-
Clematis flammula L. leaves
- CVD:
-
Cardiovascular diseases
- DES:
-
Deep eutectic solvent
- LC(ESI)/MS:
-
Liquid chromatography-electrospray ionization tandem mass spectrometry
- MDA:
-
Malondialdehydes
- MIC:
-
Minimum inhibitory concentration
- PBF:
-
Pistacia lentiscus L. black fruits
- TBARs:
-
Thiobarbituric acid reactive substances
- UAE:
-
Ultrasound-assisted extraction
References
Aidi wannes, W., Marzouk, B.: Research progress of Tunisian medicinal plants used for acute diabetes. J. Acute Dis. 5(5), 357–363 (2016). https://doi.org/10.1016/j.joad.2016.08.001
Stagos, D.: Antioxidant activity of polyphenolic plant extracts. Antioxidant 9(1), 19 (2019). https://doi.org/10.3390/antiox9010019
Rasouli, H., Farzaei, M.H., Mansouri, K., Mohammadzadeh, S., Khodarahmi, R.: Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules 21(9), 1104 (2016). https://doi.org/10.3390/molecules21091104
Swallah, M.S., Sun, H., Affoh, R., Fu, H., Yu, H.: Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. (2020). https://doi.org/10.1155/2020/9081686
Ma, J., Zheng, Y., Tang, W., Yan, W., Nie, H., Fang, J., Liu, G.: Dietary polyphenols in lipid metabolism: A role of gut microbiome. Anim. Nutrit. 6(4), 404–409 (2020). https://doi.org/10.1016/j.aninu.2020.08.002
Justino, A.B., Miranda, N.C., Franco, R.R., Martins, M.M., Silva, N.M.D., Espindola, F.S.: Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against alpha-amylase, alpha-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 100, 83–92 (2018). https://doi.org/10.1016/j.biopha.2018.01.172
Singh, M., Thrimawithana, T., Shukla, R., Adhikari, B.: Managing obesity through natural polyphenols: A review. Fut. Food 1, 100002 (2020). https://doi.org/10.1016/j.fufo.2020.100002
Chongde, S.: Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 1(1), 18–44 (2020). https://doi.org/10.1002/fft2.15
Szabo, S., Feier, B., Capatina, D., Tertis, M., Cristea, C., Popa, A.: An overview of healthcare associated Infections and their detection methods caused by pathogen bacteria in Romania and europe. Clin. Med. 11(11), 3204 (2022). https://doi.org/10.3390/jcm11113204
Milia, E., Bullitta, S.M., Mastandrea, G., Szotakova, B., Schoubben, A., Langhansova, L., Quartu, M., Bortone, A., Erick, S.: Leaves and fruits preparations of pistacia lentiscus L.: a review on the enthnopharmacological uses and implications in inflammation and infection. Antibiotics 10(4), 425 (2021). https://doi.org/10.3390/antibiotics10040425
Pachi, V.K., Mikropoulou, E.V., Gkiouvetidis, P., Siafakas, K., Argyropoulou, A., Angelis, A., Mitakou, S., Halabalaki, M.: Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): a review. J. Ethnopharmacol. (2020). https://doi.org/10.1016/j.jep.2019.112485
Saidi, R., Ghrab, F., Kallel, R., Feki, A.E., Boudawara, T., Chesne, C., Ammar, E., Jarraya, R.M.: Tunisian Clematis flammula essential oil enhances Wound Healing: GC-MS analysis, biochemical and histological Assessment. J. Oleo Sci. 67,11, 1483–1499 (2018). https://doi.org/10.5650/jos.ess18056
He, X., Yang, J., Huang, Y., Zhang, Y., Wan, H., Li, C.: Green and Efficient Ultrasonic-Assisted Extraction of Bioactive Components from Salvia miltiorrhiza by Natural Deep Eutectic Solvents. Molecules 25(1), 40 (2019). https://doi.org/10.3390/molecules25010140
Tebbi, S.O., Debbache-Benaida, N., Moulaoui, K., Zaidi, S., Kadi, R.: Optimized ultrasonic-assisted deep eutectic solvents extraction of Clematis flammula L. leaves, phytochemical screening, biological activities and the characterization of its volatile compounds. Biomass Convers. Biorefin. (2022). https://doi.org/10.1007/s13399-022-03585-9
Tebbi, S.O., Debbache-Benaida, N., Kadri, N., Kadi, R., Zaidi, S.: A novel strategy to improve the recovery of phenolic compounds from pistacia lentiscus L. fruits using design based statistical modeling for ultrasound deep eutectic solvents extraction and the evaluation of their antioxidant potential. Sustain. chem. pharm. 31, 100933 (2023). https://doi.org/10.1016/j.scp.2022.100933
De Leon, A.D., Borges, J.: Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 159, e61122 (2020). https://doi.org/10.3791/61122
Koleva, I.I., Van Beek, T.A., Linssen, J.P., De Groot, A., Evstatieva, L.N.: Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal. 13(1), 8–17 (2002). https://doi.org/10.1002/pca.611
Osman, N.I., Sidik, N.J., Awal, A., Adam, N.A., Rezali, N.I.: In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult. Ethnopharmacol. 5(4), 343–349 (2016)
Yu, Z., Yin, Y., Zhao, W., Liu, J., Chen, F.: Anti-diabetic activity peptides from albumin against alpha-glucosidase and alpha-amylase. Food Chem. 135,3, 2078–2085 (2012). https://doi.org/10.1016/j.foodchem.2012.06.088
Gueboudji, Z., Kadi, K., Nagaz, K., Addad, D., Secrafi, M., Yahya, L.B., Lachehib, B.: Phenolic compounds and biological activities of phenolic extract of olive oil mill wastewater issue from the cold extraction of olive oil from khenchela (algeria). Res. sq. (2021). https://doi.org/10.21203/rs.3.rs-360101/v1
do Cavalcante, N., Lima, A., Araujo, L.K.F., Da Silva Santos, C.M., Nascimento, F.P.D., De Castro, M.O., Rai, E.S.J.M., Feitosa, M.: Toxicity, cytotoxicity, mutagenicity and in vitro antioxidant models of 2-oleyl-1,3-dipalmitoyl-glycerol isolated from the hexane extract of Platonia insignis MART seeds. Toxicol. Rep. 7, 209–216 (2020). https://doi.org/10.1016/j.toxrep.2020.01.014
Forrester, S.J., Kikuchi, D.S., Hernandes, M.S., Xu, Q.: Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 122(6), 877–890 (2018)
Ji, M., Gong, X., Li, X., Wang, C., Li, M.: Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species- A review. Molecules. 25(4), 917 (2020). https://doi.org/10.3390/molecules25040917
Gonçalves, S., Gomes, D., Costa, P., Romano, A.: The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind. Crops Prod. 43, 465–43471 (2013). https://doi.org/10.1016/j.indcrop.2012.07.066
Durand, E., Lecomte, J., Upasani, R., Chabi, B., Bayrasy, C., Barea, B., Jublanc, E., Clarke, M.J., Moore, D.J., Crowther, J., et al.: Evaluation of the ROS inhibiting activity and mitochondrial target- ing of phenolic compounds in fibroblast cells model system and enhancement of efficiency by natural deep eutectic solvent (NADES) formulation. Pharm. Res. 34(5), 1134–1146 (2017). 10.1007/ s11095-017-2124-4
Tommasi, E., Cravotto, G., Galletti, P., Grillo, G., Mazzotti, M., Sacchetti, G., Samorì, C., Tabasso, S., Tacchini, M., Tagliavini, E.: Enhanced and selective lipid extraction from the MicroalgaP. Tricornutumby Dimethyl carbonate and supercritical CO2Using Deep Eutectic solvents and microwaves as pretreatment. ACS Sustain. Chem. Eng. 5(9), 8316–8322 (2017). https://doi.org/10.1021/acssuschemeng.7b02074
Alam, M.A., Muhammad, G., Khan, M.N., Mofijur, M., Lv, Y., Xiong, W., Xu, J.: Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 309, 127445 (2021). https://doi.org/10.1016/j.jclepro.2021.127445
Tang, Y., He, X., Sun, J., Liu, G., Li, C., Li, L., Sheng, J., Zhou, Z., Xin, M., Ling, D., Yi, P., Zheng, F., Li, J., Li, Z., Yang, Y., Tang, J., Chen, X.: Comprehensive evaluation on tailor-made deep eutectic solvents (DESs) in extracting tea saponins from seed pomace of Camellia Oleifera Abel. Food Chem. (2021). https://doi.org/10.1016/j.foodchem.2020.128243
Fawwaz, M., Pratama, M., Hasrawati, A., Sukmawati., Widiastuti, H., Abidin, R., Z: Total carotenoids, antioxidant and Anticancer Effect of Penaeus monodon Shells Extract. Biointerface Res. Appl. Chem. 11, 4: 11293–11302 (2020). https://doi.org/10.33263/briac114.1129311302
Marrelli, M., Amodeo, V., Viscardi, F., De luca, M., Statti, G., Conforti, F.: Essential oils of foeniculum vulgare subsp. piperitum and their in-vitro anti-arthiric potential. Chem. Biodiv. (2020). https://doi.org/10.1002/cbdv.202000388
Saravanan, S., Parimelazhagan, T.: In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenls of passiflora ligularis juss. Fruit pulp. Food sci. hum. Welln. 3(2), 56–64 (2014)
Mehenni, C., Atmani-Kilani, D., Dumarcay, S., Perrin, D., Gerardin, P., Atmani, D.: Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J. Food Drug Anal. 24(3), 653–669 (2016). https://doi.org/10.1016/j.jfda.2016.03.002
Kumar, K., Srivastav, S., Sharanagat, V.S.: Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultras. Sonochem. (2021). https://doi.org/10.1016/j.ultsonch.2020.105325
Mishra, A., Sharma, A.K., Kumar, S., Saxena, A.K., Pandey, A.K.: Bauhinia variegata leaf extract exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res. Int. (2013). https://doi.org/10.1155/2013/915436
Abbott, A.P., Al-Murshedi, A.Y.M.: Thermodynamics of phase transfer for polar molecules from alkanes to deep eutectic solvents. Fluid. Pha. Equil. 448, 99–104 (2017). https://doi.org/10.1016/j.fluid.2017.05.008
Dai, Y., witkamp, G.J., Choi, R. YH: Natural deep eutectic solvents as a new extraction media for phenolic metabolitesin carthamus tinctorius L. Anal. Chem. 13, 6272–6278 (2013). https://doi.org/10.1021/ac400432p
de Almeida Pontes, P.V., Ayumi Shiwaku, I., Maximo, G.J., Caldas Batista, E.A.: Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 352, 129346 (2021). https://doi.org/10.1016/j.foodchem
Letsiou, S., Trapali, M., Tebbi, S.O., Debbache-benaida, N.: A simple and robust LC-ESI single quadrupole MS-based method to analyze polyphenols in plant extracts using deep eutectic solvents. MethodsX 11, 102303 (2023). https://doi.org/10.1016/j.mex.2023.102303
Meng, Z., Zhao, J., Duan, H., Guan, Y., Zhao, L.: Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 161, 246–253 (2018). https://doi.org/10.1016/j.jpba.2018.08.048
Guo, N., Zou, Y., Li, H., Kou, P., Liu, Z., Fu, Y.: Effective extraction and recovery of linarin from Chrysanthemum indicum L. flower using deep eutectic solvent. Microchem 159, 105586 (2020). https://doi.org/10.1016/j.microc.2020.105586
Acknowledgements
The authors are thankful for the valuable comments of reviewers, which help greatly improve the manuscript.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
We confirm that there are no conflicts of interest associated with this publication.
Consent to Publish
All authors of this paper are aware of the submission and agree to its publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tebbi, S.O., Trapali, M. & Letsiou, S. Exploring the Anti-Diabetic, Antioxidant and Anti-Microbial Properties of Clematis flammula L. Leaves and Pistacia lentiscus L. Fruits Using Choline Chloride-Based Deep Eutectic Solvent. Waste Biomass Valor 15, 2869–2879 (2024). https://doi.org/10.1007/s12649-023-02360-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02360-9