Abstract
The degradation of organic dye contaminants provides a promising approach for mitigating pollutants and addressing wastewater treatment. Despite the extensive research and development of various photocatalysts with the objective of effectively degrading organic pollutants, the challenge still persists. This paper introduces a facile and sustainable technique for synthesizing silver nanoparticles (AgNPs) using kiwi fruit peel, as a bio-waste resource. A comprehensive analysis of the AgNPs was conducted using microscopic and spectroscopic techniques. The observation of a color change and measurement of UV–vis absorbance at 435 nm confirmed the development of AgNPs. FE-SEM examination demonstrated that AgNPs have homogeneous distribution of cubic structure with 10 to 70 nm. The AgNPs demonstrated photocatalytic degradation potential of 94.2% for Congo red (CR) under visible light irradiation. Additionally, synthesized AgNPs exhibited concentration depended bactericidal activity. In conclusion, as synthesized AgNPs can efficiently purify wastewater and suppress pathogens due to their strong degrading activity, reusability, and antibacterial actions.
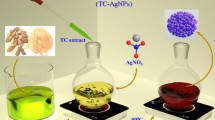
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Novelty Statement
A new and urgent need for the scientific community globally is the development of simple, and inexpensive method to synthesize effective nanoparticles using biowaste resources, as a conversion of waste to best concept. Current study aimed on the preparation of environment friendly AgNPs using kiwi fruit peel. Synthesis was confirmed through the utilization of spectroscopic and microscopic techniques. Additionally, AgNPs demonstrated a promising capabilities in terms of photocatalytic dye degradation and antibacterial activity.
Introduction
In the realm of environmental sustainability and wastewater management, innovative approaches are continually sought to address pollution and enhance water purification [1]. Different methods have been effectively employed to address the contamination caused by organic dyes into the aqueous environment [2,3,4,5]. These techniques include absorption, fenton-oxidation, and photocatalytic degradation [6]. One notable method among them is the employment of metal nanoparticles for photocatalytic reduction [7]. This approach is highly efficient owing to the robust reduction capability, exceptional degradation efficacy, quick response rate, and wide applicability in the degradation of toxic dye molecules [8, 9]. AgNPs have garnered significant focus on the field of catalysis due to their distinctive physiochemical characteristics, manageable size, and non-toxic characteristics [10, 11]. These distinctive characteristics of AgNPs, which exhibit a dimensions ranging from 1 to 100 nm, has gained considerable attention owing to their wide-ranging properties encompassing electrical, optical, and surface characteristics [12, 13].
Generally, physical and chemical processes can be used to produce AgNPs. Nevertheless, these conventional methods are often accompanied by substantial expenses and environmental concerns due to the generation of harmful byproducts and the use of harmful chemicals [14]. The importance of greener preparation of AgNPs lies in its environmentally conscious approach, which helps to reduce the potential risks associated with conventional chemical and physical synthesis methods [15,16,17]. Currently, there has been improvements in the advance of safe and eco-friendly green synthesis method for producing AgNPs [18, 19]. These methods involve the utilization of microbes and plants [20]. Phyto-mediated synthesis are gaining more and more popular because of all of its benefits. These include its non-toxic nature, eco-friendly characteristics, and cost-effectiveness [7, 21]. In contrast, microorganisms necessitate aseptic conditions for cell culture, making them less desirable [22]. Plants are commonly employed as a reductants in the preparation of AgNPs due to their rich composition of molecule compounds, including proteins, flavonoids, polyphenols, and other phytochemicals [23,24,25].
Several plant species have been employed for the preparation of AgNPs such as Theobroma cacao [26], Mentha spicata [27], Coffee arabica [28] and many more has been reported. Recently, Thomas and Thalla et al. [29] reported that the Myristica fragrans seed shell mediated green synthesized AgNPs exhibited better photocatalytic activity of ramazol brilliant blue, rhodamine B, and methyl violet under UV light [29]. AgNPs synthesized using Antidesma acidum leaf demonstrated efficient and rapid catalytic removal of congo red and methylene blue [30]. Moreover, AgNPs have the capability to effectively reduce pathogen development in the skin and wounds, as well as inhibit bacterial colonization on various device surfaces [31]. Green synthesized AgNPs using Coriandrum sativum extract displayed remarkably zone of inhibition against several pathogens [32]. An investigation conducted by Hashemi et al. [33] who have observed that AgNPs produced using Mentha pulegium and Crocus caspius extracts displayed significant antibacterial properties against E. faecalis, S. aureus, A. baumannii, P. aeruginosa, E. coli, K. pneumoniae, and P. mirabilis.
However, the utilization of food peel/kernel/pericarp waste for the preparation of AgNPs have been acknowledged as a safe, sustainable, and eco-friendly technique, when compared to conventional synthesis procedures [34]. In this study, we have used kiwi fruit peel waste as a synthesizing agent to produce AgNPs. The proposal is to implement a waste conversion system that aims to transform waste into valuable resources. This process would effectively reduce the overall volume of waste by converting it into useful products or resources [35, 36]. This study presents findings that highlight the potential of utilizing waste of kiwi fruit peel as a reductant for the eco-friendly preparation of AgNPs. The synthesized AgNPs have demonstrated promising capabilities for photocatalytic dye degradation and bactericidal applications.
Experimental
Materials
Silver nitrate, Congo red, Muller–Hinton agar (MHA) were acquired from HiMedia. Fresh fruits of kiwi were attained from a local market in Coimbatore.
Preparation of Kiwi Peel Extract
To acquire the peel extract, the fruits underwent a process of washing and drying before being peeled thinly. The peels were subsequently placed in a food dehydrator for 12 h until fully dried. Afterward, they were ground into a moderately fine powder. Next, 1 g of the powder was placed into a 50 mL beaker containing distilled water and continuously stirred for 5 h. After maceration, the mixture was transferred to a water bath set at 60 °C for 2 h. Following that, the mixture underwent filtration, and the resulting extracts were carefully preserved in an argon atmosphere for future use. The moisture content analysis was given in supplementary file.
Synthesis of AgNPs
Briefly, 10 mL of prepared peel extract was gradually mixed to a 90 mL of solution containing 1 mM silver nitrate. This mixture was subsequently kept at 27 °C for 3 h. The identification of synthesized AgNPs in the reaction mixture was initially observed through the change in color of the medium from yellow to brown. Then, formed AgNPs were purified by centrifugation at 12,000 rpm for 15 min.
Characterization of AgNPs
Optical Property
The UV–vis spectroscopy (JASCO-V530, Japan) was employed for the initial characterization of AgNPs synthesized by green method. The absorption of UV–vis within the wavelength range of 400–800 nm has been recorded.
FTIR Analysis
The several functional molecules, which are accountable for the formation of AgNPs have explored by IR analysis. This studies was conducted using a FTIR-00585, PerkinElmer spectrophotometer with a diffuse reflectance mode and performed in 400–4000 cm−1 band at a resolution of 4 cm−1 by ATR mode.
FE-SEM Analysis
Structure of the prepared AgNPs was examined by utilizing a FE-SEM (JEOL-Model JSM 6390) equipped with energy-dispersive X-ray spectroscopy (EDS). Before to detection, a thin film of platinum was coated to the sample by a sputter-coating technique.
Photocatalytic Activity
Photocatalytic assay was examined for the removal of CR in the aqueous system using AgNPs under visible light irradiation (Xe lamp with a power of 300 W). For the photocatalysis, synthesized AgNPs was introduced into a solution containing 5 ppm of CR. Prior to the exposure of light, the dye and AgNPs were thoroughly mixed in dark environment for 1 h. Subsequently, the resulting solution was subjected to light exposure. During a specific irradiation period, few aliquots of CR were removed from the dye-AgNPs mixture by centrifuging at 8,000 rpm for 5 min and subjected to absorbance analysis using UV–vis spectroscopy. The photodegradation percentage was determined by the Eq. (1),
The concentration of dye before degradation is denoted as C1, while the concentration of dye after a specific period of degradation is represented as C0.
Antibacterial Experiment
The pathogenic strains namely E. coli and S. aureus were obtained from a private microbiology center, coimbatore. According to Yousefzadeh-Valendeh et al. [37], the disc diffusion antibacterial assay was carried out. Protocol for generating agar plates involves meticulously transferring 20 mL of sterile MHA liquid into a sterile petri dish. Once the agar has solidified, a specific amount of bacterial suspension (105 CFU/mL) was evenly spreaded across the surface of the plates. The sterile discs (6 mm) were loaded with DMSO containing 10, 20 and 50 µg/mL of AgNPs and placed on solidified plates. Subsequently, the plates were kept in an incubator for 24 h at 37 °C. Then, the activity was assessed by measuring the zone of inhibition (ZOI) against the tested bacterial strains.
Results and Discussion
Formation of AgNPs
The process of AgNPs synthesis was conducted using kiwi fruit peels. The observation of a transition in color from light yellow to brown provided initial confirmation for the effective development of AgNPs. During the formation, bio-active functional components present in the aqueous peel extract might worked as a reductant for the synthesis of AgNPs [38].
Food waste is a prevalent global problem that not only results in monetary losses but also poses significant health and environmental concerns. There are multiple strategies that can be employed to address this matter, including enhancing food supply chain management, educating consumers on food protection method, and repurposing food waste to create new products. Moreover, fruit waste possesses significant potential as a treasured reservoir of bioactive compounds, which can be effectively utilized in a wide range of applications [39]. It is crucial to acknowledge that fruit and vegetable waste may potentially include hazardous substances, such as heavy metal, pesticides, and depending on the specific production and processing methods employed. It is worth mentioning that fruit and vegetable waste possesses bio-actives, which are able to act as a reductant, leading to the production of NPs. These molecules possess certain capability to attract with metal ions and facilitate their reduction, leading to the formation of NPs that exhibit unique properties [40].
Characterization of AgNPs
The UV–vis spectrum of AgNPs demonstrated the occurrence of an absorbance band at 435 nm, which demonstrating the successful production of AgNPs (Fig. 1a). Based on the previous reports, AgNPs exhibits a distinct property of UV-absorbance peak within the range of 400–480 nm. In a similar manner, AgNPs that were synthesized using W. arborea exhibited the UV-absorbance at 417 nm [41]. The UV–vis spectrum of AgNPs synthesized using Potentilla fulgens displayed an absorbance in the range of 400–450 nm [42]. A study by Ghatage et al. [43], reported that synthesized AgNPs using leaves of Aloe barbadensis miller showed UV absorbance peak at 439 nm.
Figure 1b displays the FTIR examination of AgNPs. FTIR study of synthesized AgNPs demonstrated the presence of several characteristic bands, including a distinctive N–H peak at 1632 cm−1, C=C vibration at 2115 cm−1, O–H bending at 3326 cm−1, and C–I band at 633 cm−1. The biowaste of kiwi fruit peel is a potential source for the N–H, C=C, O–H, and C–I functional groups that were found to have been acquired. The presence of these bioactive derivatives may function as a reductant, which is a necessary step in the production of AgNPs [25, 44].
Figure 2a–d depict the FE-SEM images of synthesized AgNPs. FE-SEM revealed the AgNPs have a cubic morphology, and the average size typically ranges from 10 to 70 nm. Recently, Lakkim et al. [45] observed that the AgNPs synthesized by green method displayed cubic shape with size ranging from 48 to 67 nm. Another investigation conducted by Roddu et al. [46] observed the AgNPs synthesized using Abelmoschus esculentus displayed a cubic morphology.
Additionally, EDS was conducted to investigate the elemental composition of AgNPs as shown in Fig. 2e. The EDS of AgNPs displayed a distinct peak of Ag, which providing the conformation for the synthesis of AgNPs [47]. Additional peaks including C, N, and O were observed as a result of X-ray emissions raised from the bio-compounds present in the kiwi fruit peel. The elemental configuration of the synthesized AgNPs were presented in Table 1. Nandana et al. [48] documented the occurrence of Ag, O, N, and C in the CS/Ag nanocomposite prepared through green method.
Photocatalytic Dye Degradation
CR dye molecule was used to assess the AgNPs’ capacity to degrade dye by using visible light irradiation approach. At various time intervals (0–30 min), the absorbance spectra of CR dye molecule degraded by AgNPs were measured. The degradation was confirmed by an apparent shift in the intensity of the unique absorbance region around 494 nm (Fig. 3a). The maximal CR degradation was determined to be 94.2% after 30 min, confirming the AgNPs’ good degradation characteristics (Fig. 3b). In contrast to the control of CR, the plot of C/C0 vs. time exhibited a faster decrease with a steady decline in peak strength as reaction time increased (Fig. 3c). Moreover, the reusability of the produced AgNPs was evaluated for three cycles under the same conditions. The degrading stability of AgNPs is demonstrated in Fig. 3d. Similarly, green-produced Ag NPs using S. horneri extract as a mediator demonstrated effective dye removal from Methylene blue, Rhodamine B, and Methyl orange [49].
The analysis of the kinetic degradation of CR involved the utilization of experimental models such as the pseudo-first order, pseudo-second order, and Elovich equation. Based on the findings presented in Fig. 4a–c, it can be observed that the mean deviation obtained from the kinetic models indicates a strong fit between the experimental data and the Elovich model. This is primarily due to the high rate (R2 = 0.93458) observed in comparison to other models. These results suggest that the diffusion of CR over AgNPs is the main contributing factor to the degradation process, making the Elovich model the most suitable for explaining the degradation characteristics of CR by AgNPs. Figure 4d depicts the suggested mechanism for photocatalytic reaction. When the dye solution is subjected to visible light, the electrons (e−) within its valence band (VB) get energized and shifted into the conduction band (CB), resulting in the emission of a photon with energy exceeding the band gap. This process results in the development of vacancies in the VB. Holes that are present in the VB will undergo a reaction with H2O, resulting in the formation of OH· radicals. Those radicals function as potent oxidizing agents. Additionally, e− located in the CB will react with O2 to generate O2· radicals, which possess superoxidase properties. The presence of these radicals helps to inhibit the recombination and neutralization of the system. Consequently, the system will produce a sufficient quantity of OH· radicals, facilitating the catalytic breakdown of the dyes and leading to the formation of smaller organic compounds water, CO2, and residues) through the subsequent mineralization of the degraded products [50,51,52].
Antibacterial Applications
The phenomenon of bacterial inhibition by Ag has been widely acknowledged. The implementation of green preparation methods can enhance biocompatibility and decrease significant impacts on human health [53]. Bacterial inhibition property of AgNPs were examined in relation to their effectiveness against E. coli and S. aureus. Synthesized AgNPs demonstrated potential inhibitory character against both the pathogens, which were dependent on the concentration. Figure 5a and b shows the plates of well diffusion experiment featuring various quantity of AgNPs (10, 20, and 50 µg/mL). A clear zone of inhibition were observed around the discs embedded with AgNPs, against both the examined pathogens. The bactericidal ZOI showed an increase with the increasing concentration of NPs. For S. aureus, the ZOI was found to be 8 mm for 10 µg/mL, 12 mm for 20 µg/mL and 14 mm for 50 µg/mL of AgNPs, although E. coli exhibited 10 mm ZOI for 10 µg/mL, 13 mm for 20 µg/mL and 16 mm for 50 µg/mL of AgNPs. These outcomes of the bactericidal action performed by AgNPs may vary based on the specific membrane characteristics of the bacteria. Similarly, AgNPs produced through the utilization of Rumex nervosus demonstrated potential pathogen inhibition activity [54]. AgNPs synthesized using an extract derived from Bixa orellana seeds exhibited antibacterial properties towards S. aureus, E. coli, S. dysen, and S. boydii in a concentration-dependent manner [55].
Figure 5c depicts the mechanism of bactericidal activity of AgNPs. The degradation of the cell wall and the disruption of the membrane are two fundamental processes that could potentially account for the bactericidal activity of AgNPs [56]. The initial phase of contact between AgNPs and bacteria involves the attachment of AgNPs to the bacterial membrane. This adherence leads to morphological changes, ultimately resulting in permeability change, membrane depolarization, and compromise of the cell wall’s integrity. The internal contents of bacterial pathogens are released into the surrounding environment due to depolarization and morphological damage and thus leading to cell death. The second method entails the development of reactive oxygen species, such as superoxide, hydroxyl radicals, and hydrogen peroxide. These agents efficiently interact with the genetic materials present in the bacterial cells, thereby impeding their growth mechanisms [13, 25, 57, 58].
Conclusions
The current study aims to explore the application of bio-waste of kiwi fruit peel extract for the development of AgNPs by a green method. The peel extract contains phytochemical molecules that have the potential to function as an effective reductant in the formation AgNPs. Further, AgNPs were tested by UV–vis absorbance, FTIR, FE-SEM, and EDS. Synthesized AgNPs had cubic shape with a size of 10–70 nm. Green-synthesized AgNPs demonstrated a remarkable 94.2% degradation ability towards CR, and also demonstrating excellent stability. In addition, the AgNPs demonstrated dose-dependent inhibition activity against E. coli and S. aureus. Additionally, our research findings indicate that generated AgNPs possess significant potential and efficacy as a viable option for application in both the industrial and biological sectors. They exhibit notable proficiency in the removal of dyes found in wastewater, as well as in the prevention of pathogen contamination.
Data availability
All data generated or analysed during this study are included in this published article.
References
Chandraseagar, S., Abdulrazik, A.H., Abdulrahman, S.N., Abdaziz, M.A.: Aspen plus simulation and optimization of industrial spent caustic wastewater treatment by wet oxidation method. IOP Conf. Ser. Mater. Sci. Eng. 702, 012011 (2019). https://doi.org/10.1088/1757-899X/702/1/012011
Annam Renita, A., Sathish, S., Kumar, P.S., Prabu, D., Manikandan, N., Iqbal, M., Rajesh, A., Rangasamy, G.: Emerging aspects of metal ions-doped zinc oxide photocatalysts in degradation of organic dyes and pharmaceutical pollutants – A review. J. Environ. Manage. 344, 118614 (2023). https://doi.org/10.1016/j.jenvman.2023.118614
Zafar, S., Bukhari, D.A., Rehman, A.: Azo dyes degradation by microorganisms—an efficient and sustainable approach. Saudi J. Biol. Sci. 29, 103437 (2022). https://doi.org/10.1016/j.sjbs.2022.103437
Varjani, S., Rakholiya, P., Ng, H.Y., You, S., Teixeira, J.A.: Microbial degradation of dyes: an overview. Bioresour. Technol. 314, 123728 (2020). https://doi.org/10.1016/j.biortech.2020.123728
Rafiq, A., Ikram, M., Ali, S., Niaz, F., Khan, M., Khan, Q., Maqbool, M.: Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 97, 111–128 (2021). https://doi.org/10.1016/j.jiec.2021.02.017
Shabir, M., Yasin, M., Hussain, M., Shafiq, I., Akhter, P., Nizami, A.S., Jeon, B.H., Park, Y.K.: A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 112, 1–19 (2022). https://doi.org/10.1016/j.jiec.2022.05.013
Parmar, M., Sanyal, M.: Extensive study on plant mediated green synthesis of metal nanoparticles and their application for degradation of cationic and anionic dyes. Environ. Nanatechnol. Monit. Manag. 17, 100624 (2022). https://doi.org/10.1016/j.enmm.2021.100624
Hashmi, S.S., Shah, M., Muhammad, W., Ahmad, A., Ullah, M.A., Nadeem, M., Abbasi, B.H.: Potentials of phyto-fabricated nanoparticles as ecofriendly agents for photocatalytic degradation of toxic dyes and waste water treatment, risk assessment and probable mechanism. J. Indian Chem. Soc. 98, 100019 (2021). https://doi.org/10.1016/j.jics.2021.100019
Sankar Sana, S., Haldhar, R., Parameswaranpillai, J., Chavali, M., Kim, S.C.: Silver nanoparticles-based composite for dye removal: a comprehensive review. Clean. Mater. 6, 100161 (2022). https://doi.org/10.1016/j.clema.2022.100161
Khan, S.A., Jain, M., Pandey, A., Pant, K.K., Ziora, Z.M., Blaskovich, M.A.T., Shetti, N.P., Aminabhavi, T.M.: Leveraging the potential of silver nanoparticles-based materials towards sustainable water treatment. J. Environ. Manag. 319, 115675 (2022). https://doi.org/10.1016/j.jenvman.2022.115675
Nandhini, N.T., Rajeshkumar, S., Mythili, S.: The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 19, 101138 (2019). https://doi.org/10.1016/j.bcab.2019.101138
Hossain, N., Islam, M.A., Chowdhury, M.A.: Synthesis and characterization of plant extracted silver nanoparticles and advances in dental implant applications. Heliyon 8, e12313 (2022). https://doi.org/10.1016/j.heliyon.2022.e12313
Ljaz, L., Bukhari, A., Gilani, E., Nazir, A., Zain, H., Saeed, R., Hussain, S., Hussain, T., Bukhari, A., Naseer, R., Yasra, R., Aftab, R.: Green synthesis of silver nanoparticles using different plants parts and biological organisms, characterization and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 18, 100704 (2022). https://doi.org/10.1016/j.enmm.2022.100704
Gopu, M., Kumar, P., Selvankumar, T., Senthilkumar, B., Sudhakar, C., Govarthanan, M., Kumar, S., Selvam, R.: Green biomimetic silver nanoparticles utilizing the red algae Amphiroa rigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioprocess. Biosyst. Eng. 44, 217–223 (2021). https://doi.org/10.1007/s00449-020-02426-1
Bhattarai, B., Zaker, Y., Bigioni, T.P.: Green synthesis of gold and silver nanoparticles: challenges and opportunities. Curr. Opin. Green Sustain. Chem. 12, 91–100 (2018). https://doi.org/10.1016/j.cogsc.2018.06.007
Balachandar, R., Navaneethan, R., Biruntha, M., Kumar, A., Govarthanan, K.K., Karmegam, M.: Antibacterial activity of silver nanoparticles phytosynthesized from Glochidion candolleanum leaves. Mater. Lett. 311, 131572 (2022). https://doi.org/10.1016/j.matlet.2021.131572
Valarmathi, N., Ameen, F., Almansob, A., Kumar, P., Arunprakash, S., Govarthanan, M.: Utilization of marine seaweed Spyridia filamentosa for silver nanoparticles synthesis and its clinical applications. Mater. Lett. 263, 127244 (2020). https://doi.org/10.1016/j.matlet.2019.127244
Roy, N., Gaur, A., Jain, A., Bhattacharya, S., Rani, V.: Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environ. Toxicol. Pharmacol. 36, 807–812 (2013). https://doi.org/10.1016/j.etap.2013.07.005
Thatoi, P., Kerry, R.G., Gouda, S., Das, G., Pramanik, K., Thatoi, H., Patra, J.K.: Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B Biol. 163, 311–318 (2016). https://doi.org/10.1016/j.jphotobiol.2016.07.029
Balaraman, P., Balasubramanian, B., Kaliannan, D., Durai, M., Kamyab, H., Park, S., Chelliapan, S., Lee, C.T., Maluventhen, V., Maruthupandian, A.: Phyco-synthesis of silver nanoparticles mediated from marine algae Sargassum myriocystum and its potential biological and environmental applications. Waste Biomass Valoriz. 11, 5255–5271 (2020). https://doi.org/10.1007/s12649-020-01083-5
Rani, N., Singh, P., Kumar, S., Kumar, P., Bhankar, V., Kumar, K.: Plant-mediated synthesis of nanoparticles and their applications: a review. Mater. Res. Bull. 163, 112233 (2023). https://doi.org/10.1016/j.materresbull.2023.112233
Sampath, G., Govarthanan, M., Rameshkumar, N., Vo, D.V.N., Krishnan, M., Sivasankar, P., Kayalvizhi, N.: Eco-friendly biosynthesis metallic silver nanoparticles using Aegle marmelos (Indian bael) and its clinical and environmental applications. Appl. Nanosci. 13, 663–674 (2023). https://doi.org/10.1007/s13204-021-01883-8
Kakakhel, M.A., Sajjad, W., Wu, F., Bibi, N., Shah, K., Yali, Z., Wang, W.: Green synthesis of silver nanoparticles and their shortcomings, animal blood a potential source for silver nanoparticles: a review. J. Hazard. Mater. Adv. 1, 100005 (2021). https://doi.org/10.1016/j.hazadv.2021.100005
Nadaf, S.J., Jadhav, N.R., Naikwadi, H.S., Savekar, P.L., Sapkal, I.D., Kambli, M.M., Desai, I.A.: Green synthesis of gold and silver nanoparticles: updates on research, patents, and future prospects. OpenNano 8, 100076 (2022). https://doi.org/10.1016/j.onano.2022.100076
Ahmed, A., Usman, M., Ji, Z., Rafiq, M., Yu, B., Shen, Y., Cong, H.: Nature-inspired biogenic synthesis of silver nanoparticles for antibacterial applications. Mater. Today Chem. (2023). https://doi.org/10.1016/j.mtchem.2022.101339
Herrera-Marín, P., Fernández, L., Pilaquinga, F., Debut, F., Rodríguez, A., Espinoza-Montero, A.: Green synthesis of silver nanoparticles using aqueous extract of the leaves of fine aroma cocoa Theobroma cacao Linneu (Malvaceae): optimization by electrochemical techniques. Electrochim. Acta. (2023). https://doi.org/10.1016/j.electacta.2023.142122
Moosavy, M.H., de la Guardia, M., Mokhtarzadeh, A., Khatibi, S.A., Hosseinzadeh, N., Hajipour, N.: Green synthesis, characterization, and biological evaluation of gold and silver nanoparticles using Mentha spicata essential oil. Sci. Rep. 13, 1–15 (2023). https://doi.org/10.1038/s41598-023-33632-y
Harsha Haridas, E.S., Bhattacharya, S., Varma, M.K.R., Chandra, G.K.: Bioinspired 5-caffeoylquinic acid capped silver nanoparticles using coffee arabica leaf extract for high-sensitive cysteine detection. Sci. Rep. 13, 1–12 (2023). https://doi.org/10.1038/s41598-023-34944-9
Thomas, T., Thalla, A.K.: Synthesis of silver nanoparticles using Myristica fragrans seed shell: assessment of antibacterial, antioxidant properties and photocatalytic degradation of dyes. J. Environ. Chem. Eng. 11, 109585 (2023). https://doi.org/10.1016/j.jece.2023.109585
Basumatary, S., Kumar, K.J., Daimari, J., Mondal, A., Kalita, S., Dey, K.S., Deka, A.K.: Biosynthesis of silver nanoparticles using Antidesma acidum leaf extract: its application in textile organic dye degradation. Environ. Nanatechnol. Monit. Manag. 19, 100769 (2023). https://doi.org/10.1016/j.enmm.2022.100769
Paladini, F., Pollini, M.: Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials (Basel) (2019). https://doi.org/10.3390/ma12162540
Atrooz, O., Al-Nadaf, A., Uysal, H., Kutlu, H.M., Sezer, C.V.: Biosynthesis of silver nanoparticles using Coriandrum sativum L. extract and evaluation of their antibacterial, anti-inflammatory and antinociceptive activities. S. Afr. J. Bot. 157, 219–227 (2023). https://doi.org/10.1016/j.sajb.2023.04.001
Hashemi, Z., Mizwari, Z.M., Alizadeh, S.R., Habibi, M., Mohammadrezaee, S., Ghoreishi, S.M., Mortazavi-Derazkola, S., Ebrahimzadeh, M.A.: Anticancer and antibacterial activity against clinical pathogenic multi-drug resistant bacteria using biosynthesized silver nanoparticles with Mentha pulegium and Crocus caspius extracts. Inorg. Chem. Commun. 154, 110982 (2023). https://doi.org/10.1016/j.inoche.2023.110982
Lee, K.J., et al.: Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater. Lett. 105, 128–131 (2013)
Mythili, R., et al.: Utilization of market vegetable waste for silver nanoparticle synthesis and its antibacterial activity. Mater. Lett. 225, 101–104 (2018)
Govarthanan, M., et al.: Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artif. Cells Nanomed. Biotechnol. 44, 1878–1882 (2016)
Yousefzadeh-Valendeh, S., Fattahi, M., Asghari, B., Alizadeh, Z.: Dandelion flower-fabricated Ag nanoparticles versus synthetic ones with characterization and determination of photocatalytic, antioxidant, antibacterial, and α-glucosidase inhibitory activities. Sci. Rep. 13, 1–17 (2023). https://doi.org/10.1038/s41598-023-42756-0
Thulasinathan, B., Ganesan, V., Manickam, P., Kumar, P., Govarthanan, M., Chinnathambi, S., Alagarsamy, A.: Simultaneous electrochemical determination of persistent petrogenic organic pollutants based on AgNPs synthesized using carbon dots derived from mushroom. Sci. Total Environ. 884, 163729 (2023). https://doi.org/10.1016/j.scitotenv.2023.163729
Abdullah, T.S.H.: Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 271, 116295 (2021)
Gowda, B.H.J., et al.: Current trends in bio-waste mediated metal/metal oxide nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 71, 103305 (2022)
Habeeb, H., Thoppil, J.E.: Green synthesis of silver nanoparticles from Wrightia arborea: physiochemical profiling and genotoxic potency. Inorg. Chem. Commun. 156, 111179 (2023). https://doi.org/10.1016/j.inoche.2023.111179
Bharali, A., Sarma, H., Biswas, N., Kalita, J.M., Das, B., Sahu, B.P., Prasad, S.K., Laloo, D.: Green synthesis of silver nanoparticles using hydroalcoholic root extract of Potentilla fulgens and evaluation of its cutaneous wound healing potential. Mater. Today Commun. 35, 106050 (2023). https://doi.org/10.1016/j.mtcomm.2023.106050
Ghatage, M.M., Mane, P.A., Gambhir, R.P., Parkhe, V.S., Kamble, P.A., Lokhande, C.D., Tiwari, A.P.: Green synthesis of silver nanoparticles via Aloe barbadensis Miller leaves: anticancer, antioxidative, antimicrobial and photocatalytic properties. Appl. Surf. Sci. Adv. 16, 100426 (2023). https://doi.org/10.1016/j.apsadv.2023.100426
Aisida, S.O., Ugwu, K., Akpa, P.A., Nwanya, A.C., Nwankwo, U., Botha, S.S., Ejikeme, P.M., Ahmad, I., Maaza, M., Ezema, F.I.: Biosynthesis of silver nanoparticles using bitter leave (Veronica Amygdalina) for antibacterial activities. Surf. Interfaces 17, 100359 (2019). https://doi.org/10.1016/j.surfin.2019.100359
Lakkim, V., Reddy, M.C., Pallavali, R.R., Reddy, K.R., Reddy, C.V., Bilgrami, A.L., Lomada, D.: Green synthesis of silver nanoparticles and evaluation of their antibacterial activity against multidrug-resistant bacteria and wound healing efficacy using a murine model. Antibiotics 9, 1–22 (2020). https://doi.org/10.3390/antibiotics9120902
Roddu, A.K., Wahab, A.W., Ahmad, A., Taba, P.: Green-route synthesis and characterization of the silver nanoparticles resulted by bio-reduction process. J. Phys. Conf. Ser. 1341, 032004 (2019). https://doi.org/10.1088/1742-6596/1341/3/032004
Bharathi, D., Ranjithkumar, R., Nandagopal, J.G.T., Djearamane, S., Lee, J., Wong, L.S.: Green synthesis of chitosan/silver nanocomposite using kaempferol for triple negative breast cancer therapy and antibacterial activity. Environ. Res. 238, 117109 (2023). https://doi.org/10.1016/j.envres.2023.117109
Nandana, C.N., Christeena, M., Bharathi, D.: Synthesis and characterization of chitosan/silver nanocomposite using rutin for antibacterial, antioxidant and photocatalytic applications. J. Clust. Sci. 33, 269–279 (2022). https://doi.org/10.1007/s10876-020-01947-9
Song, W.C., Kim, B., Park, S.Y., Park, G., Oh, J.W.: Biosynthesis of silver and gold nanoparticles using Sargassum Horneri extract as catalyst for industrial dye degradation. Arab. J. Chem. 15, 104056 (2022)
Bustos-Guadarrama, S., Nieto-Maldonado, A., Flores-López, L.Z., Espinoza-Gomez, H., Alonso-Nuñez, G.: Photocatalytic degradation of azo dyes by ultra-small green synthesized silver nanoparticles. J. Taiwan Inst. Chem. Eng. 142, 104663 (2023). https://doi.org/10.1016/j.jtice.2022.104663
Krishna, A.S., Rani, S.K., Vasimalai, N.: Bio-fabricated silver nanocatalyst for photocatalytic degradation and organic transformation of toxic pollutants. Next Mater. (2023). https://doi.org/10.1016/j.nxmate.2023.100023
Marimuthu, S., Antonisamy, A.J., Malayandi, S., Rajendran, K., Tsai, P.C., Pugazhendhi, A., Ponnusamy, V.K.: Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 205, 111823 (2020). https://doi.org/10.1016/j.jphotobiol.2020.111823
Bharathi, D., Vasantharaj, S., Bhuvaneshwari, V.: Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Mater. Res. Express (2018). https://doi.org/10.1088/2053-1591/aac2ef
Alshameri, A.W., Owais, M., Altaf, I., Farheen, S.: Rumex nervosus mediated green synthesis of silver nanoparticles and evaluation of its in vitro antibacterial, and cytotoxic activity. OpenNano 8, 100084 (2022). https://doi.org/10.1016/j.onano.2022.100084
Maitra, B., Halima Khatun, M., Ahmed, F., Ahmed, N., Kadri, J., Zia Uddin Rasel, H., Kanti Saha, M., Hakim, B., Kabir, M., Habib, S.R., Rabbi, M.R.: Biosynthesis of Bixa orellana seed extract mediated silver nanoparticles with moderate antioxidant antibacterial and antiproliferative activity. Arab. J. Chem. 16, 104675 (2023). https://doi.org/10.1016/j.arabjc.2023.104675
Bharathi, D., Ganesh, J., Nandagopal, T., Lee, J., Ranjithkumar, R.: Facile synthesis and characterization of chitosan functionalized silver nanoparticles for antibacterial and anti-lung cancer applications. Polymers 15, 2700 (2023)
Rajeshkumar, S., Bharath, L.V.: Mechanism of plant-mediated synthesis of silver nanoparticles—a review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 273, 219–227 (2017). https://doi.org/10.1016/j.cbi.2017.06.019
Das, C.G.A., Kumar, V.G., Dhas, T.S., Karthick, V., Govindaraju, K., Joselin, J.M., Baalamurugan, J.: Antibacterial activity of silver nanoparticles (biosynthesis): a short review on recent advances. Biocatal. Agric. Biotechnol. 27, 101593 (2020)
Acknowledgements
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R398) King Saud University, Riyadh, Saudi Arabia.
Funding
This study was funded by King Saud University (RSP2023R398), Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
DB: conceptualization, methodology, writing-original draft preparation, review and editing, supervision. JL: formal analysis and discussion. PK: conceptualization, supervision. RM: formal analysis and validation, SD: funding acquisition and resources. MSA: funding acquisition and resources.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bharathi, D., Lee, J., Karthiga, P. et al. Kiwi Fruit Peel Biowaste Mediated Green Synthesis of Silver Nanoparticles for Enhanced Dye Degradation and Antibacterial Activity. Waste Biomass Valor 15, 1859–1868 (2024). https://doi.org/10.1007/s12649-023-02328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02328-9