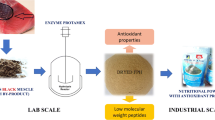
Abstract
Purpose
Fish by-products serve as a major source of protein that can promote the state of health. These by-product wastes have attracted much attention of food biotechnologists due to the availability of large quantities of raw material for the process, and presence of high protein content with good amino acid balance and bioactive peptides. Controlled enzymatic hydrolysis of protein-rich fish wastes is believed to be a better way to produce bioactive peptides. Under this context, this work is to show that swordfish head can be a good source of proteins that can be valorised as functional ingredients.
Methods
Tow swordfish (Xiphias gladius) head muscle protein hydrolysates (XHMPHs) were produced by the separate action of alcalase® (HM-Al) and savinase® (HM-Sa). The physico–chemical characteristics of hydrolysates, such as proximal composition, size, AAs and RP-HPLC profiles, in vitro antioxidant and functional properties were determined.
Results
XHMPHs showed different degrees of hydrolysis (19.46% with savinase and 11.04% with alcalase). Both hydrolysates had a high protein content (61.82 in HM-Al and 63.61% in HM-Sa) and proportion of small peptides (< 1000 Da) (42.34% in HM-Sa, 38.65% in HM-Al). However, HM-Sa showed the highest content of hydrophobic peptides than HM-Al. In the two hydrolysates, glutamic acid, aspartic acid, arginine and lysine have an individual concentration greater than 5%. Essential amino acids (EAA) accounted for 42% and 39% of the mass of HM-Sa and of HM-Al, respectively. Enzymatic hydrolysis improved solubility at pH values ranged from pH 2.0 to pH 10.0, significantly as well as emulsifying and foaming properties of Swordfish proteins. The emulsifying activity of XHMPHs decreased with increasing concentrations. Conversely, the foaming abilities increased as the hydrolysate concentrations increased. The tow hydrolysates showed dose-dependent antioxidant activities. HM-Sa displayed the highest protection against hydroxyl radical induced oxidation.
Conclusions
The results suggested that HM-Sa and HM-Al could be used as a source of promising nutritional and pharmaceutical applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food-derived bioactive peptides represent potential functional food ingredients roles that promote health. In addition to their nutritional importance as a source of essential amino acids (AA) for the growth and maintenance of the organism, peptides exhibit a high functionality, in the determination of various organoleptic and rheological characteristics of foods [1]. Indeed, due to the presence of essential nutrients and bioactive components in fish protein hydrolysates, these find place in various industrial applications including pharmaceutical products, cosmetics, and animal nutrition. Thus, the demand for protein has increased and is expected to continue to increase. Due to limited natural protein resources, it will be impossible to meet market demand without more careful management of available resources. One of the main actions to be carried out in this area is the recovery of non-traditional protein sources (waste, insects, etc.). Marine by-product proteins may have particular interest, not only because of their wide quantitative availability (171 million tons in 2016) (The State of World Fisheries and Aquaculture 2020); but, also, because they are likely to be better accepted by the consumer than proteins from other sources such as insects. In addition, the use of fish waste as a source of protein avoids their rejection in nature, which has a positive impact on the environment.
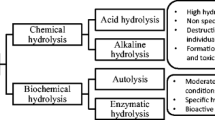
Bioactive peptides can be derived from the fish and its by-products or waste employed by various chemical or biochemical methods, including acid-alkaline hydrolysis, enzymatic hydrolysis, and fermentation [2, 3]. The enzymatic hydrolysis is the best approach to hydrolyze proteins from fish and its by-products due to the short reaction time, ease of scalability and predictability [3, 4]. Indeed, the enzymatically extracted proteins proved to be of better quality (less oxidized) than their counterparts obtained by modifying the pH [5], and without negative impact on the perceptions of appetite or regulatory hormones [6]. In addition, this process leads to the improvement of AA bioavailability [7], biological [8,9,10,11] and functional (Physico-chemical) properties [10,11,12] Thus, enzymatic treatment of fish proteins increases their economic value.
Functional and biological activities of hydrolysates are variable due to the change of several factors such as the origin of the substrate proteins, the treatment of the sample prior to hydrolysis, the specificity of the enzymatic activity and the conditions of hydrolysis (DH, ratio between enzyme and substrat [E/S], etc.) [11]. These parameters influence the sites and the intensity of the hydrolysis, and therefore the number and the structure of the generated peptides, which are the main actors of the properties. Indeed, the enzyme to substrate ratio is an important factor to consider obtaining a good degree of hydrolysis. Peptide sequences and their biological activities may differ depending on the type of enzyme used. Hydrolysis exhibits the excellent solubility of protein hydrolysate along with other functional properties like emulsifying and foaming properties.For example, the protein hydrolysate of Bluewing Searobin (Prionotus punctatus), generated by alcalase and having a DH of 34.7%, showed greater solubility and a higher index of emulsifying activity than the hydrolysate of DH 30% obtained from the same proteins by action of Flavorzyme [12]. On the other hand, the first hydrolysate had the lowest water and oil retention capacity. In the same way, salmon protein hydrolysates, prepared by alcalase or savinase, showed an oil-binding capacity which decreases with increasing DH [13]. At different DH (5%, 10% and 15%), hydrolysates generated by alcalase had the best capacity. According to Liu et al. [14], the effect of DH and enzyme specificity on functional properties is a consequence of their effect on structure characteristics (hydrophobicity, polarity, molecular size…). Depending on their physical and chemical characteristics, peptides express one or more biological activities such as antioxidant, antihypertensive, cholesterol-lowering and antimicrobial activity. Such potential of peptides has been the subject of particular interest for several years, especially since traditional synthetic bioactive compounds are refused by the general public because of their undesirable side effects [15]. Several proteins have been used to produce hydrolysates with bioactive potential, in particular an antioxidant activity. Among the proteins used in this field, several came from maritime organisms such as sardines [8], Thornback ray [16], Boops boops, Triggerfish, Boops boops, smooth hound and cuttlefish [17].
It seems obvious that the potential application of the hydrolysate requires an optimal balance between several of its properties [18]. In fact, the properties expressed by the hydrolysates seem to be a “modeling” of a particular protein, using specific protease acting under specific conditions. Thus, the hydrolysis of the same proteins by different enzymes and/or under different conditions is likely to produce hydrolysates with particular and different properties.
The swordfish (X. gladius) is a cosmopolitan species present in the Atlantic Ocean and the Mediterranean, including along the Tunisian coasts. In 2002, the catch of this fish was 1138 tonnes [19]. The head of this fish, which represents a significant part of the harvested quantity, is generally rejected in nature, thus constituting a source of pollution. However, this by-product contains a large amount of muscle that can serve as a source for useful substances.
This is the first research that deals with the preparation of value-added head protein hydrolysates from swordfish using two different enzymatic activities (alcalase, savinase). The molecular weight distribution, as well as the physico-chemical and and functional properties of both hydrolysates was investigated and compared.
Materials and Methods
Fish Muscle Preparation
The head of fresh X. gladius comes from the fish market of Sfax (Tunisia). Head muscle was separated, stripped of its skin, rinsed with cold distilled water, and then stored in sealed plastic bags at − 20 °C until further use. Chemicals and solvent reagents of analytical grade were obtained from different commercial sources. Commercial alcalase 2.4 L and savinase, purchased from Novo Nordisk (Bagsverd, Denmark), were used for the production of protein hydrolysates.
Preparation of Protein Hydrolysates
Swordfish head muscle (500 g), previously chopped in a grinder (Moulinex), were placed in 1 L of distilled water, and then cooked at 90 °C for 20 min. Muscle and cooking water were transferred to a blender Moulinex and homogenized for 2 min. pH and temperature were adjusted to the optimum conditions for each enzyme: Alcalase 2.4 L® (pH 8.0 and 50 °C) and Savinase® (pH 9.0 and 50 °C). The hydrolysis reaction was started by the addition of the enzyme at a 3:1 (U of enzyme/mg of protein) enzyme/protein ratio. During hydrolysis, temperature was kept stable and the pH was maintained to optimal value by 4 N NaOH solution addition. The hydrolysis was stopped (by adjusting the pH to 12) when the reaction medium pH remains stable. Then, head Swordfish protein hydrolysates were centrifuged at 5000 g for 20 min at 4 °C. The soluble fraction was collected, lyophilized and, stored at − 20 °C, for later use. Control experiments without the addition of enzymes are also carried out.
Determination of the Degree of Hydrolysis
The degree of hydrolysis (DH), defined as the percent ratio of the number of peptide bonds broken (h) to the total number of peptide bonds in the substrate studied (htot), in each case, was calculated from the amount of base (NaOH) added to keep the pH constant during the hydrolysis as given below:
B is the amount of base consumed (mL) to keep the pH constant during the reaction. Nb is the normality of the base, MP is the mass (g) of protein (N × 6.25, where N is the nitrogen mass as determined by kjeldhal method), and α is the average degree of dissociation of the α-NH2 groups released during hydrolysis expressed as:
pH and pK represent the values at which the proteolysis was conducted. The total number of peptide bonds (htot) in a fish protein concentrate was assumed 8.6 meq/g.
Physico–Chemical Characterization
To estimate protein content, the total nitrogen content determined by the Kjeldhal method [20] was multiplied by the factor 6.25. Lipids was determined gravimetrically, after Soxhlet extraction with hexane of the dried samples [21]. The protein and fat contents are expressed in dry weight. Moisture and ash content was determined according to the AOAC methods 930.15 and 942.05, respectively [22]. All measurements were carried out in triplicate.
Molecular Weight Distribution
The distribution of the peptide molecular weight (MW) of the different hydrolysates was determined by gel filtration chromatography on a Superdex Peptide PE 7.5/300 column (GE Healthcare, Uppsala, Sweden) according to the procedure described by Dupas et al. [23] using a Shimadzu-Nexera XR HPLC. Briefly, sample (50 µL) elution was carried out at 35 °C for 120 min using 30% (V/V) of 0.1% (V/V) trifluoroacetic acid (TFA) in acetonitrile and 70% (V/V) of 0.1% (V/V) TFA in water (flow rate: 0.25 mL/min). Peptides were detected by spectrophotometry at 215 nm. In the same way, standard peptides [cytochrome C (12 400 Da), aprotonin (6500 Da), substance P (1348 Da), glycine 6 (360 Da), glycine 3 (189 Da) and glycine (75 Da)] were used to establish a calibration. In the different hydrolysates, the peptides were sorted into 4 major fractions regarding their MW. The relative areas of each fraction were given as a percentage of the total area (LabSolutions software, version 5.73).
Reverse Phase High Performance Liquid Chromatography
In order to complete peptides profiles obtained by gel filtration in term of hydrophobicity vs. size, an analysis was performed on a C18 Omnispher column (250 mm × 4.6 mm; 5 μm) (Agilent Technology, Les Ulis, France) installed on a Shimadzu-Nexera XR HPLC. Used method was previously described by Parrot et al. [24]. Briefly, samples (100 µL; 1 mg/mL) were loaded into the column and eluted at for 160 min with 30% of 0.1% TFA in acetonitrile and 70% of 0.1% TFA in water. The flow rate was 1 mL/min, and the absorbance was carried out at 215 nm.
Amino Acid Composition
A volume (50 µL) of hydrolysate solutions (1 mg/mL) were mixed with 0.5 mL of 6 N HCl at 112 °C for 24 h on a heating block, and then filtered through a 0.45 μm membrane filter prior to analysis. Ten microliters of each treated sample were derivatized using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate Waters AccQ·Fluor Reagent Kit (according to the Waters AccQ·Tag Chemistry Package Instruction Manual). HPLC analyses were then performed with a Waters 2996 Separation Module equipped with a Waters 2475 multi-wavelength fluorescence detector: amino acids were separated on a Waters AccQ·Tag amino acid analyzing Column. For each detected amino acid, the concentration content was expressed as a percentage of total amino acids (TAA) in the sample.
Determination of Functional Properties
Solubility
The solubility of X. gladius head muscle protein hydrolysates (XHMPHs) was performed according to Tsumura et al. [25] with slight modifications. The pH of the sample was adjusted to different values from 2.0 to 10.0 using solutions of 2 N HCl or 2 N NaOH. After stirring for 30 min at ambient temperature (25 ± 1 °C), the samples were centrifuged at 8000×g for 10 min and the nitrogen content of the supernatant was determined by the method of Lowry et al. [26]. The nitrogen solubility of the sample was determined by the following equation:
Emulsifying Properties
The emulsion activity index (EAI) and the emulsion stability index (ESI) of the.
hydrolysates were determined according to the method of [27]. Ten milliliters of soybean oil and 30 ml of hydrolysate solutions at different concentrations (0.5%, 1% and 2%) were homogenized for 1 min at room temperature (22 ± 1 °C) (Moulinex-R62 homogenizer). Aliquots of the emulsions (50 µL) were pipetted from the bottom of the container at 0 and 10 min after homogenization and diluted 100 times with a 0.1% SDS solution and their absorbance was immediately measured at 500 nm. The EAI and the ESI were calculated according to the following equations [27]:
where \(\Delta A\) is (A0 − A10) and t = 10 min. Where A0 and A10 are the absorbance of the sample at 0 or 10 min, respectively. All determinations are means of three measurements.
Foaming Capacity and Foam Stability
The foaming properties [capacity (FC) and stability (FS)] of hydrolysate samples were performed following the method of Shahidi et al. [28]. Twenty millilitres of protein hydrolysate solutions (0.5%, 1% and 2% w/v) was homogenised using g Ultraturrax T18 model homogenizer at a speed of 20,000 rpm to incorporate the air for 1 min at room temperature (25 ± 1 °C). The whipped sample was then immediately transferred into a 50 mL graduated cylinder, and the total volume was measured at 30 min after whipping. Foam expansion was expressed as percentage of volume increased after homogenization at 0 min, which was calculated according to the following equation:
VT is the total volume after whipping (mL), V0 is the volume before whipping.
Foam stability was calculated as the volume of foam remaining after 30 min.
Vt is total volume after whipping (mL), V0 is the original volume before whipping, and Vt is total volume after leaving at room temperature for 30 min.
All determinations are means of at least two measurements.
Water-Holding Capacity
Water-holding capacity (WHC) was determined using the method described by McConnell et al. [29]. Protein samples (500 mg) were mixed with 50 mL of distilled water using a stirrer. The protein suspension was maintained at room temperature for 30 min then, centrifuged at 5000 g for 30 min. The supernatant was decanted and the tube drained at a 45° angle for 10 min. WHC was determined from the weight difference.
Oil-Holding Capacity
Oil-holding Capacity (OHC) was determined using the method described by Lin et al. [30]. Samples (500 mg) of were vortexed with 10 mL of maize oil for 30 s. The emulsion produced was kept at 37 °C for 1 h, and then centrifuged at 2500 g for 30 min. The free oil was decanted and drained at a 45° angle for 20 min. The fat absorption of the sample was determined from the weight difference.
Antioxidant Activity
DPPH Radical-Scavenging Assay
The DPPH free radical-scavenging activity of XHMPHs was measured by the method of Bersuder et al. [31]. BHA was used as positive control. The test was carried out in triplicate and DPPH radical-scavenging activity was calculated as follows:
where A control is the absorbance of the control reaction and A sample is the absorbance in the presence of sample. DPPH has absorption at 517 nm, which disappear upon reduction by an antiradical compound.
Ferrous Chelating Activity
The chelating activity of samples towards ferrous ion (Fe2+) was determined according to the method of [32]. The method involves inhibition of the formation of Fe2–ferrozine complex afterer the addition of the sample. The chelating activity of the antioxidant for Fe2+ was calculated according to the following formula:
where A control is the absorbance of the control reaction and A sample is the absorbance of samples. EDTA was used as a standard. The test was carried out in triplicate.
Total Antioxidant Capacity
This assay is based on the reduction of Mo (VI) to Mo (V) by the sample and the subsequent formation of a green phosphate/Mo (V) complex at acidic pH [33].
The activity of the hydrolysate was expressed as α-tocopherol equivalents using the following linear equation:
where A: absorbance at 695 nm and B: the concentration as the α-tocopherol equivalent (mmol/mL).
Statistical Analysis
All experiments were conducted in triplicate. Data were expressed as means with standard deviations. Data were analyzed using analysis of variance (ANOVA).
Results and Discussion
Preparation of Protein Hydrolysates
Peptides composition may depend on the enzyme formulation as well as the treatment conditions of the parent protein within which bioactive peptides could be processed to retain their bioactivity. Indeed, bioactivity was enhanced by choosing a defined set of hydrolysis which includes screening of the best enzyme, degree of hydrolysis, optimization of hydrolysis conditions, purification and characterization, including molecular weight, amino acid composition, and sequence [34]. The hydrolysates of the present study were produced using savinase or alcalase whose action was maintained until the final DH was obtained. These two subtilisin endopeptidases were chosen due to the high hydrolysis efficiency they have shown with many different proteins [24, 26].
The Shape of hydrolysis curves (Fig. 1) is similar to those previously published for hydrolysates [8, 27, 28]. Protein hydrolysis was characterized by a high rate of hydrolysis during the first 30 min followed by a slowing phase and then a stationary phase. The last two phases can be justified by enzymatic inactivation and/or the production, at high DH, of inhibitory peptides [13].
Alcalase and savinase resulted in a final DH of 11.04% and 19.46%, respectively. These DHs indicate a greater efficiency of savinase than of alcalase in the hydrolysis of swordfish head muscle proteins. A presence of savinase action sites in greater number and/or better accessibility could justify the highest DH of HM-Sa. In addition, a inhibitors peptides in greater quantity and/or more effective may be produced by alcalase [29, 31].
Physico–Chemical Properties of Hydrolysates
Because the constituents of a product determine its biological and functional properties, and therefore its mode of application, the proximal and amino acid composition, the size and hydrophobicity profiles of the peptides, as well as the functional properties of the two hydrolysates were analyzed.
Chemical Composition
The chemical composition allows not only to choose the right food, but also to assess its potential for development and application. The proximal composition of coproduct, undigested proteins and hydrolysates is listed in Table 1.
Based on the composition of muscle swordfish heads, which were 11.68% for proteins, 34.15% for lipids, 56.57% for moisture and 1.79% in ash, swordfish head muscle was clearly different from flesh muscle of many other fish. For example, the heads of bighead carp [34] and sockeye salmon head [35] had higher moisture content (77.31% and 69.6%, respectively) and lower lipids (1.68% and 14.5%, respectively). The protein content of swordfish was lower than bighead carp fish muscles heads protein (15.78%) and comparable with that of salmon head muscles (11.9%). In agreement with the literature, the moisture and protein content were increased, while that of lipids has decreased [36].
There were significant differences in proximate chemical composition of the prepared hydrolysates using savinase (HM-Sa) and alcalase (HM-Al). The protein content of dried hydrolysates ranged from 61.82 to 63.61%. These values were lower than that of undigested proteins (UHMP).This content was similar to other hydrolysates such as salmon head protein hydrolysates [35]. However, a higher protein content was reported in hydrolysates of a large number of aquatic protein such as the proteins of by-products (frame, dark muscle, cut offs, viscera, skin, scales, small bones and fins) (73–82.66%) [37] bighead carp fresh muscles (80.58–85.27%) [34] and those from herring protein (77.0%) [36].
Fat content of hydrolysates (10.07% and 11.39% for HM-Al and HM-Sa, respectively) were lower than those of UHMP (59.12%).
In hydrolysates, protein and lipid levels move in opposite directions [38]. The hydrolysates present displayed more lipid and less protein than other hydrolysates such as those of bighead carp muscle protein (lipid: 2.84–4.59%; protein 80.58–85.27%) [34] and thorny skate (lipids: 0.10–1.19%; proteins 71-74%) [39] or fish by-products (lipids: 0.44–0.87%; proteins: 73.51-82.66%) [37]. High ash content was found in HM-Sa and HM-Al (21.24–24.52% respectively). Similar content was reported in some earlier hydrolysates such as Leiognathus bindus protein hydrolysate prepared by Johnrose et al. [40], but lower levels have been reported in other hydrolysates. For example, ash constituted 5.97–6.69% of fresh muscle protein hydrolysates from bighead carp [34], 4–7.7% of salmon head protein hydrolysates [35] and 9.71–17.74% of thornback ray muscle hydrolysates [39].
Compared to HM-Sa and HM-Al, non-hydrolyzed proteins contain significantly lower ash content (7.52%). The amount of NaOH added to the reaction medium to keep the pH constant during the hydrolysis can justify the increase of ash in the hydrolysates. On this basis, we can reasonably assume an enrichment of the hydrolysates in Na, which decreases their quality. Indeed, an increased intake of this mineral promotes the development of various pathologies (diseases of the kidneys, brain, vascular system, etc.) [41]. Consequently, it appears appropriate to subject these hydrolysates to a desalination step before using them as a food supplement.
Amino Acid Composition
The AA composition is an important determinant of the nutritional, biological and functional properties of hydrolysates. In particular, its nutritional value depends on its content of essential amino acids (EAA), and its biological and functional properties were related to its content of particular AA or groups of AA. Indeed, due to their structural properties, their composition and their amino acid sequences, proteins and peptides have particular properties of water retention, texture, gelation, whipping and emulsification [12].
The total amino acids of HM-Sa and HM-Al constituted 69.91 g/100 g and 63.46 g/100 g, respectively (Table 2). The sum of total amino acids did not equal the value of total protein (N × 6.25). This is probably due to the imprecision of the used nitrogen/protein factor (6.25). The higher amino acid content in the stronger DH hydrolyzate (HM-Sa) is consistent with observations made by other authors [34]. Cysteine was not identified in the hydrolysates present. This observation is in agreement with the very low level of this AA reported in other maritime co-products. For example, it constitutes less than 0.4% of the AA totals of rainbow trout heads and skipjack heads [42]. When the two current hydrolysates are compared, only a minor difference in their amino acid compositions was observed. The clearest difference was the higher level of leucine in HM-Sa (5.76 g/100 g) than in HM-Al (2.74 g/100 g).
The non-essential amino acid (NEAA) fractions were dominated by glutamic acid followed by aspartic acid. This was also the case in many other hydrolysates such as those prepared from bighead, mesopelagic carp, Baltic herring or roach and salmon head proteins, respectively [33, 35].
The EAA profile of HM-Sa and HM-Al was dominated by lysine which accounts for 27.50% and 31.31% of essential amino acids, respectively. This EAA was also the most abundant essential amino acid in protein hydrolysates from sardinella, Cuttlefish, Smooth hound and Boops boops [17] and white shrimp [43]. In HM-Al and HM-Sa, the Lys content was 7.71 mg/g hydrolysate and 8.15 mg/100 g hydrolysate, respectively. Similar level was reported in sockeye salmon protein hydrolysate (71.4–83.2 mg/gram of protein) [35], from Baltic herring (70.67–74.68 mg/g of protein) and roach (73.94–81.71 mg/g of protein) [44]. Methionine was the minor EAA in HM-Al and HM-Sa, reaching about 1.19 g/100 g and 1.44 g/100 g, respectively) and leucine was the EAA that showed the greatest variation between the two hydrolysates.
In present study, EAA/NEAA was 0.73 for HM-Sa and 0.63 for HM-Al. This value is similar or lower than that of the standards ratio of commercial fish protein hydrolysates (0.75) [38, 40] and of other fish protein hydrolysates. For example, this ratio was 0.65–0.85 in roach (Rutilus rutilus) and Baltic herring protein hydrolysates produced by action of Protamex, Neutrase or Corolase [44] and 1.08–1.12 in bighead carp protein hydrolysates prepared by ficin enzyme [34].
The EAA/TAA ratio was higher in HM-Sa than in HM-Al. This observation could be justified by its greater DH. Indeed, five of the EAAs are hydrophobic (Val, Met, Phe, Leu and Ile) which tend to occupy the core of the native protein can be better recovered under the condition of more extensive hydrolysis. Indeed, these hydrophobic EAAs as well as total hydrophobic AAs were more present in HM-Sa (15.89 g/100 g and 22.85 g/100 g, respectively) than in as well as constitutes 15.89 g/100 g of HM-Sa and HM- Al (11.85 g/100 and 19.15 g/100 g, respectively).
Nutritional Value
The nutritional value of proteins depends on their EAA composition. According to the FAO/WHO, the EAA/TAA ratio must be greater than or equal to 0.40 and the NEAA/EAA ratio must be greater than or equal to 0.60. The two prepared hydrolysates contained all the EAAs except tryptophan, which could be departed during the acid treatment. The EAA/TAA and NEAA/EAA ratios were, in order, 0.42 and 1.36 in HM-Sa and 0.39 and 1.57 in HM-Al.
In eggs and milk proteins, the NEAAs/EAAs ratio is equal to one. In HM-Sa and HM-Al, this ratio is greater than 1; but its value is lower than that observed in vegetable proteins (soya, wheat, peas, hemp, etc.) [45].
The ability of the hydrolysates to meet the body’s demand for each of the different EAAs detected in the hydrolysates was evaluated by calculating their respective percentage in the hydrolysate relative to the reference protein (AA score). The results are shown in Table 3. With the exception of leucine, which is a limiting AA in the HM-Al, all EAAs are present in the two hydrolysates at a higher level than their values in the reference protein. According to the results, HM-Sa appeared to be applicable as dietary protein supplements to improve diets deficient in one and/or other of EAAs. This is for example the case of diets based on plant products whose proteins are generally deficient in Lys. In addition, HH-Al had appeared potentially useful to respond to the deficiency of certain results proposed HM-Al to correct dietary deficits in EAAs except those deficient in Leucine. The correction of such diets requires the combination of another leu-rich product with HM-Al. Based on their EAA content, HM-Sa and HM-Al can be used as supplements to improve the EAA status of certain inadequate diets. However, HM-Al could not be used alone when the diet is leucine deficient.
As shown in Table 1, the level of lipids in HM-Sa and HM-Al is significant. Based on their maritime origin and their high level of w3 polyunsaturated fatty acids (PUFA), previously reported in the muscle tissues of Tunisian swordfish [46], we can logically assume that HM-Sa and HM-Al lipids are rich in w3 lipids. This is one more reason for using these hydrolysates as a supplement. Indeed, w3 lipid deficiency is another common type of malnutrition.
Molecular Weight
Molecular weight is an important parameter reflecting the hydrolysis of proteins, which further correlates with the functional and biological activity of hydrolysate [47]. The molecular weight profiles of our hydrolysates were investigated using gel filtration chromatography. Obtained profiles are shown in Fig. 2; Table 4. Swordfish head muscle proteins hydrolysis process using alcalase or savinase resulted in comparable global molecular weight profiles. The constituents of the two hydrolysates were distinguished into different size populations: I, II, III and IV that represented fractions > 10,000 Da, 10,000 − 5000 Da, 5000 − 1000 Da and < 1000 Da, respectively.
The cited size populations had a different importance in the two hydrolysates (Table 4). In particular, the smaller population (< 1000 Da) was more present in HM-Sa then HM-Al and values were 42.34% and 38.68%, respectively. This is in agreement with previous studies which reported an increase in the proportion of small peptides when the DH increases [34]. However, the proportion of the fraction < 1 KDa observed in HM-Al and HM-Sa was significantly lower than that reported in hydrolysates of other proteins, such as that of burbot head (53%), burbot viscera ( 74%) [47], skin of Theragra chalcogramma (85.95%) [48] and Chinese sturgeon [49].
Reversed-Phase High Performance Liquid Chromatography
The hydrophilic/hydrophobic peptide ratio strongly affects the biological and functional properties of hydrolysates [50]. The RP-HPLC method allows the separation of proteins/peptides according to their hydrophobicity. Under the parameters used, a longer retention time indicates greater hydrophobicity and molecular weight heterogeneity [14, 25]. Thus, the lowest retention times (< 10 min) generally correspond to very hydrophilic and small-sized peptides (< 15 residues) while the highest retention times (greater than 60 min) correspond to proteins. Between 10 and 60 min the more the retention time is high, the more the peptides are large and/or hydrophobic.
The separation profiles of the samples are shown in Fig. 3. The many detected peaks illustrate the heterogeneous composition of the samples. The constituents of the UHMP were eluted before 10 min, whereas the elution of those of the hydrolysates required about one hour. This indicates the greater heterogeneity of the hydrolysates.
Compared to HM-Al, HM-Sa is essentially distinguished by a greater presence of peptides with high hydrophobicity. This can be justified by its greater DH. Indeed, more extensive hydrolysis is likely to implicate more binding of the core (“rich in hydrophobic AAs”) of the protein, and consequently produces an increase in the hydrophobic character of the generated peptides.
Hydrophobicity plays an essential role in many functional (bitterness, foaming and emulsion stability, etc.) and bioactive (alpha-glucosidase inhibition, antioxidant, anti-ACE, antimicrobial, etc.) properties of peptides of food origin [26, 27]. Thus, each of the two hydrolysates produced would have particular properties and would be more suitable for particular applications.
Determination of Functional Properties
Functional properties are the intrinsic physicochemical properties, which influence the behavior of a product during the preparation, processing, storage, and consumption. The study of the functional properties of fish protein hydrolysates are important, particularly if they are intended for use as ingredients in food products.
Solubility
Solubility is an essential functional property of proteins and peptides. Indeed, it affects many of the other functional properties influencing the usefulness of hydrolysates (and any other ingredients), such as emulsification and foaming. The solubility of hydrolysates at different pH ranging from 2 to 10 is shown in Fig. 4. It can be noted that pH and hydrolysis process influenced the solubility of the tested Swordfish proteins samples. The solubility of all studied samples was minimal at pH 5, which indicates that their pHi was equal or close to this value. As the pH became more acidic or more basic, solubility of all present samples increases. Minimal solubility of tilapia fish protein hydrolysates [51] was observed at a pH 5 and pH 4.5–5.5 for Bighead Carp [34]. Other hydrolysates showed a lowest solubility at pH 4 [28, 30, 32] or pH 6.0 [52].
In accordance with our findings, a large number of studies reported that higher solubility of protein hydrolysates was obtained at strongly acidic or alkaline pH [43].
Throughout the used pH range, the hydrolysates were significantly more soluble than UHMP (p < 0.05). HM-Al and HM-Sa solubility were 53 to 90% and 75 to 95%, respectively. Similar solubility was reported for bighead carp protein hydrolysates [34] and octopus protein hydrolysates [52]. However, hydrolysates with higher [53] and lower [35] solubility have been reported.
The differences of solubility between HM-Al and HM-Sa can be justified by their different DH. Indeed, many previous studies have reported that solubility increases when DH increases [30] [52]. The enzymatic hydrolysis could cause a decrease in the protein molecular size and a release of small soluble peptides and maintain the exposure of more charged and polar groups which could form stronger hydrogen bonds with water such as previously reported for Cobia (Rachycentron canadum) frame hydrolysate prepared by Amiza et al. [54].
High solubility is a criterion requested by the food industry. Indeed, it improves appearance and smooth mouthfeel of the product and allows its use in formulated food systems. Limited hydrolysis is therefore a valuable approach to improve the potential of swordfish head proteins in the food industry. The hydrolyzate generated by savinase, which shows great solubility even at pHi, may be of particular interest in this field. Indeed, it can be a valid and usable ingredient in the whole pH range tested (pH 2–10).
Emulsifying Properties
Emulsifying activity index (EAI) (ability to form emulsion) and emulsion stability index (ESI) (ability to maintain the formed emulsion) are two parameters generally used to evaluate the emulsifying capacity. The EAI (m2/g) and ESI (min) of swordfish UHMP and hydrolysates at different powder concentrations (0.5%, 1%, 2%) were determined. As shown in Table 5, emulsification activity was enhanced by enzymatic hydrolysis but was reduced by increasing sample concentration. The difference between UHMP and hydrolysates was significant at used concentrations (p < 0.05). The same observation has been reported by many previous studies [28, 32]. Limited hydrolysis enhances exposure of hydrophobic amino acid residues (which can interact with oil) and the number of hydrophilic groups that allow more interaction with lipids and with water, respectively. The decrease in emulsifying power at high concentration could result from the interaction of hydrophobic groups with each other, which reduces their concentration of at the interface [55].
Previous studies [53] reported a negative relationship between EAI and DH. This relationship was assigned, at least, to a decrease in hydrophobicity that accompanies the increase in DH [56]. In the present study, the strongest DH hydrolysate, namely HP-Sa, was also the richest in hydrophobic peptides. The negative relationship between DH and EAI was not observed but the greater richness in hydrophobic peptides was accompanied by an improvement in ESI. Their greater hydrophobicity allows them to form more stable emulsions.
Although the key role of many caracteristics des peptides, such as the molecular weight and hydrophobic character, in their emulsifying ability is obvious, their relative importance is unknown. None of the parameters allows, on its own, the evaluation of functional properties.
Foaming Capacity and Foam Stability
Foams are colloidal systems with a liquid phase and a gas phase. They are of particular interest in the food industry and their stability is essential for their acceptance by consumers. The foaming properties of an ingredient are generally expressed in terms of foaming and stability, and they depend on several of its characteristics such as solubility, hydrophilicity/hydrophobicity balance, flexibility and the presence of charged or polar groups. To form a good foam, a protein must be able to rapidly migrate across the air-water interface, and reorganize at the interface. Foam expansion at 0 min after whipping indicates foaming capacity (FC) and foam expansion at 30 min after whipping indicates the stability of the foam (FS). FC and FS of UHMP and hydrolysates powders at different concentrations are shown in Table 6.
Consistent with literature data, FC and FS of swordfish proteins were improved both by limited proteolysis [28, 53] and by increasing sample concentration [54] (p < 0.05). The effect of hydrolysis may be a consequence of the reduction in the size of the peptides which allows them to diffuse more easily and to reach the air-water interface more quickly to encapsulate air bubbles and develop a foam. Also, hydrolysis can declutter the hydrophobic AAs initially in the core which contribute to stabilizing the foam.
Indeed, the literature reports that foaming power decreases with increasing degree of hydrolysis [52]. In agreement with this observation, the present study reveals that the hydrolysate with the lowest DH (HM-Al) had with the highest FC and FS. However, the lower FS of HM-Sa seems to disagree with its higher content of more hydrophobic peptides. It can be assumed that the negative effect of certain structural characteristics of peptides on foaming properties would outweigh the positive effect of their hydrophobic character.
Water-Holding Capacity
The water holding capacity (WHC) expresses the amount of water absorbed per unit weight of the sample. It influences rheological properties, mechanical strength, elasticity, plasticity and is essential for the food industry [57]. This property is sensitive to several factors including amino acid composition, protein conformation, surface polarity and surface hydrophobicity [55]. Several studies have shown that limited hydrolysis of fish protein hydrolysates improves water retention capacity, which is confirmed by the current results. Indeed, the two hydrolysates presented a WHC significantly higher than that of the intact proteins (p < 0.05) (Table 7).
This property is due in particular to the interaction of water with polar amino acids[58]. Thus, its enhancement caused by limited protein hydrolysis can be explained, at least in part, by the increase in the number of carboxyl and amine groups resulting from the cleavage of peptide bonds. In accordance with this idea, the hydrolysate with the strongest WHC is the one resulting from the cleavage of a greater number of peptide bonds (HM-Sa). A positive relationship between WHC and DH has also been reported by Alahmad et al. [34].
The WHC expressed by actual hydrolysates is higher than that of some hydrolysates such as that of yellow-striped scad proteins (Selaroides leptolepis) prepared by Fawzya et al. [59] but lower than that of other hydrolysates such as those of bighead carp (2.18–3.93 g/g) [34], Octopus (2.96–4.20 g/g) [52].
Oil-Holding Capacity
The oil-binding capacity (OHC) of protein hydrolysate contributes to the taste and palatability of food products and their emulsifying power. This ability is due to the hydrophobic interaction between the non-polar amino acid and the lipid hydrocarbon chain.
The oil absorption of the hydrolysates prepared using alcalase and savinase and of UHMP were 1.85 g/g, 1.50 g/g and 1.25 g/g, respectively (Table 7). Haldar et al. [60], reported that the fat absorption capacity of freshwater mussel protein hydrolysates increases with hydrolysis time. This is in agreement with our current observation. Indeed, HM-Sa has a higher DH and a higher OHC than HM-Al. Other previous studies report, on the contrary, a negative relationship between DH and OHC [35].
Compared to other hydrolysates, such as herring protein (7.3 mL/g) [61] and rainbow trout (5.1 mL/g) [58], actual hydrolysates showed weaker OHCs. This can be justified by their relative richness in lipids.
In Vitro Antioxidant Properties of the XHMPHs
DPPH Radical-Scavenging Assay
The results presented in Fig. 5A clearly indicate that HM-Sa and HM-Al possessed significant efficacy in scavenging the DPPH radical, and that this efficacy is dose-dependent. The increase of the antioxidant activity against DPPH and the scavenging activity of all hydrolysates increased with increasing the hydrolysates concentration has been observed in several previous studies [16, 17, 52].
Antioxidant activity: A scavenging effect on DPPH free radical, B ferrous ion chelating activity, C total antioxidant activities, BHA and EDTA were used as positive control. Values are given as mean ± SD from triplicate determinations (n = 3). UHMP: Undigested proteins. X. gladius heads muscle protein hydrolysates were obtained by treatment with Savinase (HM-Sa) and Alcalase (HM-Al). a–e: Different small letters indicate significant differences at different concentrations in the same sample. (p < 0.05). A–D: Different capital letters designate differences between samples at the same concentration (p < 0.05).
The greatest efficacy against this radical was observed with HM-Sa at 5 mg/mL (72.22 ± 3.1%) (57.83 ± 2.39% for HM-Al and 21.65 ± 0. 83% for UHMP), suggesting that HM-Sa is the richest sample in electron-donating peptides which could react with free radicals to convert them to more stable products and terminate the radical chain reaction.
Ferrous Ion Chelating Activity
The ferrous ion chelating activities of XHMPHs, UHMP and EDTA were assayed at different concentrations. The results obtained are presented in Fig. 5B. At all concentrations tested, the hydrolysates showed higher Fe2 + ion chelating activity than UHMP. At a concentration of 3 mg/ml, two hydrolysates and EDTA showed the same activity which is very clearly stronger than that of UHMP. Depending on their respective activity when present at a lower concentration, the present samples had the following classification: EDTA > HM-Al > HM-Sa > UHMP. At 1 mg/mL EDTA, the activity of EDTA was 100% while that of HM-Al and HM-Sa was 63.70% and 50.31%, respectively. The different activity of the samples could be due to the difference in the characteristics of their constituents like, for example, the presence of acidic and basic amino acids with carboxyl and amino groups in the side chains [62].
Total Antioxidant Capacity
The total antioxidant capacity of XHMPHs and the BHA, evaluated with a quantitative Method, is depicted in Fig. 5C. All hydrolysates showed increasing antioxidant activity with increasing concentration, and HM-Al showed the greatest antioxidative efficacy (174.88 µmol/mL α-tocopherol equivalents) at 5 mg/ml, followed by HM-Sa (158.55 µmol/mL α- tocopherol equivalents). The undigested muscle exhibited the lowest antioxidant activity (90.16 µmol/mL α-tocopherol equivalents). The results are comparable to that proved by bougatef et al. [63]. BHA, as positive control, was found to be more efficient (205 µmol/mL α-tocopherol equivalents) at the same concentration (p < 0.05).
Conclusion
In the present study, two different DH hydrolysates are prepared by subjecting swordfish head proteins to the action of savinase or alcalase. The hydrolysates proved to be of advantageous composition, in particular by their high protein content and their amino acid profile. Moreover, they exhibited improved functional properties and significant antioxidant activity. Indeed, the differences between the antioxidant activities of the protein hydrolysates might be due to the fact that peptides are different in terms of chain length as well as amino acid sequences. HM-Sa was found to be effective antioxidants in different in vitro assays. However, the results observed reveal a sensitivity of hydrolysate properties to the enzymatic specificity and/or to the DH. Although both hydrolysates were effective, but HM-Sa, with the higer DH and more hydrophobic acids, exibet the higher antioxydant activity.
Actual hydrolysates could be used as food supplements and natural additives to correct the EAA status, improve the rheological properties and prevent the oxidation process of food products. However, it may be useful to adapt the choice of the enzyme and of the DH to the properties sought. thus, an optimization of the hydrolysis conditions is beneficial to produce more active peptides.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Martins, C.P.C., Ferreira, M.V.S., Esmerino, E.A., Moraes, J., Pimentel, T.C., Rocha, R.S., Freitas, M.Q., Santos, J.S., Ranadheera, C.S., Rosa, L.S., Teodoro, A.J., Mathias, S.P., Silva, M.C., Raices, R.S.L., Couto, S.R.M., Granato, D., Cruz, A.G.: Chemical, sensory, and functional properties of whey-based popsicles manufactured with watermelon juice concentrated at different temperatures. Food Chem. 255, 58–66 (2018). https://doi.org/10.1016/j.foodchem.2018.02.044
Kumoro, A.C., Wardhani, D.H., Kusworo, T.D., Djaeni, M., Ping, T.C., Ma’rifat Fajar Azis, Y.: Fish protein concentrate for human consumption: a review of its preparation by solvent extraction methods and potential for food applications. Ann. Agric. Sci. 67, 42–59 (2022). https://doi.org/10.1016/j.aoas.2022.04.003
Gao, R., Yu, Q., Shen, Y., Chu, Q., Chen, G., Fen, S., Yang, M., Yuan, L., McClements, D.J., Sun, Q.: Production, bioactive properties, and potential applications of fish protein hydrolysates: developments and challenges. Trends Food Sci. Technol. 110, 687–699 (2021). https://doi.org/10.1016/j.tifs.2021.02.031
Thirukumaran, R., Anu Priya, V.K., Krishnamoorthy, S., Ramakrishnan, P., Moses, J.A., Anandharamakrishnan, C.: Resource recovery from fish waste: prospects and the usage of intensified extraction technologies. Chemosphere 299, 134361 (2022). https://doi.org/10.1016/j.chemosphere.2022.134361
Kakkos, S., Kirkilesis, G., Caprini, J.A., Geroulakos, G., Nicolaides, A., Stansby, G., Reddy, D.J.: Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst. Rev. (2022). https://doi.org/10.1002/14651858.CD005258.pub4
Lees, M.J., Carson, B.P.: The potential role of fish-derived protein hydrolysates on metabolic health, skeletal muscle mass and function in ageing. Nutrients 12, 2434 (2020). https://doi.org/10.3390/nu12082434
Morifuji, M., Ishizaka, M., Baba, S., Fukuda, K., Matsumoto, H., Koga, J., Kanegae, M., Higuchi, M.: Comparison of different sources and degrees of hydrolysis of dietary protein: effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 58, 8788–8797 (2010). https://doi.org/10.1021/jf101912n
Barkia, A., Bougatef, A., Khaled, H.B., Nasri, M.: Antioxidant activities of sardinelle heads and/or viscera protein hydrolysates prepared by enzymatic treatment. J. Food Biochem. 34, 303–320 (2010). https://doi.org/10.1111/j.1745-4514.2009.00331.x
Henriques, A., Vázquez, J.A., Valcarcel, J., Mendes, R., Bandarra, N.M., Pires, C.: Characterization of protein hydrolysates from fish discards and by-products from the North-West Spain fishing fleet as potential sources of bioactive peptides. Mar. Drugs 19, 338 (2021). https://doi.org/10.3390/md19060338
Lassoued, I., Jridi, M., Nasri, R., Dammak, A., Hajji, M., Nasri, M., Barkia, A.: Characteristics and functional properties of gelatin from thornback ray skin obtained by pepsin-aided process in comparison with commercial halal bovine gelatin. Food Hydrocoll. 41, 309–318 (2014). https://doi.org/10.1016/j.foodhyd.2014.04.029
Park, P.-J., Jung, W.-K., Kim, S.-K., Jun, S.-Y.: Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limandaaspera) frame protein. Eur. Food Res. Technol. 219, 20–26 (2004). https://doi.org/10.1007/s00217-004-0882-9
dos Santos, S.D., Martins, V.G., Salas-Mellado, M., Prentice, C.: Evaluation of functional properties in protein hydrolysates from bluewing searobin (Prionotuspunctatus) obtained with different microbial enzymes. Food Bioprocess. Technol. 4, 1399–1406 (2011). https://doi.org/10.1007/s11947-009-0301-0
Kristinsson, H., Rasco, B.: Fish protein hydrolysates: production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 40, 43–81 (2000). https://doi.org/10.1080/10408690091189266
Liu, Y., Li, X., Chen, Z., Yu, J., Wang, F., Wang, J.: Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem. 151, 459–465 (2014). https://doi.org/10.1016/j.foodchem.2013.11.089
Ito, N., Hirose, M., Fukushima, S., Tsuda, H., Shirai, T., Tatematsu, M.: Studies on antioxidants: their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem. Toxicol. 24, 1071–1082 (1986). https://doi.org/10.1016/0278-6915(86)90291-7
Imen, L., Imen, E., Fatma, H., Ahmed, B.: Thornback ray gelatin hydrolysate as an alternative preservative: effect of hydrolysis degree. (AJNFS). pp. 9–14. (2022). https://fac.umc.edu.dz/inataa/revue/files/ajnfs0202002.pdf
Lassoued, I.: Evaluation of four fish protein hydrolysates as a source of antioxidants and amino acids. Journal of Cleaner Production. Vol. 37. (2022). https://doi.org/10.1016/j.jclepro.2022.131303.
McClements, D.J., Jafari, S.M.: General aspects of nanoemulsions and their formulation. In: Nanoemulsions, pp. 3–20. Elsevier (2018). https://doi.org/10.1016/B978-0-12-811838-2.00001-1
Hattour, A., Gaamour, A., Ben Smida, M.A.: Etude préliminaire de l’âge et de la croissance de l’espadon <Xiphias gladius> des eaux tunisiennes. Preliminary study of the age and growth of the swordfish (Xiphias gladius) of tunisian water. (2005)
AOAC: Official methods of analysis. association of official analytical chemists, 14th edn. AOAC, Arlington (1984)
Soxhlet, F.: Die gewichtsanalytische bestimmung des milchfettes. Dinglers Polytechnisches J. 232, 461–465 (1879)
AOAC: pdf, (2000). http://webpages.icav.up.pt/PTDC/CVT-NUT/4294/2012/AOAC%202000.pdf
Dupas, C., Adt, I., Cottaz, A., Boutrou, R., Molle, D., Jardin, J., Jouvet, T., Degraeve, P.: A chromatographic procedure for semi-quantitative evaluation of caseinphosphopeptides in cheese. Dairy Sci. Technol. 89, 519–529 (2009). https://doi.org/10.1051/dst/2009027
Parrot, S., Degraeve, P., Curia, C., Martial-Gros, A.: In vitro study on digestion of peptides in emmental cheese: analytical evaluation and influence on angiotensin I converting enzyme inhibitory peptides. Nahrung 47, 87–94 (2003). https://doi.org/10.1002/food.200390032
Tsumura, K., Saito, T., Tsuge, K., Ashida, H., Kugimiya, W., Inouye, K.: Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT - Food Sci. Technol. 38, 255–261 (2005). https://doi.org/10.1016/j.lwt.2004.06.007
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951). https://doi.org/10.1016/S0021-9258(19)52451-6
Pearce, K.N., Kinsella, J.E.: Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agric. Food Chem. 26, 716–723 (1978). https://doi.org/10.1021/jf60217a041
Shahidi, F., Han, X.-Q., Synowiecki, J.: Production and characteristics of protein hydrolysates from capelin (Mallotusvillosus). Food Chem. 53, 285–293 (1995). https://doi.org/10.1016/0308-8146(95)93934-J
McConnell, A.A., Eastwood, M.A., Mitchell, W.D.: Physical characteristics of vegetable foodstuffs that could influence bowel function. J. Sci. Food Agric. 25, 1457–1464 (1974). https://doi.org/10.1002/jsfa.2740251205
Lin, M.J.Y., Humbert, E.S., Sosulski, F.W.: Certain functional properties of sunflower meal products. J. Food Sci. 39, 368–370 (1974). https://doi.org/10.1111/j.1365-2621.1974.tb02896.x
Bersuder, P., Hole, M., Smith, G.: Antioxidants from a heated histidine-glucose model system. I: investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J. Amer Oil Chem. Soc. 75, 181–187 (1998). https://doi.org/10.1007/s11746-998-0030-y
Decker, E.A., Welch, B.: Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 38, 674–677 (1990). https://doi.org/10.1021/jf00093a019
Prieto, P., Pineda, M., Aguilar, M.: Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341 (1999). https://doi.org/10.1006/abio.1999.4019
Alahmad, K., Xia, W., Jiang, Q., Xu, Y.: Effect of the degree of hydrolysis on nutritional, functional, and morphological characteristics of protein hydrolysate produced from bighead carp (Hypophthalmichthysnobilis) using ficin enzyme. Foods 11, 1320 (2022). https://doi.org/10.3390/foods11091320
Sathivel, S., Smiley, S., Prinyawiwatkul, W., Bechtel, P.J.: Functional and nutritional properties of red salmon (Oncorhynchusnerka) enzymatic hydrolysates. J. Food Sci. 70, c401–c406 (2005). https://doi.org/10.1111/j.1365-2621.2005.tb11437.x
Liceaga-Gesualdo, A.M., Li-Chan, E.C.Y.: Functional properties of fish protein hydrolysate from herring (Clupeaharengus). J. Food Sci. 64, 1000–1004 (1999). https://doi.org/10.1111/j.1365-2621.1999.tb12268.x
Muzaifa, M., Safriani, N., Zakaria, F.: Production of protein hydrolysates from fish by-product prepared by enzymatic hydrolysis. Aquac. Aquar. Conserv. Legis. 5, 36–39 (2012)
Thammapat, P., Raviyan, P., Siriamornpun, S.: Proximate and fatty acids composition of the muscles and viscera of asian catfish (Pangasiusbocourti). Food Chem. 122, 223–227 (2010). https://doi.org/10.1016/j.foodchem.2010.02.065
Lassoued, I., Mora, L., Nasri, R., Aydi, M., Toldrá, F., Aristoy, M.-C., Barkia, A., Nasri, M.: Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Rajaclavata) muscle. J. Proteom. 128, 458–468 (2015). https://doi.org/10.1016/j.jprot.2015.05.007
Johnrose, P., Vincent, S., Joseph, S., Magdalene, J.: Bioactive and functional properties of fish protein hydrolysate from leiognathus bindus. Asian J. Pharm. Clin. Res. 9, 277 (2016). https://doi.org/10.22159/ajpcr.2016.v9i5.14002
Hunter, R.W., Dhaun, N., Bailey, M.A.: The impact of excessive salt intake on human health. Nat. Rev. Nephrol. 18, 321–335 (2022). https://doi.org/10.1038/s41581-021-00533-0
Li, L., Jiang, Y.: Proximate composition and nutritional profile of rainbow trout (Oncorhynchusmykiss) heads and skipjack tuna (KatsuwonusPelamis) heads. Molecules 24, 3189 (2019). https://doi.org/10.3390/molecules24173189
Latorres, J.M., Rios, D.G., Saggiomo, G., Wasielesky, W., Prentice-Hernandez, C.: Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeusvannamei). J. Food Sci. Technol. 55, 721–729 (2018). https://doi.org/10.1007/s13197-017-2983-z
Kakko, T., Damerau, A., Nisov, A., Puganen, A., Tuomasjukka, S., Honkapää, K., Tarvainen, M., Yang, B.: Quality of protein isolates and hydrolysates from Baltic Herring (Clupea harengus membras) and Roach (Rutilus rutilus) produced by pH-Shift processes and enzymatic hydrolysis. Foods. 11, 230 (2022). https://doi.org/10.3390/foods11020230
Gorissen, S.H.M., Crombag, J.J.R., Senden, J.M.G., Waterval, W.A.H., Bierau, J., Verdijk, L.B., van Loon, L.J.C.: Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50, 1685–1695 (2018). https://doi.org/10.1007/s00726-018-2640-5
Smida, M.A.B., Marzouk, B., Cafsi, M.E.: The composition of fatty acids in the tissues of tunisian swordfish (Xiphiasgladius). Food Chem. 115, 522–528 (2009). https://doi.org/10.1016/j.foodchem.2008.12.084
Vázquez, J.A., Sotelo, C.G., Sanz, N., Pérez-Martín, R.I., Rodríguez-Amado, I., Valcarcel, J.: Valorization of aquaculture by-products of salmonids to produce enzymatic hydrolysates: process optimization, chemical characterization and evaluation of bioactives. Mar. Drugs 17, 676 (2019). https://doi.org/10.3390/md17120676
Jia, J., Zhou, Y., Lu, J., Chen, A., Li, Y., Zheng, G.: Enzymatic hydrolysis of alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food. Agric. 90, 635–640 (2010). https://doi.org/10.1002/jsfa.3861
Noman, A., Ali, A.H., AL-Bukhaiti, W.Q., Mahdi, A.A., Xia, W.: Structural and physicochemical characteristics of lyophilized chinese sturgeon protein hydrolysates prepared by using two different enzymes. J. Food Sci. 85, 3313–3322 (2020). https://doi.org/10.1111/1750-3841.15345
Wilding, P., Lillford, P.J., Regenstein, J.M.: Functional properties of proteins in foods. J. Chem. Technol. Biotechnol. 34, 182–189 (2008). https://doi.org/10.1002/jctb.280340307
Foh, M., Amadou, I., Kamara, M., Foh, B., Xia, W.: Effect of enzymatic hydrolysis on the nutritional and functional properties of nile tilapia (Oreochromis niloticus) proteins. Am. J. Biochem. Mol. Biol. 1, 54–67 (2011). https://doi.org/10.3923/ajbmb.2011.54.67
Ben Slama-Ben Salem, R., Bkhairia, I., Abdelhedi, O., Nasri, M.: Octopusvulgaris protein hydrolysates: characterization, antioxidant and functional properties. J. Food Sci. Technol. 54, 1442–1454 (2017). https://doi.org/10.1007/s13197-017-2567-y
Bordbar, S., Ebrahimpour, A., Zarei, M., Hamid, A.A., Saari, N.: Alcalase-generated proteolysates of stone fish (Actinopygalecanora) flesh as a new source of antioxidant peptides. Int. J. Food Prop. 21, 1541–1559 (2018)
Amiza, M.A., Kong, Y.L., Faazaz, A.L.: Effects of degree of hydrolysis on physicochemical properties of cobia (Rachycentron canadum) frame hydrolysate. Inte. Food Research Journal. ProQuest 19(1), 199-206 (2012).
Lawal, O.S.: Functionality of african locust bean (Parkiabiglobossa) protein isolate: effects of pH, ionic strength and various protein concentrations. Food Chem. 86, 345–355 (2004). https://doi.org/10.1016/j.foodchem.2003.09.036
Souissi, N., Bougatef, A., Triki-Ellouz, Y., Nasri, M.: Biochemical and functional properties of sardinella (Sardinella aurita) by-product hydrolysates. Food Technol. Biotechnol. 45(2), 187–194 (2007)
Ge, Y., Sun, A., Ni, Y., Cai, T.: Some nutritional and functional properties of defatted wheat germ protein. J. Agric. Food Chem. 48, 6215–6218 (2000). https://doi.org/10.1021/jf000478m
Taheri, A., Anvar, S.A.A., Ahari, H., Fogliano, V.: Comparison the functional properties of protein Hydrolysates from poultry byproducts and rainbow trout (Onchorhynchus mykiss) viscera. Iran. J. Fish. Sci. 12(1) 154-169 (2013). https://hdl.handle.net/1834/37351
Fawzya, Y.N., Nursatya, S.M., Susilowati, R., Chasanah, E.: Characteristics of fish protein hydrolysate from yellowstripe scad Selaroidesleptolepis produced by a local microbial protease. E3S Web Conf. 147, 03017 (2020). https://doi.org/10.1051/e3sconf/202014703017
Haldar, A., Das, M., Chatterjee, R., Dey, T.K., Dhar, P., Chakrabarti, J.: Functional properties of protein hydrolysates from fresh water mussel Lamellidens marginalis (Lam). Indian J. Biochem. Biophys. 55, 9 (2018)
Sathivel, S., Bechtel, P., Babbitt, J., Smiley, S., Crapo, C., Reppond, K.D., Prinyawiwatkul, W.: Biochemical and functional properties of herring (Clupea harengus) byproduct hydrolysates. J. Food Sci. 68, 2196–2200 (2003). https://doi.org/10.1111/j.1365-2621.2003.tb05746.x
Wiriyaphan, C., Xiao, H., Decker, E.A., Yongsawatdigul, J.: Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 167, 7–15 (2015). https://doi.org/10.1016/j.foodchem.2014.06.077
Bougatef, A., Hajji, M., Balti, R., Lassoued, I., Triki-Ellouz, Y., Nasri, M.: Antioxidant and free radical-scavenging activities of smooth hound (Mustelusmustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 114, 1198–1205 (2009). https://doi.org/10.1016/j.foodchem.2008.10.075
Acknowledgements
The authors greatly appreciate the Ministry of Higher Education and Scientific Research-Tunisia, for its providing financial support for the realization of this study.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elgaoud, I., Hamed, F., Lassoued, I. et al. Production of Hydrolysates from Swordfish (Xiphias gladius) Head Muscle as New Protein Source: Evaluation of Nutritional, Antioxidant and Functional Properties. Waste Biomass Valor 15, 1065–1080 (2024). https://doi.org/10.1007/s12649-023-02224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02224-2