Abstract
In this study, carotenoprotein from Pacific white shrimp (Litopenaeus vannamei) processing waste was extracted with the aid of alcalase (CP-A) and pepsin (CP-P) at various levels (0–4 units/100 g waste) for different times (0–240 min). Recovery of carotenoids and protein increased with 3 unit/g of enzyme and hydrolysis times until 180 min (p < 0.05). The extracted carotenoprotein by pepsin and alcalase consisted of 72.11–75.32% protein and carotenoids content was in the range of 330–530 µg/g samples. The phenylalanine, lysine, methionine, and valine as essential amino acids were higher at CP-A and CP-P. The dominant non-essential amino acids in carotenoproteins were aspartic acid, glutamic acid, glycine, and alanine. It was rich in mono and polyunsaturated fatty acids. The CP-A showed higher content of docosahexaenoic acid and eicosapentaenoic acid (8.52 and 6.49%) than CP-P (5.55 and 5.49%). The saturated fatty acids were reduced after enzymatic hydrolysis and contents were higher in carotenoproteins. The extracted samples showed a significant amount of mineral contents. Sodium, phosphorus, magnesium, and potassium contents were found higher in CP-A. The lead and copper were reduced as a result of hydrolysis. Therefore, carotenoprotein from processing residue of pacific white shrimp could be used as the value-added nutritious enriching food or feed powder.
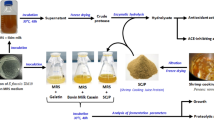
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Extraction of carotenoprotein could be valorized shrimp waste and reduce environmental problems. These residues are one of the problems of shrimp processors in developing countries. Lots of researchers evaluated the extraction of carotenoprotein under various conditions in their work. In the present work, carotenoproteins were prepared by two types of common enzymes.
The obtained data can be useful in the option of the find a suitable method to producing carotenoprotein for animal feed or food. It suggests that other ways are better to investigate and a comparison could be helpful.
Introduction
Annually, a substantial content of residues, by-products, and wastes produces by the fish and crustacean processing industry. This discards, burden severe environmental and ecological influences, also have considerable effects on the economic viability of the fisheries sectors [1, 2]. In recent years, growing considerations have been given to the waste of shrimp processing [3, 4]. The shrimp processing generates considerable quantities of waste in the form of heads, tails, and cephalothorax; which these large amounts of discards cause some problems [5, 6].
In 2018, the farmed shrimp represented 6 million tonnes; which the share of whiteleg shrimp between the all farmed crustaceans was 52.9%. Between 1998 and 2018, the share of whiteleg shrimp in the world production of farmed shrimp increased from 20.4 to 82.7% and the annual production of farmed whiteleg shrimp was 20.6% [7]. Approximately 50–70% of the whole weight of the raw material estimates as the wastes of shrimp processing. These residues have been explored as a potential and rich source of chitin, protein, carotenoid, shrimp hydrolysate, shrimp flavorant, nutritive components, PUFA, and enzymes [5, 8, 9]. In Iran, a small quantity of the waste is being used as manure and animal feed mix. Thus, the large quantity of this organic waste is a problem for processors while can be a potential source of bioactive products.
Today, many researchers no longer bring up shrimp by-products as waste and instead it is known as a raw material that is prepared for usage in other processes [10]. Carotenoid and carotenoproteins have been known as bioactive compounds that have the potential to use as natural colorants, antioxidants, antimicrobial agents in different industries such as food, pharmacology, textiles, chemicals, and feed [5, 9, 11, 12]. Carotenoproteins are protein–pigment complexes as stable products; which carotenoids by binding to the protein (hydrophobic sites) will be more stable [13]. Therefore, using such discards shows the economic value and has drawn more attention in recent years [3, 11]. Some investigates have been carried out on the isolation of carotenoproteins from shrimp and crab processing discards by using different methods such as autolytic hydrolysis [5], enzymatic hydrolysis [2, 14], and fermentation [15]. Shrimp waste hydrolysate could be a biological source of active peptides with considerable potential in pharmacology, anti-cancer activity, and/or as growth-stimulating agents in animal feeds [16, 17]. In a study, extracted alkaline proteinases from fish viscera had applied to extract carotenoproteins [9, 18], and the extracted were studied as an antioxidant agent in system models [19].
According to previous studies, enzymatic hydrolysis seems one of the suitable methods to increase the efficiency extraction of caroteneproteins [9]. The hydrolysis condition is effective on the yield and characteristics of carotenoprotein from shrimp processing waste. Therefore, the present study investigates the impact of hydrolysis using Alcalase and pepsin on the extraction of carotenoproteins from Pacific white shrimp waste processing. Furthermore, this study aimed to describe some biochemical characteristics, mineral content, and nutritional characteristics such as amino and fatty acids contents of extracted carotenoproteins.
Materials and Methods
Chemicals
Alcalase (Bacillus licheniformis, 2.4 AU-A/g) was obtained from Novozymes (Bagsvaerd, Denmark) and pepsin (porcine gastric mucosa), bovine serum albumin (BSA), Boron trifluoride (BF3), fatty acid standards, were obtained from Sigma-Aldrich (St Louis, Mo, USA). All other chemicals were of analytical grade and supplied by AppliChem (GmbH, Darmstadt, Germany) and Merck (KGaA, Darmstadt, Germany).
Preparation of Raw Material
Shrimp processing waste (a mixture of cephalothorax, shell, and tail) were obtained Litopenaeus vannamei (Shilabzi shrimp processing Co., Golestan, Iran). The waste was transported to the laboratory within 30 min; upon arrival, the raw waste was grounded by a food processor (Panasonic, Malaysia Sdn Bhd.), was packed and stored in polyethylene bags at − 20 °C until the experiment.
Extraction of Carotenoprotein Using Proteases
Carotenoproteins (CP-A and CP-P) extraction from shrimp waste was performed according to Chakrabarti [26] and Sila et al. [2]. At the first, the optimal concentration of enzymes (pepsin and alcalase) and time of hydrolysis were determined. 25 g of homogenized wastes were mixed with EDTA buffer solution (0.5 M) in a ratio of 1:2.5 and the pH of the mixture was adjusted (pepsin, 2 and alcalase, 8). Enzymes were added (0 to 4 U/g) and the mixture was incubated for 0, 60, 120, 180, and 240 min (rpm 200) (IKA-Werke GmbH & Co. KG, Staufen Germany). In the end, the mixture was filtered via a multilayer cleaning cloth; the pH was adjusted to 4.5 (HCl 2 M) and was centrifuged at 3913 g (4 °C, 20 min), then it was dissolved in 20 ml of 5 mmol sodium phosphate buffer (pH 7) and lyophilized (Christ, ALPHA 2–4 LSC, Gmb, Germany). The dried CPs were stored in tightly closed plastic containers. The optimal condition of hydrolysis was determined based on the recovery protein and carotenoid contents.
Total Carotenoid
Total carotenoid in the waste and CPs was measured. 1 g of the sample was mixed with acetone (25 ml) and homogenized (2 min, 16,000 rpm); then filtered (Whatman paper filter, No.1) and was drained in a separating funnel and was subdivided with an equal volume of petroleum ether. The separator funnel was shaken and stands for 30 min (24 °C). The top layer was washed twice with an equal volume of water. The obtained petroleum ether layer was passed and dehydrated by anhydrous Na2SO4. All petroleum ether was evaporated by rotary at vacuum condition. The residue was appropriately diluted in petroleum ether (10 ml) and the absorbance was read at 468 nm. The carotenoid content (µg/g) was calculated by the Eq. (1); of which 0.2 is the absorbance of standard astaxanthin (1 mg/ml).
Protein Recovery of Hydrolyzed Shrimp Waste
Protein solubility was measured at different times according to the biuret method [20]. 100 mg of the sample was mixed with 0.5 M NaOH (10 ml) at 85 °C. After 1 h incubation, the samples were centrifuged (3913 × g, 20 min); the supernatant was tested for protein content. The bovine serum albumin (BSA) was applied as the standard and the results were reported in mg/mL by BSA standard curve [12].
Proximate Analysis and Chitin Content
The moisture, protein, fat, and ash contents of the uniformly grounded waste and CPs were estimated by standard methods [21]. To determine of chitin content, 2 g of sample was mixed by 30 ml of NaOH (2%, w/v) for 6 h at 25 C. After incubation (6 h), the mixture was filtered (Whatman paper filter, No. 1) and was incubated with HCl (1 mM) for 30 min (25 °C). After the filtration, samples were washed and the pigment removed by vigorously homogenized (Ultraturax, IKA-Werke GmbH & Co. KG, Staufen Germany) with cold acetone (14,000 rpm, 5 min). Then, it was washed with water and mixed with NaOCl (0.3%). After incubation for 6 h at 25 °C, the sample was filtered and washes with distilled water and was dried (24 h, 60 °C). The chitin content was expressed as a percentage.
Determination of Color
The color was measured using a Lovibond CAM-System 500, Colorimeter Imaging (Tintometer Ltd., Amesbury, UK). The color (L*, lightness; a*, redness/greenness; and b*, yellowness/blueness) indexes were reported in the CIE system.
Determination of Amino Acid
The amino acids composition of SW and CPs was analyzed. The sample was analyzed through hydrolysis of samples using 6 M HCl (1 h, 110 °C). After cooling, an aliquot of the sample was subjected to neutralization (pH 1.5–5). Derivatization of amino acids was done by phenyl isothiocyanate. The amino acid standard and hydrolysates were dissolved in 100 mL buffer; 1–10 mL of the solutions were analyzed by reverse-phase HPLC (PerkinElmer, Shelton, CT, USA). Tryptophan was measured, separately.
Determination of Fatty Acid Composition
Fatty acid compositions of SW and CPs were defined as fatty acid methyl esters (FAMEs) according to Metcalfe and Schmitz [22] using a gas chromatograph (GC Hewlett Packard 5890 series, Avondale, PA., USA); which provided a flame ionization detector. MEs were acquired from fatty acids in the lipid extract of samples through Boron trifluoride esterification. The MEs were afterward dissolved in n-heptane and were injected into the GC and breakdown was carried out on a fused silica capillary column (30 m × 0.32 mm, 0.25 μm; Supelco, Bellefonte, PA, USA). Helium was used as a carrier gas. The initial temperature of the oven was introduced at 160 °C and went up to 200 °C (3 °C/min) and continued for 1 min. It was more increased to 220 °C at 3 °C/min and the temperature was kept for 17 min. The temperature of the detector and injection was planned at 240 and 250 °C, respectively. Identification of chromatographic peaks was carried out to the retention time of FAME standards. In the end, fatty acid compositions were shown as the percentage of total fatty acids.
Determination of Mineral and Heavy Metals
The minerals and heavy metals (calcium, sodium, potassium, iron, manganese, zinc, magnesium, copper, cadmium, mercury, and lead) content of shrimp waste and carotenoproteins were determined. The nitric acid and H2O2 were used to digest the samples. The analysis was determined by Optima 8000 ICP-OES Spectrometer (PerkinElmer, Inc., Waltham, USA).
Statistical Analysis
The results were analyzed using a one-way analysis of variance in the completely randomized design. To compare the means Tukey’s test was carried out. The analysis was accomplished by IBM SPSS Statistics for Windows (Version 22.0., IBM Corp. Armonk, NY., USA).
Results and Discussion
Protein Solubility of Shrimp Waste
The Protein solubility of Vannami shrimp waste at different pH was determined (Fig. 1). This was similar to the isoelectric pH of other species of shrimp [14, 23]. At pH 4 to 7, the solubility of the protein was low and in the range of 1–3 and 8–10, protein had a higher solubility. At pH 6, the solubility was the lowest (1.8 mg/ml). This pH was determined at 5.78 for the brown shrimp [14]. This solubility is extremely related to pH; which at the isoelectric point, the lowest protein solubility is happening. At this point, protein has a net charge to zero, and the highest isoelectric point is at high acid or basic pH [24].
According to Latorres et al. [25], The low solubility of protein is related to the firm structure of macromolecule to subunits; which are bounded by multiple inter and intramolecular disulfide bonds and hydrophobic interactions. Therefore, hydrolysis of shrimp waste may increase the solubility of the protein.
Effect of Different Types and Levels of Enzymes and Hydrolysis Times on the Recovered Carotenoprotein from Shrimp Waste
The effect of different levels of enzymes (alcalase and pepsin) and times of hydrolysis incubation on the recovery of protein and carotenoid from shrimp waste has shown in Figs. 2 and 3.
As expected, with increasing time of hydrolysis and concentration of enzymes, recovery of carotenoid and protein was increased. These recoveries significantly increased at higher units of the enzyme (3 U/g). The control batch proved that enzymes at all levels had positive results on the proteolysis of shrimp waste and carotenoid extraction. Nevertheless, in protein recovery, a higher level of the enzymes (> 2 U/g) was more effective (p < 0.05); which was previously reported by Sila et al. [2]. No significant differences in protein and carotenoid recoveries were considerable at levels of 3 and 4 U/g enzymes. In both enzymes, the hydrolysis of the waste was increased after 2 h; then, a slower rate of hydrolysis was found 180 min up to 240 min and reached the equilibrium phase (p > 0.05). No significant differences in recoveries were observed after 180 min of hydrolysis. The comparison of enzymes showed that the alcalase was more effective than pepsin in the recovery of compounds from white shrimp waste (p < 0.05). The highest recovery of protein by 3 U/g of pepsin and alcalase was 55.85 and 50.1%, respectively; which had no significant difference by 4 units of the enzymes. In the case of carotenoid recovery, the higher content was 532.14 and 531.21 mg/g for alcalase and pepsin at a concentration of 3.0 U/g of Barse et al. [26] reported that trypsin was more effective than papain and pepsin for extracting carotenoprotein from shrimp shells. Related results were also observed by Klomklao et al. [27], Sila et al. [2], and Senphan et al. [12] when different concertation and type of proteases were applied for hydrolysis of shrimp wastes to the recovery of carotenoid and protein. The enzyme levels are various in different studies related to the activities of the enzyme. In the current study, 3 units of enzymes showed acceptable results of recovery. It was inconsistent with the results of pink shrimp shells hydrolysis by a barbell and bovine trypsin (2 unit/g shell) [2]; while 1.2 units of trypsin enhanced the carotenoprotein extraction from tiger shrimp exoskeleton [27].
Proximate Composition of Shrimp Waste and Carotenoproteins
The proximate compositions and chitin contents of shrimp waste were 35.71% protein, 1.75% lipid, 39.42% ash, and 22.48% (Table 1).
CP-P showed a significantly higher yield (4.10%) than CP-A carotenoproteins (3.10%). Protein in various shrimp shells has been reported about 31 to 49% [2, 12, 23]. Due to the great protein content of the waste, this source could be used directly to the formulations for feed [23]. The max protein content in the CP-A and CP-P was 75.32 and 72.11%, respectively (p > 0.05); which is within the range of 71.09 to 80.05 reported by previous studies [2, 28]. Higher protein content in carotenoproteins than the raw waste could be attributed to the removal of insoluble solid matter besides protein solubilization by enzymatic hydrolysis [15, 29]. The carotenoid content in the SW was close to 24.06 µg/g, which was in the range of 35.8 µg/g tiger a prawn (P monodon) from 23 to 331 µg/g in the exoskeleton reported by previous studies. It depends on species, nutritional, and rearing conditions. Due to the effect of proteases, the total carotenoid in CP-A (530.37 µg/g) and CP-P was significantly higher than raw material; which is in agreement with the other findings [19, 27]. Sila et al. [2] reported 140 µg/g carotenoid in carotenoprotein extracted from pink shrimp waste.
Proteins of shellfish such as shrimp and crabs have nutritional worth; which is caused by a complex formation of the proteins with astaxanthin, and it has a mighty antioxidant activity [12, 30, 31]. Therefore, higher content of carotenoid will cause the antioxidant activity of the CPs. It was also observed about lipid content in CPs compared with that found in SW; which CP-A had a higher content of carotenoid and lipid in comparison to CP-P. The chitin and ash contents were relatively lower in CP-P and CP-A (2.47 and 1.61%) compared with SW (22.48%); which was inconsistent with Chakrabarti [14] also found that carotenoprotein from tropical brown shrimp shell waste extracted by trypsin, had lower ash and chitin contents than shrimp waste. Our findings are in line with previous works reported by Sila et al. [2], and Senphan et al. [12] who found high chitin at shrimp residue and the lower content in recovered CP of pink and white shrimps. It has been described that extraction by protease promotes the outgoing of protein, and lipid from shrimp residues, whiles chitin is maintained in the shell [12, 27]. According to these results, enzyme-derived CP can be used as a source of pigments, protein, and fats for human dietary supplements and salmon breeding [27].
Color of Shrimp Waste and Carotenoproteins
The color parameters of SW and recovered CPs are presented in Table 2. L* value (Lightness) was found higher in the SW (p < 0.05); which was significantly lower in CP-A and CP-P. SW had significantly lower a* and b*values (p < 0.05).
The recovered CP-A and CP-P samples were orange–red and CP-A showed higher redness of 38.60 (a*) and yellowness of 42.90 (b*). Higher values of a* and b* were related to the extracted astaxanthin along with protein. Seafood discards are rich in proteins, polyunsaturated fatty acids (PUFA), and other nutrients including carotenoids, minerals, vitamins, squalene, glycosaminoglycans, and others having various health benefits.
These results agreed with the reports by Senphan et al. [12] and Sila et al. [28] in a study on obtained carotenoproteins by proteases; which reported lower lightness and higher a* and b* in CPs than shrimp waste. Crustacean color depends on the astaxanthin content that causes a range of colors from green, yellow, blue to brown. The deposited astaxanthin is the responsibility of the color and hue saturation of crustaceans [32]; which could be used as a coloring agent in feed or maybe food.
Amino Acid Composition
The essential and non-essential amino acids were found at Pacific white shrimp waste (Table 3). The shrimp waste has a significant content of essential amino acids such as phenylalanine, lysine, valine, and leucine (155.34, 73.04, 59.00, and 58.05 mg/g sample, respectively). The non-essential amino acids such as glutamic acid, arginine, aspartic acid, and glycine also have been identified in amino acid profiles of shrimp waste (109.35, 82.05, 73.04, and 62.07 mg/g sample, respectively). Carotenoproteins extracted from shrimp shells had higher total non-essential amino acid (660.06 and 606.23 mg/g) level than shrimp waste (529.67 mg/g). The CPs were a significant source of the non-essential amino acids such as arginine (101.05 and 93.20 mg/g sample, respectively); which was higher in alcalase treated waste.
Due to degradation during hydrolysis, tryptophan was not in any sample. Carotenporoteins recovered by alcalase and pepsin were a significant source of the non-essential amino acids glutamic acid, aspartic acid, glycine, alanine (p < 0.05). The phenylalanine, lysine, and valine as essential amino acids were high at alcalase treated waste; while phenylalanine and lysine were at a higher concentration (124.74 and 54.24 mg/g) in CP-P. The non-essential amino acids aspartic acid, glutamic acid, glycine, and alanine were found in higher concentrations in both carotenoproteins; which alcalase treated waste displayed higher content amino acids. It was inconsistent by Pattanaik et al. [13] reporting between all amino acids, glutamic acid was in the main and high amount in all the CPs extracted from different shrimp species. It was also in agreement with the findings of Senphan et al. [12], Klomklao et al. [27] who reported that the CPs were abundant in these amino acids. Messina et al. [31] reported the size and the presence of some polar amino acids such as tyrosine, histidine, and lysine in protein hydrolysate of seafood processing waste could contribute to high antioxidant capacity. De Silva et al. [33] reports biotransformation of shellfish waste to carotenoprotein. The results showed autolysis was effective at the extraction of carotenoproteins contained small peptide fragments.
In a similar study, the essential amino acids in carotenoprotein of L. vannami shrimp waste were reported higher than in the current study [12]. Armenta and Guerrero-Legarreta [15] showed that carotenoprotein extracted from fermented vannami shrimp waste was rich in aspartic acid and glutamic acids. Cremades et al. [34], expressed that carotenoproteins could develop diets for patients who are suffering from specific diseases such as cancer–anorexia-cachexia syndrome and renal damage.
Fatty Acid Composition
Fatty acid compositions of shrimp waste and extracted carotenoprotein by different enzymes are presented in Table 4. The fatty acid profile of shrimp shells could be related to species differences and also farming and/or feeding conditions.
Shrimp waste exhibited a desirable content of PUFAs (33.85%); which were found to be more abundant than MUFAs and SFAs (27.37 and 29.85, respectively). The most abundant SFAs in SW were Palmitic (C16:0) (15.29%) and Stearic acids (C18:0) (6.20%) and the main MUFA was oleic acid (C18:1 n-9); among the PUFAs, DHA (C22:6n-3) and EPA (C20:5n-3) were the main PUFAs (5.50 and 4.79%, respectively) in shrimp waste. It was inconsistent with similar studies on pink shrimp shell fatty acids; which palmitic, stearic, and oleic acid was prevail in the shells. Total PUFA in this study was more than the reported of pink shrimp shells (22.48%) [2]. The SFAs were reduced after enzymatic hydrolysis and MUFA and PUFA contents were higher in carotenoproteins (p < 0.05). The high content of PUFA and the ratio of PUFA/SFA descending orders were: CP-A > CP-P > WS. The lowest ratio of total ω6 to ω3 was in CP-A samples. Palmitic acid, oleic acid, DHA, and EPA were found to be higher than other fatty acids of CP-A and CP-P; which CP-A showed higher DHA and EPA (8.52 and 6.49%) than CP-P (5.55 and 5.49%). According to, Cremades et al. [34] recovered CP from crayfish contained significant content of ω3 and ω6 fatty acids which bound to protein and make this compound as a nutraceutical product.
Mineral and Heavy Metal Composition
Table 5 shows the mineral and heavy metal compositions of the shrimp waste and recovered carotenoproteins. Minerals are essential to several important biochemical and physiological functions in the body [35]. In the present study, SW contained substantial amounts of calcium, phosphorus, and sodium.
It was likely due to the existence of wide concentrations of the minerals in the raw material. Sodium, phosphorus, magnesium, and potassium contents were found to be higher in CPs. The mineral content in CPs was affected by substrate and enzyme. Chalamaiah et al. [35] also considered the effect of enzyme and substrate type on the mineral type and content in hydrolyzed roe samples. The minerals in the SW were higher than CPs; which calcium is mainly found in higher quantities in waste. Furthermore, except for sodium, the mineral contents no significant difference was observed among CP-P and CP-A. Due to the neutralization of acidic pH by addition NaOH; sodium was mainly in higher quantity in CP-P [36]. In CP-A and CP-P, phosphorus and magnesium contents were significantly reduced than raw material. Regarding the heavy metals, the lead was in SW; which was eliminated in the CPs (Table 3). The elements decreased significantly after enzymatic hydrolysis. The presence of heavy metals in shrimp waste depends on the content of metals in the water and the breeding area. Copper, zinc, and iron are among essential heavy metals. According to Wu and Yang [37], the amount of Zn and Fe elements are reflect in aquatic environments. These essential heavy metals decreased in CPs; which were higher in CP-A samples.
Conclusions
The optimum conditions for the higher content of extracted carotenoid and protein of shrimp residue by alcalase and pepsin were studied. The major efficiency was found by the alcalase at 3 U/g during 180 min of incubation time, and a higher yield of extraction was found by pepsin. The results showed the raw shell of Litopenaeus vannami can be a source of protein and mineral contents. The carotenoproteins had a higher content of fatty acids and essential and non-essential amino acids and it confirmed that hydrolysis could be a valuable upgrading process for shrimp residue. This bio-macromolecule contained an acceptable level of mineral contents. The co-extraction of protein and carotenoid by a green method, as a bioactive and antioxidant powder, could be used as an additive, supplement, or functional ingredient in a variety of marketable foods, and more studies in this area are recommended.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CPs:
-
Carotenoproteins
- CP-A:
-
Carotenoprotein Recovered by Alcalase
- CP-P:
-
Carotenoprotein Recovered by Pepsin
- SW:
-
Shrimp Waste
- SFA:
-
Saturated Fatty Acids
- MUFA:
-
Mono Unsaturated Fatty Acids
- PUFA:
-
Poly Unsaturated Fatty Acids
- BSA:
-
Bovine Serum Albumin
- BF3:
-
Boron triFluoride
- FAMEs:
-
Fatty Acid Methyl Esters
References
Morales-Medina, R., et al.: Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem. 194, 1208–1216 (2016)
Sila, A., Nasri, M., Bougatef, A.: Isolation and characterisation of carotenoproteins from deep-water pink shrimp processing waste. Int. J. Biol. Macromol. 51(5), 953–959 (2012)
Sila, A., et al.: Ability of natural astaxanthin from shrimp by-products to attenuate liver oxidative stress in diabetic rats. Pharmacol. Rep. 67(2), 310–316 (2015)
Gómez-Estaca, J., et al.: Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 216, 37–44 (2017)
Sowmya, R., Rathinaraj, K., Sachindra, N.M.: An autolytic process for recovery of antioxidant activity rich carotenoprotein from shrimp heads. Mar. Biotechnol. 13(5), 918–927 (2011)
Sachindra, N.M., Bhaskar, N., Mahendrakar, N.S.: Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 26(10), 1092–1098 (2006)
FAO: The state of world fisheries and aquaculture. In: Sustainability in Action. FAO, Rome (2020)
Takeungwongtrakul, S., Benjakul, S.: Oxidative stability of lipids from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) as affected by essential oils incorporation. Eur. J. Lipid Sci. Technol. 116(8), 987–995 (2014)
Nasri, R., et al.: Digestive alkaline proteinases from Serranus scriba viscera: characteristics, application in the extraction of carotenoproteins from shrimp waste, and evaluation in laundry commercial detergents. Biocatal. Agric. Biotechnol. 4(3), 355–361 (2015)
Mechri, S., et al.: A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environ. Sci. Pollut. Res. 27(13), 15842–15855 (2020)
Sowmya, R., Sachindra, N.M.: Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chem. 134(1), 308–314 (2012)
Senphan, T., Benjakul, S., Kishimura, H.: Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci. 5, 54–63 (2014)
Pattanaik, S.S., et al.: Characterization of carotenoprotein from different shrimp shell waste for possible use as supplementary nutritive feed ingredient in animal diets. Aquaculture 515, 734594 (2020)
Chakrabarti, R.: Carotenoprotein from tropical brown shrimp shell waste by enzymatic process. Food Biotechnol. 16(1), 81–90 (2002)
Armenta, R.E., Guerrero-Legarreta, I.: Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 112(2), 310–315 (2009)
Kannan, A., et al.: Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation. J. Sci. Food Agric. 91(10), 1920–1924 (2011)
Gildberg, A., Stenberg, E.: A new process for advanced utilisation of shrimp waste. Process Biochem. 36(8), 809–812 (2001)
Poonsin, T., et al.: Albacore tuna spleen trypsin: potential application as laundry detergent additive and in carotenoprotein extraction from Pacific white shrimp shells. Biocatal. Agric. Biotechnol. 17, 638–646 (2019)
Poonsin, T., et al.: Carotenoprotein from Pacific white shrimp (Litopenaeus vannamei) shells extracted using trypsin from albacore tuna (Thunnus alalunga) spleen: antioxidant activity and its potential in model systems. J. Food Biochem. 42(2), e12462 (2018)
Gornall, A.G., Bardawill, C.J., David, M.M.: Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177(2), 751–766 (1949)
AOAC: Official Methods of Analysis. AOAC, Gaithersburg (2002)
Metcalfe, L.D., Schmitz, A.A.: The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 33(3), 363–364 (1961)
Sánchez-Camargo, A.P., et al.: Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 102(1), 87–93 (2011)
Gómez-Estaca, J., Montero, P., Gómez-Guillén, M.C.: Chemical characterization of wash water biomass from shrimp surimi processing and its application to develop functional edible films. J. Food Sci. Technol. 55(10), 3881–3891 (2018)
Latorres, J.M., et al.: Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 55(2), 721–729 (2018)
Barse, A.V., et al.: Endocrine disruption and metabolic changes following exposure of Cyprinus carpio to diethyl phthalate. Pestic. Biochem. Physiol. 88(1), 36–42 (2007)
Klomklao, S., et al.: Extraction of carotenoprotein from black tiger shrimp shells with the aid of bluefish trypsin. J. Food Biochem. 33(2), 201–217 (2009)
Sila, A., et al.: Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 148, 445–452 (2014)
Ghelichi, S., Shabanpour, B., Pourashouri, P.: Properties of fish sausages containing common carp (Cyprinus carpio) roe oil and defatted roe protein hydrolysate during refrigerated storage. J. Aquat. Food Prod. Technol. 27(2), 185–199 (2018)
Sasidharan, A., Venugopal, V.: Proteins and co-products from seafood processing discards: their recovery, functional properties and applications. Waste Biomass Valoriz. 11(11), 5647–5663 (2020)
Messina, C.M., et al.: Vitro bioactivity of astaxanthin and peptides from hydrolisates of shrimp (Parapenaeus longirostris) by-products: from the extraction process to biological effect evaluation, as pilot actions for the strategy “from waste to profit.” Mar. Drugs 19(4), 216 (2021)
Wade, N.M., Gabaudan, J., Glencross, B.D.: A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquacult. 9(2), 141–156 (2017)
de Silva, M.P.K.S.K., Senaarachchi, W.A.R.K.: Efficiency of biotransformation of shellfish waste to carotenoprotein by autolysis and crab-shrimp endo-enzymes. J. Aquat. Food Prod. Technol. 30(5), 526–534 (2021)
Cremades, O., et al.: Isolation and characterization of carotenoproteins from crayfish (Procambarus clarkii). Food Chem. 82(4), 559–566 (2003)
Chalamaiah, M., et al.: Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 31(2), 388–398 (2015)
Chalamaiah, M., et al.: Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res. Int. 52(1), 221–229 (2013)
Wu, X.-Y., Yang, Y.-F.: Heavy metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. J. Food Compos. Anal. 24(1), 62–65 (2011)
Author information
Authors and Affiliations
Contributions
PP: supervision, conceptualization, formal analysis, writing—reviewing and editing; HM: methodology, data curation, resources; AK: visualization, investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pourashouri, P., Mirsadeghi, H. & Khodanazary, A. Extracting and Physicochemical Properties of Carotenoprotein from Shrimp Processing Waste by Proteases-Mediated Hydrolysis. Waste Biomass Valor 13, 1169–1178 (2022). https://doi.org/10.1007/s12649-021-01561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01561-4