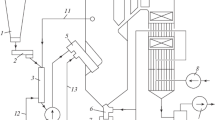
Abstract
The aim of this paper is to test the applicability of upgraded agricultural biomass feedstock such as torrefied sunflower husks during combustion in small and medium heating applications. Sunflower husk is formed in large quantities at enterprises producing sunflower oil and can be used as biofuel. However, big problems arise due to the low bulk density of husks and the rapid growth of ash deposits on the heating surfaces of boilers. In order to solve these problems, it was proposed to produce pellets from husks, and to subject these pellets to torrefaction. After torrefaction, net calorific value was increased by 29% while the risk of high temperature corrosion of boilers was reduced. Signs of ash softening neither occurred in combustion of raw nor in combustion of torrefied sunflower husk pellets. High aerosol emissions, already present in raw sunflower husk pellets, could not be mitigated by torrefaction. First combustion results at medium scale furnaces indicated that sunflower husk pellets (both raw and torrefied) in a commercial boiler < 400 kW, operated in a mode with low primary zone temperatures (< 850 °C), meet current emission limits. Regarding the future upcoming emission limits according to the European Medium Combustion Plant Directive, additional measures are required in order to comply with the dust limits.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
Initial results from lab scale and commercial scale combustion tests are presented regarding the use of torrefied sunflower husk pellets, an agricultural fuel that even though is available in large quantities in Russia, is not exploited appropriately. Although several available studies describe the impact of torrefaction on fuel properties, there is absence of adequate literature that directly compares the combustion behaviour of raw (thermally untreated) and torrefied sunflower husk pellets. Therefore, this study aims to determine the applicability of sunflower husk pellets as fuel in small and medium scale furnaces after having being upgraded through pelletisation and torrefaction. The suggested process is expected to affect the costs related to storage and transportation of this biofuel especially when referring to long distances.
Introduction
Agricultural solid residual biomass is available in vast amounts for sustainable energy generation. For instance, in Russia, an abundant supply of biomass in the form of wood, crop residues and agricultural animal waste is available. However, the actual biofuel share in the overall energy production matrix, is with an estimate of only 1.2% insignificant, and biomass accounts for only 0.5% (USDA 2016) [1]. In Russia, the production of vegetable oil from oil seeds is one of the main branches of agro-industrial production. The annually raising production is accompanied by a simultaneous increase of production wastes in the form of sunflower husk. Its amount exceeds 3.5 million tons per year [2].With heating values between 13 and 17.5 MJ/kg, moisture content between 9.1 and 11.6%, ash content between 1.7 and 3.8% and volatiles between 70.4 and 77.1% [3] sunflower husk has promising properties for an extended utilization as fuel for renewable energy production.
Currently, low-temperature vortex technology is applied for the husk combustion in Russia as the least expensive one by rule [4,5,6]. However, the physicochemical characteristics of the husk do not allow the combustion process in the boiler furnace to be accomplished. More specifically, the combustion process frequently leads to rapid growth of ash deposits in the convective zone of the boiler, with related decreases in boiler productivity by 40–50% and rapid damage of the boiler due to corrosion of its convective heating surfaces.
The compression of husk into pellets has been suggested as one promising option for improved thermal utilisation. This reduces significantly the entrainment of coarse fly ash during combustion of sunflower husks and ensures a prolonged stable operation of the boiler [7]. However, biomass pellets are hydrophilic and thus losing their mechanical durability at the slightest contact with moisture. As a result pelletisation alone increases the overall costs of storage and transportation [8].
Thermal pretreatment of biomass has been suggested as another way to improve the properties of residual biomass in order to provide more appropriate fuel qualities for combustion and gasification applications. Torrefaction of biomass is a mild form of pyrolysis at temperatures typically between 200 and 320 °C [9]. The solid’s yield of torrefaction varies between 60 and 90% on a weight basis and the energy yield is typically high, sometimes exceeding 90% [10]. According to Bergmann et al. [11], torrefaction leads to a dry product with no biological activity such as rotting. The authors came to the conclusion that torrefaction combined with densification provides an attractive fuel with high energy density. Apart from the increase in energy content and heating value of the biomass, the main advantages of torrefaction include the lower biodegradability, improved transportability, improved grindability resulting in reduced energy consumption for comminution and also the improved mechanical durability for torrefied pellets [12, 13]. Ibrahim et al. [14] indicated that through torrefaction the increased degree of hydrophobicity and grindability can be traced to changes in chemical structure of the fuel.
Singh et al. [15] reported for torrefied eucalyptus biomass better fuel properties as compared to raw biomass and improved properties for co-combustion with coal. The value of Fuel Ratio increased and Combustibility index decreased significantly with severity of torrefaction. The thermal stability of lignin decreased significantly for moderate and severe torrefaction. Torrefied eucalyptus can be used to generate energy through various thermochemical processes such as pyrolysis, gasification, and combustion due to improved physicochemical properties. Similar results were reported also for torrefaction of Acacia nilotica [16], for which the optimum value of temperature, residence time and heating rate to obtain maximum value of HHV (higher heating value) and energy yield combined, were 252 °C, 60 min, and 5 °C/min, respectively. It was noted that the effect of temperature on torrefaction was significant, while the effect of residence time and heating rate were minimal. Based on the results of Singh et al. [17], there is a clear indication that temperature is a significant operating parameter in torrefaction as compared to residence time in terms of yield and improving the physicochemical properties for pigeon pea stalk. Results indicated that mild torrefaction is more suitable for pigeon pea stalk as compared to severe torrefaction. In another study [18], the results obtained for torrefied agricultural waste (sugarcane bagasse) indicated a net reduction in the hydrogen and oxygen contents to 3.4% and 45.8%, respectively, and an increase in the total carbon content, HHV and true density to 50.3%, 24.0 MJ/kg and 1.12 g/cc, respectively, under optimized torrefaction conditions (temperature of 300 °C and residence time of 60 min).
According to Moreno et al. [19], the HHV (17.84 MJ/kg) of sunflower husk is similar to that of other commercially available solid biofuels, which demonstrates its potential for thermal energy generation. However, due to its low volume/energy ratio, it is necessary to densify this biofuel to reduce transport costs. In addition, replacing a 430 kW fuel oil boiler with a biomass boiler of the same capacity fed by this biofuel can avoid the emission of 254.09 tons of CO2 per year, as well as obtain an annual energy saving of 75%.
In Russia several sunflower oil production plants are already accompanied by plants that valorize their production waste of sunflower husk for the production of pelletised biomass energy carrier, which are overwhelmingly exported so far. The potential of sunflower husk can be further exploited by thermal treatment. There, torrefaction technology has already been studied and established in form of demonstration plants in the region e.g. in previous projects dealing with co-torrefaction of biomass an coal sludge [20]. Two processing schemes are possible in principle. The first one is thermal treatment of raw biomass followed by pelletisation, the second option is to pelletise the raw biomass followed by torrefaction. Taking into account the advantages of existing regional infrastructure and know-how as well as the specific needs of existing technologies for densification and thermal treatment of biomass, option two has been chosen. Installed equipment for processing of straw and husks has been used without the necessity of extensive modifications in pelletisation equipment for thermally untreated material. An existing torrefaction reactor operating effectively with free-flowing biomass (which is not the case for raw sunflower husk) and biomass layers with sufficiently large porosity (0.5–0.6) was available.
Despite the above advantages, which can be obtained by torrefaction pretreatment of biomass, maturation and market introduction of torrefaction technologies have gone slower than anticipated [21]. One of the reasons may be the energy consumption of the torrefaction process, which is about 3 to 4 MJ/kg of the initial biomass. The energy consumption of the torrefaction process can be reduced by using secondary energy resources for this process, for example, the exhaust gases of a gas piston power generating unit, as described by Zaichenko et al. [22]. Furthermore, it is possible to use the heat of exothermic reactions that occur when the biomass is heated [23, 24]. The torrefaction process of sunflower husk pellets specifically can be seen as co-pyrolyzing husk biomass and a certain amount of sunflower oil still contained in the husk. The presence of oil speeds up the pyrolysis process [25]. Since the oil content in husk depends on the type of sunflower seed, the torrefaction process of husk pellets requires stricter control of reactors temperature and residence time than for other biomass fuels.
Another reason for slow market introduction—besides unfavorable comparative price trends for fossil fuels and CO2 certificates—is generally a lack of investigations into the utilization of torrefied biomass fuels in small and medium scale applications. Specifically, we perceive only scarce literature [26, 27] that directly compares the combustion behaviour of raw and thermally treated sunflower husk pellets.
We present here a structured approach to investigate the applicability of sunflower husk pellets as fuel in small and medium scale furnaces after upgrading through torrefaction. Chemical fuel analysis and lab-scale combustion tests are applied to pre-evaluate the effect of torrefaction on relevant combustion properties of sunflower husk pellets, which are discussed with the help of fuel indices. Commercial scale combustion tests with raw and torrefied sunflower husk pellets with a 180 kWth, state-of-the-art grate furnace technology with adaptable process parameters are conducted. The applicability of a state-of-the-art grate furnace technology under modern process conditions like flue gas recirculation are demonstrated by meeting emission limits without the necessity of costly secondary measures for gas cleaning.
Experimental Section
Experimental Unit for Pelletisation
In the experiments conducted, raw and torrefied sunflower husk pellets were utilized. Sunflower husk pellets were produced by “Clean Energy” LLC (Russia) in an experimental unit (capacity of 3 tn/h) that was an outcome of the project “TORRECOMB_ID#213_ERA.NET RUS Plus—’A novel approach for the implementation of TORrefaction in Residential and COMmunal heating Boilers”. Heat treatment of the pellets was carried out in a moving bed reactor described in the next section, using the heat produced from exothermic reactions of biomass.
Pilot Unit for Biomass Torrefaction
The torrefaction unit consists of the gas-piston power plant, the gas–water heat exchanger, the vertical torrefaction column, the afterburner and the forced air heater [28]. The combustion products (CP) of the gas piston engine in this complex, play the part of the heat carrier. The fuel for the gas engine is natural gas from the network. The temperature of the CP at the engine outlet is 600–700 °C. Immediately after the engine, the CP are separated into two streams: the first is directly fed to the mixing zone (lower part of torrefaction column), and the second enters the heat exchanger, where it is cooled to a given temperature. Furthermore, the mixing of cooled and initial CP in a certain proportion takes place (provided by a programmable valve), which allows to achieve the desired torrefaction temperature mode (250–300 °C). In the central part of the vertical torrefaction column, the process of torrefaction occurs with the periodic movement of pellets through the processing zone. The afterburner serves for the disposal of volatile products released during the heat processing of the initial pellets, preventing from air pollution with volatile pyrolysis products. To eliminate the possibility for pyrolysis products to be released into the environment through the top loading device, an air shutter is provided.
Lab-Scale Combustion
The combustion behaviour of raw (SFH) and torrefied sunflower husk pellets (TSFH) was investigated through the application of a multi-step laboratory approach (chemical analyses, fuel indices and fixed-bed lab-scale reactor combustion tests).
The chemical composition and relevant properties of the fuel have been determined by application of the following methods. The moisture content was determined according to ISO 18134-2. The fuel sampling and ash processing for the following analyses were carried out according to ISO 14780. The ash content was determined by ashing the fuel at 550 °C (ISO 18122). Calcium-rich fuels tend to form considerable amounts of carbonates at 550 °C. During combustion, however, practically no formation of carbonates occurs; oxides are formed exclusively instead, due to the prevailing high combustion temperatures. As a result, the ash content determined by the standard is overestimated in comparison to the amount of ash produced in the conventional systems. Due to this fact, the total inorganic carbon (TIC) value was used to calculate the so-called “corrected ash content” based on EN 13137. The C, H and N concentrations of fuel are determined according to ISO 16948 by means of combustion and subsequent gas chromatographic separation with an elementary analyser. Chlorine content of fuel is determined according to ISO 16994 and concentrations of main and secondary elements in fuel and ash according to ISO 16967 and ISO 16968.
The chemical analysis results, form the basis for fuel indices calculation that may be applied for a pre-evaluation of the combustion behaviour. In the present study, the sum of K, Na, Zn and Pb (evaluation of the aerosol emissions expected), the molar Si/K ratio (indicator regarding the K release), the molar 2S/Cl ratio (indicator for high temperature corrosion), the molar Si/(Ca + Mg) and (Si + P+K)/(Ca + Mg + Al) ratio (as indicators concerning ash melting behavior) have been used. In a former study of Sommersacher et al. [29], the above mentioned fuel indices and respective background information for the derivation as well as boundary conditions of application of these indices were presented.
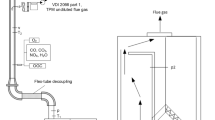
During the following step, raw (SFH) and torrefied sunflower husk pellets (TSFH) were combusted in a fixed-bed lab-scale reactor. The fixed-bed lab-scale reactor is a discontinuously operated cylindrical pot furnace in which conditions well comparable to grate-fired plants (> 50 kW thermal output) prevail. Biomass is introduced into a cylindrical holder (100 mm height and 95 mm inner diameter) placed inside an electrically heated cylindrical retort. The initial sample mass is, depending on the fuel density, commonly between 100 and 400 g. Dry air is imported in a porous plate at the bottom of the fuel bed. Five thermo-couples measure the bed temperature at different positions and a balance continuously records the mass loss during the experiments. Flue gas composition is measured above the fuel bed by means of the following methods:
(I) Fourier transform infrared spectroscopy (FTIR) (Ansyco Series DX 4000) for CO2, CO, H2O, NO, NH3, HCN, NO2, N2O, CH4, light hydrocarbons and low condensable matters. (II) Flue gas analysis (Emerson NGA 2000), which employs paramagnetism for O2, nondispersive infrared analysis (NDIR) for CO2 and CO and heat conductivity for H2. (III) Flame ionization detector (FID) (Messer Griesheim Austria Model VE7) for carbon amount in organic gaseous hydrocarbons (OGC). (VI) Chemiluminescence detector (CLD) (ECO Physics CLD 700 EL ht) for NO and NOx. (IV) Wide band λ sensor for the estimation of O2-concentration.
The reactor setup has been previously described in detail by Sommersacher et al. [29] and Brunner et al. [30]. The accuracy of FTIR measurements for NH3, HCN, HCl and SO2 has been checked by parallel sampling of flue gas with the impinger method and quantitation by photometry (NH3, HCN) and ion chromatography (HCl, SO2). NDIR, FID and CLD instruments have been calibrated on a daily basis before test runs by zero-point adjustment with N2 and test gas mixtures in N2 (300 ppm CO, 10.0 vol% CO2 and O2; 900 ppm propane; 340 ppm NO).
Experiments with the fixed-bed lab-scale reactor provide information concerning the combustion behavior, duration of drying, release of volatiles and char burnout. The release behavior of volatile and semi-volatile ash forming elements during the combustion process can be determined by mass calculation and element balances for the ash forming elements. On the basis of this evaluation, the emissions of aerosols, SO2 and HCl can also be estimated. Optical evaluation of ash residues allows preliminary assessment of slagging tendencies.
Calculation of Release Rates of Aerosol Forming Elements
On the basis of elementary balances based on weight measurements, chemical analyses of fuel and ash, the release rates for S, Cl, K, Na, Zn and Pb can be calculated according to Eq. 1.
where: mInput = the mass of fuel in g in the reactor, cx,Input = the concentration of the respective element in the fuel in mg/kg, mOutput = the mass of ash in g db, after the experiment and cx, Output = the concentration of the respective element in the ash in mg/kg.
Combustion in a Commercial Fixed-Bed Grate Furnace
Combustion tests with raw (SFH) and torrefied sunflower husk pellets (TSFH) were performed in a 180 kWth pilot-scale biomass combustion plant. The pilot-scale grate furnace consists of a fuel feeding system, including a sliding bar conveyor, a screw conveyor and a stoker screw. The horizontally moving grate furnace with a primary and secondary combustion zone has a separately adjustable combustion air supply. The combustion zone walls are covered with refractory material and are thus not cooled. The combustion zone temperature can be controlled through flue gas recirculation below the grate and into the primary combustion zone. The air cooled horizontally moving grate has an area of approximately 0.43 m2 (typical specific grate area load of approximately 500 kWHu/m2 for wood chips with a moisture content of 35 wt%, w.b.), whereas the two-pass hot water fire tube boiler with upstream flame tube has a nominal capacity of 180 kWth.
Prior to test runs, the combustion zone and boiler of the pilot-scale biomass combustion plant were cleaned to remove ash deposits. During test runs, representative fuel samples were received by taking 0.5 L subsamples every half hour directly from the continuous fuel feeder. Bottom ash was collected in ash bins in total and was given time to cool down over night. Sample volumes of fuel and bottom ash were reduced representatively via a sample splitter to volumes of 2 L. Furnace and boiler ash was removed and sampled after each test run.
Flue gas was sampled in the flue gas duct after the boiler outlet for analysis of O2, CO, NOx, SOx, HCl, total fly ash emissions and aerosols. Total fly ashes were determined applying the gravimetric method according to VDI 2066. Flue gas was sucked through a silica wool package plugged into a filter jacket located in the flue gas duct and an additional plane filter. Fly ash concentration in the flue gas was calculated by dividing the mass of the retained fly ash (mg) by the flue gas flow through the filter (m3). Aerosols with particle sizes < 1 µm (PM1) were determined by a Dekati gravimetric cascade impactor after separating coarse dust particles by a cyclone.
Plant performance data as furnace temperatures and pressure, flue gas temperatures, energy output, feed and return temperatures, oxygen content in the flue gas as well as mass flows of primary and secondary combustion air (hot wire anemometer), flue gas and recirculated flue gas (velocity measurement with Prandtl pipe and differential pressure sensor in the respective flue gas duct) were recorded by a process control system, subsequently processed and evaluated.
Operating Conditions
The release of inorganic species from the fuel during combustion as well as possible ash related problems on the grate (e.g. slagging) are influenced by the temperature in the fuel bed. The temperature in the primary combustion zone directly above the fuel bed, was used as indicator for the fuel bed temperature. In Table 1, the applied Flue Gas Recirculation (FGR) mode as well as the average temperature in the primary combustion zone during the evaluation period are given.
The temperature in the primary combustion zone is mainly affected by the FGR rate below the grate. For sunflower husk pellets, the temperature was reduced from 1021 to 776 °C by applying FGR above and below the grate. Torrefied sunflower husk pellets have been treated with three different ratios of FGR (below and above grate) resulting to primary combustion zone temperatures ranging from 942 to 788 °C.
Boiler Efficiency
The boiler efficiency has been determined during all combustion tests for specific evaluation periods lasting about three hours. The boiler efficiency is defined as the ratio between the boiler’s thermal output Qdot and the maximum enthalpy flow that could be provided by the mass flow of fuel fed to the system Hdot_in.
Both variables are calculated as follows [31]:
where: Qdot = thermal output of the boiler in W, cp = 4196.3 J/kg K is the specific heat capacity of water at 353.15 K (typical mean value of the feed and return temperature), Tfeed = feed temperature in K, Tret = return temperature in K
where: mdot_fuel = mass flow of wet fuel fed to the boiler in kg/s, Hu = lower heating value of the fuel in J/Kg.
Since a continuous measurement of the fuel mass flow is not installed, it has been calculated from the measured flue gas volume flow (in Nm3/h) and the specific flue gas volume Vf (in Nm3/kg biomass) [32] where
Fuel Indices
Based on the elemental compositions of biomass fuels, elemental ratios (“fuel indices”) can be calculated in order to pre-evaluate the combustion behavior. The following indices were especially developed and evaluated for new biomass fuels like wheat straw, olive kernels, maize spindle or grass pellets [29].
The nitrogen concentration in fuels is an indicator for NOx emissions. The sum of K, Na, Zn and Pb is an indicator for the estimation of aerosol emissions. A portion of the partially volatile elements S, Cl, K, Na, Zn and Pb is released from the fuel into the gas phase during combustion. In the gas phase, these elements are exposed to homogeneous gas phase reactions. Due to oversaturation in the gas phase, vapors begin to nucleate or condense on the surfaces of existing particles or heat exchanger tubes. In general, aerosol emissions increase with higher values of the index [29].
On the basis of the molar 2S/Cl ratio in the fuel, the risk of high temperature corrosion can be assessed [29]. It was indicated that there is a correlation between the molar 2S/Cl ratio in the fuel and the molar 2S/Cl ratio in the aerosols. It may be assumed that elemental compositions of aerosols resemble elemental patterns in deposits on heat exchanger tubes. High temperature corrosion risk increases with increasing Cl content in the deposits. Low corrosion risk is expected with a 2S/Cl ratio > 4 while the risk of high temperature corrosion can be neglected for values above 8. The molar Si/K ratio provides a first proxy for the release tendency of potassium from the fuel into the gas phase. Higher index values are linked with increased incorporation of K into silicate phases and corresponding lower K release.
The indices (Si)/(Ca + Mg) and (Si + P+K)/(Ca + Mg + Al) were used to evaluate ash melting behavior. Silicon forms low-melting eutectic phases with potassium and phosphorus has the ability to form melting phases at rather low temperatures [33]. On the contrary, Ca, Mg and Al tend to raise the melting point. Therefore, indices with smaller values are related with high ash melting temperatures [29]. The indices are summarized in Table 2.
Combustion Indices
Changes in combustion indices such as fuel ratio (FR), combustibility index (CI), and volatile ignitability (VI) were determined to assess the torrefaction performance and fuel quality. These indices were calculated using the following equations:
According to Makino and Tanno et al. [34] and Ohm et al. [35], FR ranging from 0.5 to 2.0 are commonly used in coal fired power plants, while CI ranging from 14 to 23 MJ/kg is recommended. Meanwhile, it was recommended that the volatile ignitability should have a specific calorific value of at least 14 MJ/kg [36].
Results and Discussion
Fuel Characterization
Chemical Properties
The chemical composition of the investigated fuels (“sunflower husk pellets” and “torrefied sunflower husk pellets) and reference fuel values for sunflower husk pellets extracted from relevant literature, are given in Table 3.
According to Table 3 the values of the sunflower husk pellets are well fitted with literature values. It can also be observed that sunflower husk net calorific value (NCV) is increased by 29% by torrefaction process. This increase can induce specific cost reduction for transport and storage. The combustion heat of the pellets after torrefaction increases due to a decrease in the moisture content of the pellets and an increase in the carbon content of pellets after heat treatment. The reduction of more than 20% of the volatiles content in pellets after heat treatment is also notable. This, obviously, is due to the synergistic effect that is observed with the combined pyrolysis of biomass and oil, contained in the husk [25]. Bulk density of raw sunflower husk pellets was 610 kg/m3 and was reduced to 550 kg/m3 after torrefaction. In addition, torrefied fuels behave hydrophobically and thus do absorb less water [37,38,39], which is another great characteristic of torrefied fuels. More specifically, it was found that the hygroscopicity of the initial pellets from sunflower husk was 22% whereas the hygroscopicity of pellets from sunflower husk after torrefaction at temperatures of 230 °C, 250 °C and 275 °C was reduced to 13%, 12.2% and 11% respectively. Specific energy needs for milling is also reduced for torrefied fuels while co-combustion with coal is facilitated (Tumuluru et al. [40]).
Fuel and Combustion Indices Results
The calculated values for the investigated fuels are summarized in Table 3. In general, a slight increase in fuel N content is found after torrefaction compared to the raw fuel. A lower conversion rate of fuel N to N in NOx emissions is expected for the torrefied fuels compared to raw fuel, since such a trend is also reported by Sommersacher et al. [29]. According to correlations published in this work, the potential of NOx emissions can be estimated to be in the range of 200 mg/Nm3 (dry flue gas at 13 vol% O2).
Due to the value of K + Na + Zn + Pb fuel index, high aerosol emissions are expected for the raw fuel. An even further increase in aerosol emissions is very likely for the torrefied fuel. Due to the molar 2S/Cl ratio in the fuel, the risk of high temperature corrosion is relatively low for the sunflower husk. It is even further reduced by the torrefaction process as a portion of Cl is transferred to the torrefaction gas and removed from the fuel.
Due to low Si/K sunflower husks ratio, a significant K release is expected, which results in high aerosol emissions.
The indices (Si)/(Ca + Mg) and (Si + P+K)/(Ca + Mg + Al) used to evaluate ash melting behavior show similar values for raw and torrefied fuel. Here, only a slight increase of the index is found regarding the torrefied fuel, indicating slightly lower characteristic temperatures of ash melting behavior. The relatively low values for sunflower husk pellets indicate a high ash melting point thus no problems with ash melting are expected.
Regarding combustibility indices, it has been observed that biomass subjected to torrefaction at 250–300 °C resulted in increase in fuel ratio (0.59 from 0.12) and volatile ignitability (20.04 from 18.62 MJ/kg) and a decrease in combustibility index (40.83 from 166.70 MJ/kg). This strongly implies that torrefaction temperature and time strongly affect the combustion indices FR, VI and CI. This is because these indices are dependent on volatile matter and its conversion to fixed carbon. In general, combustion indices can be used to assess the torrefaction performance and quality of torrefied biomass.
Lab-Scale Combustion Results
The lab procedure for the combustion of biomass with the laboratory scale combustion reactor is exemplified in more detail with the following representative test run. In this test, raw (untreated) sunflower husk pellets are burnt. In general, the test runs are carried out in triplicate in order to guarantee representative results. For the evaluation test data and chemical analyses, three repetitions were used. Similar results were obtained for the repetitions, which is why only one test run is described in detail. For this test run, the fuel mass was 380 g.
Figure 1 depicts the fuel mass loss, the temperatures in the fuel bed, the average flue gas temperature, the concentrations of the most important components and the NOx precursors in the flue gas as a function of the test duration.
The total test time was 3500 s. Drying of the fuel can be identified in the beginning with the low mass loss, low fuel bed temperatures and water release. Thermal conversion starts after 500 s which can be recognized by the decrease of O2 and increase of the CO2 concentration in the flue gas. The main decomposition phase starts with a significant decrease in O2 to zero after 750 s. The release of volatile components and the parallel gasification of the charcoal are carried out up to a time of 1600 s. High mass losses, an increase in the fuel bed temperatures and a combustion air ratio of less than one are typical for this phase. Mainly CO2, CO, H2O and CH4 but also small amounts of other hydrocarbons are released into the gas phase. After 1500 s, CH4 disappears, which can be used as an indication of the end of volatile constituent’s release and the start of charcoal combustion. The above mentioned phase is characterized by a lower proportion of CO and an increase in the CO2 concentration in the flue gas, as well as an increase in oxygen concentration. At the beginning of this phase, typically the maximum fuel bed temperatures (1092 °C, in this experiment) are observed. At the end of the experiment, the mass of remaining ash is determined and an ash sample is transferred to the lab for further analysis.
Durations of total reaction, the start of the release of volatile constituents and charcoal gasification, the start of charcoal combustion, the duration of each phase and the maximum bed temperature for the fuel (sunflower husk pellets and torrefied sunflower husk pellets) tested are summarized in Table 4.
Durations of total reaction were 2311 s and 2218 s for sunflower husks and torrefied sunflower husks respectively; the duration of the volatilization and charcoal gasification was 1254 s for “SFH” and 837 s for “TSFH”; the duration of charcoal combustion was 1057 s and 1381 s for “SFH” and “TSFH” respectively; the maximum bed temperature for the sunflower husk pellets was 1151 °C and 1213 °C for torrefied sunflower husk pellets.
These results apparently show that the phase of volatiles release and charcoal gasification is shortened for the torrefied fuel. This is obvious, since some of the volatile constituents have already been transferred from the fuel into the torrefaction gas during the torrefaction process. In return, charcoal burnout of torrefied fuel takes longer. This can be explained by the higher (fixed) carbon content of the torrefied fuel. The increased carbon content also causes higher maximum fuel bed temperatures for the torrefied fuel, which are generally reached at the beginning of char burnout.
Optical Evaluation of Ashes
According to the experimental results, no slag formation was found for the sunflower husk pellets (raw and torrefied). The structure of the individual pellets was still preserved after the test run, (but could be destroyed by the application of a low force) and a loose ash was obtained.
Release of NOx Precursors
Figure 2a depicts the release of the NOx precursors. It can be observed that the majority of the NOx precursors is formed as NH3. A considerable part is released as NO from the fuel into the gas phase. Brunner et al. (2013) [30] reported that NOx precursors are dominated by NH3 for agricultural fuels, whereas HCN is the predominant NOx precursor species for wood. This has been confirmed for the agricultural residues (sunflower husk pellets) tested in the experiments. Figure 2a depicts a large proportion of the NOx precursors occurring during the release of volatile constituents and charcoal gasification. It is also observed that, in total, fewer NOx precursors are released for the torrefied fuel compared to the raw fuel. A higher N content was determined for the torrefied fuel compared to the raw fuel (Table 3). These data serve as valuable input parameters for CFD simulations on NOx formation in biomass combustion.
a Conversion of fuel N to N in NOx precursors for sunflower husk pellets (raw and torrefied)—(upper left). b Release rates of aerosol forming elements for raw (SFH) and torrefied sunflower husk pellets (TSFH)—(upper right). c Estimated aerosol emissions for sunflower husk pellets (raw and torrefied)—(bottom)
Release Rates of Aerosol Forming Elements
Three test runs were evaluated for sunflower husk pellets (SFH and TSFH). The release rates from the test runs are shown in Fig. 2b. For K, Zn and Pb, similar release rates are determined for the torrefied and the untreated, raw fuel. S and Cl have higher release rates for the raw fuel than for the torrefied. The above mentioned behavior can be explained by a relatively high S and Cl release during torrefaction, which reduces the concentration of these elements in the fuel. In the case of Na, very low concentrations are present for sunflower husk pellets (Table 3). Possible inhomogenity in the fuel as well as inaccuracies in analysis can be responsible for the fact that no Na release has been determined in torrefied sunflower husk.
Aerosol Emissions
Based on the release rates of the main aerosol forming elements K, Na, Zn and Cl, the potential for aerosol formation can be estimated. These elements are assumed to be solid K2SO4, KCl, Na2SO4, NaCl and ZnO. In addition, the formation of carbonates (K2CO3 and Na2CO3) is possible if there is not enough S and Cl to completely bind the K and Na as chlorides and sulfates (Sommersacher [41]). Normally an excess of S and Cl to K and Na is present, resulting in gaseous chlorine and sulfur emissions. Assuming that the total sulfur reacts with the K and Na present, the potential of maximum aerosol emissions can be calculated. Assuming that all Cl is bound to Na and K, the potential of minimal aerosol emissions can be calculated. This estimation of the aerosol emissions does not take into account the losses caused by the condensation of ash-forming vapors on the walls and the formation of deposits in boilers of large plants. In addition, particle losses by the reaction of ash-forming vapors with coarse fly ash particles and the gaseous emissions of S (e.g. SOx) and Cl (e.g. HCl) are not taken into account.
The outcome of this estimation is shown in Fig. 2c. It is apparent that aerosol emissions for sunflower husk pellets are clearly dominated by K—other elements have a negligible influence. For sunflower husk pellets, the released amounts of K are sufficient to bind the total available S and Cl in the form of particulate emissions. In addition, formation of particulate K2CO3 is expected.
For sunflower husk pellets, gaseous SO2 and HCl emissions are theoretically negligible, since large amounts of K are released by this fuel and the entire S and Cl can be bound in the form of particulate emissions.
Combustion Results in a Commercial Fixed-Bed Grate Furnace
Test Run Evaluation
After introduction of sunflower husk pellets/torrefied sunflower husk pellets and adjustment of operating conditions, a period of about 2.5 h was required to reach stable operation. Then, the evaluation period along with the discontinuous particulate matter emission measurements started. Mean values of relevant data of all evaluation test runs obtained during the evaluation periods are shown in the graphs of Fig. 3. Figure 3a depicts the most relevant data from the combustion plant during an evaluation test run lasting from 8:00 until 17:30. The data shown represent the evaluation test runs No 4 (Evaluation period 1) and No 5 (Evaluation period 2). The data shown include the thermal power and the temperatures in the primary and secondary combustion zone. Figure 3b illustrates relevant data from the emission measurements. Data include concentrations of O2, CO, NOx as well as total suspended particulate matter (TSP) and PM1.
Mean values of relevant data of all evaluation test runs obtained during the evaluation periods are summarized in Table 5.
For the combustion of sunflower husk pellets a plant efficiency of 83% was calculated whereas for the torrefied sunflower husk pellets the plant efficiency was increased to 85%. The increase in plant efficiency was mainly due to the reduction of the fuel moisture content during torrefaction from 11.4 wt% (wet basis) in husks to 6.0 wt% in the torrefied product.
Emissions
All emissions presented were evaluated regarding the current emission limits in Austria. These limits are regulated within the “Feuerungsanlagenverordnung” (FAV) from 2011 [42]. The limits given in Appendix are valid for combustion plants for the incineration of straw or similar agricultural substances (e.g. cereal plants, cereal grains, grasses, miscanthus) depending on the fuel heat capacity. The testing plant used for the evaluation test runs had a fuel heat capacity of 180 kW. For real-scale applications also, higher fuel heat capacities up to 10 MW are of relevance. Therefore, emission limits for fuel heat capacities from < 0.4 MW up to 10 MW were considered within the evaluation of the results.
Carbon Monoxide
CO emissions for the evaluation test runs No 1, No 2, No 4 and No 5 were below the detection limit of the measurement system used. Therefore, a complete burnout of the gas phase was present. Only for evaluation test run No 3, measureable CO concentrations with a mean value of 111.6 mg/Nm3 (11% O2) were determined. However, the CO concentration was still well below the emissions limits according to FAV (Table 5).
Nitric Oxides
NOx emissions determined during the evaluation period as well as the FAV limits are presented in Fig. 4a. The NOx emissions were ranging from 216 mg/Nm3 (11% O2) to 372 mg/Nm3 (11% O2). These values are below the emission limits according to FAV for all heat capacity ranges considered.
Regarding the SFH pellets combustion, the application of FGR below the grate increased the NOx emissions from 216 to 310 mg/Nm3 (11% O2). For the combustion of TSFH pellets, NOx emissions were ranging from 256 to 372 mg/Nm3 (11% O2). Nevertheless, all NOx emissions were below the FAV limits. By performing dedicated test runs using air staging strategies, a further reduction of NOx emissions is considered to be possible. Therefore, secondary measures for NOx reduction should not be necessary if air staging is applied and optimized.
HCl and SOx
HCl and SOx emissions were determined during the evaluation period whereas the FAV limits are presented in Fig. 4b. The emissions limit for SO2 is 350 mg/Nm3 (11% O2) and the limit for HCl is 30 mg/Nm3 (11% O2).
Between SFH and TSFH pellets no significant difference in the emission values of HCl and SΟx was observed. For both fuels, HCl and SOx emissions were clearly below FAV limits and were therefore not considered questionable.
The application of FGR below grate increased HCl and SOx emissions. The main reason for this trend is given by the decreased release of alkali species (mainly K and Na) with decreasing temperatures in the primary combustion zone. Therefore, less reaction partners for Cl and S are available to bind this species which results in increased HCl and SOx emissions. This trend is exemplarily shown in Fig. 4(c), where SOx emissions in dependence of PM1 emissions are presented. For high PM1 emissions almost no SOx emissions were present.
Particulate Emissions
Particulate emissions originating from grate furnaces are typically comprised of aerosols (particulate matter < 1 µm) or PM1 emissions and coarse fly ashes. With the measurement equipment applied, particulate emissions were determined as PM1 and TSP (total suspendered particulate matter). TSP emissions comprise of PM1 and coarse fly ashes.
TSP and PM1 emissions were determined during the evaluation period and compared with emission limits in FAV in Fig. 4d. Emission limits are only available for TSP with values ranging from 150 (< 0.4 ΜW) to 20 mg/Nm3 (2–10 ΜW) for 11%O2. The emissions are presented in dependence of the temperature in the primary combustion zone.
The data presented in Fig. 4d indicate that the temperature in primary combustion zone (PCZ) is the main influencing factor on the release of ash forming species. No significant differences between combustion of raw and torrefied sunflower husk pellets were observed.
The TSP emission limit in Austria for furnaces with fuel heat capacity ranging from 2 to 10 MW is 20 mg/Nm3. This limit was not met in any of the evaluation test runs. For smaller furnaces with fuel heat capacities below 0.4 MW, evaluation test runs No 2, No 4 and No 5 showed dust emissions below the limit of 150 mg/Nm3. These evaluation tests run with a primary combustion zone temperature below 850 °C.
The results indicated that in order to minimize dust emissions, flue gas recirculation below the grate is necessary. To meet FAV limits of larger furnaces above 2 MW, secondary measures for the reduction of dust emissions are most likely necessary.
Guideline values for dust separation measures and achievable dust concentration after the separation are as following:
-
1.
Multicyclone: ~ 150 mg/Nm3
-
2.
Flue gas condensation: ~ 50 mg/Nm3
-
3.
ESP: ~ 20 mg/Nm3
-
4.
Baghouse filter: ~ 10 mg/Nm3
Composition of Ash Fractions
Grate ash, TSP and PM1 fractions sampled during the evaluation test runs were analyzed for their chemical composition subsequently to the test runs. The results of the chemical analyses are presented in Fig. 5a (TSP), b (PM1) and c (grate ash).
Si, Ca, Mg, P and K are prevailing in grate ash samples. An enrichment of Si, Ca and Mg is typical for grate ashes since these elements are not released into the gas phase during combustion, remain in the ash and form coarse ash particles mainly found in the grate ash. Also, P is typically not released into the gas phase during combustion and hence mainly found in the grate ash.
The literature commonly agrees that the release of potassium generally increases with temperature [43,44,45]. As shown above in Table 5 the variation of the flue gas recirculation ratios has a direct influence on the temperature in the primary combustion zone and thereby on the amount of released particulate matter (Fig. 4d). The elemental composition of TSP and PM1 samples was almost identical and dominated by K, S, and Cl.
Conclusions
Chemical analyses of raw and torrefied sunflower husk pellets, allowed a pre-evaluation of combustion related problems, followed by calculating fuel specific indices concerning corrosion risk, aerosol formation and slagging tendency. Combustion experiments were conducted on a fixed-bed laboratory reactor where the conditions of a grate furnace are simulated. Experiments confirmed a lower N conversion to NOx for TSFH pellets than that for raw SFH pellets as predicted by the NOx emission index. High aerosol emissions (according to the index K + Na + Zn + Pb) are expected for raw fuels. Aerosol emissions for the torrefied fuel appeared to be even higher. From the molar 2S/Cl ratio, a low high temperature corrosion risk for SFH pellets is predicted. For SFH pellets, it is likely according to the ash melting indices that no obstacles regarding ash melting will be observed.
Lab-scale combustion tests also reveal shorter periods of volatile release for the torrefied fuels compared with raw fuels. This is coherent since a part of the volatile constituents is already released during torrefaction process. Char burnout of torrefied fuels has longer lasting due to their increased quantity of fixed carbon. Aerosol emissions are clearly dominated by potassium. The released amount of K is sufficient enough to bind the total available S and Cl in the form of particulate emissions resulting in negligible gaseous SO2 and HCl emissions.
The net calorific value (NCV) was increased by 29% for SFH pellets by torrefaction which can lead to specific cost reductions during transport and storage. In addition, TSFH pellets behave hydrophobically and thus do absorb less water, which is especially a storage issue. In the case of SFH pellets, a low risk of high temperature corrosion is expected for raw fuel which is further minimized by torrefaction. The extremely high aerosol emissions that are already present for raw SFH pellets are spread even further by torrefaction. The emission limits have to be taken into account especially in the case of fine particulate matter (PM) emissions. Signs of ash softening occurred neither in combustion of raw nor in combustion of TSFH pellets.
Commercial scale fixed-bed grate furnace tests showed that SFH pellets and TSFH pellets even more, combust in high temperatures due to low moisture contents. Emission from TSFH pellets combustion of CO, NOx, HCl, SOx comply with related limit values whereas high dust emissions occurred which can be controlled by flue gas recirculation below the grate. An automatic cleaning system of the boiler and flue gas recirculation is recommended whereas flue gas recirculation below and above the grate is necessary in order to control the temperature and avoid furnace damage. Of course, in order to meet future upcoming emission limits according to the European Medium Combustion Plant Directive [46] secondary measures are most likely necessary.
In general, the technical potential and viability of TSFH pellets application in small and medium scale furnaces was examined with promising results. For sure, more research and development investigations are required in order to increase the net calorific value, durability and hydrophobicity of torrefied biomass as well as the profitability of the combustion plants in operation. The torrefaction process seems to be a preferable option for long distances, because of the environmental benefits earned from the transportation process as the energy density of the torrefied biofuel is higher. However, the above issue may be further studied in the future, in terms of total costs and life cycle analysis.
References
Russian Federation: Biofuels annual, biofuels update. In: Gray, R. (ed.) GAIN Report. vol. RS1631, p. 20. USDA Foreign Agricultural Service, Moscow (2016)
Marketing research of the sunflower oil market for 2016-2020. Forecast for 2020–2024, vol. 60634, p. 40. TK Solutions, Moscow (2020)
Maj, G., Krzaczek, P., Kuranc, A., Piekarski, W.: Energy properties of sunflower seed husk as industrial extrusion residue. Agric. Eng. 21(1), 77–84 (2017). https://doi.org/10.1515/agriceng-2017-0008
Puzyrev, E.M., Shchurenko, V.P.: Cyclone furnace. Russian Federation Patent 2105239, 20.02.1998
Puzyrev, E.M., Shchurenko, V.P., Sharapov, M.A.: Device for burning solid fuel. Russian Federation Patent 2126113, 10.02.1999
Puzyrev, E.M., Shchurenko, V.P., Shcherbakov, F.V.: Vortex furnace. Russian Federation Patent 2126932, 27.02.1999
Sharapov, A.M.: Conversion of biofuel boilers. Heat Supply News Magazine No. 12, 160 (2013) https://www.rosteplo.ru/Tech_stat/stat_shablon.php?id=3186
Graham, S., Ogunfayo, I., Hall, M.R., Snape, C., Quick, W., Weatherstone, S., Eastwick, C.: Changes in mechanical properties of wood pellets during artificial degradation in a laboratory environment. Fuel Process. Technol. 148, 395–402 (2016). https://doi.org/10.1016/j.fuproc.2016.03.020
Stelt, M.J.C., Gerhauser, H., Kiel, J.H.A., Ptasinski, K.J.: Biomass upgrading by torrefaction for the production of biofuels: a review. Biomass Bioenerg. 35(9), 3748–3762 (2011). https://doi.org/10.1016/j.biombioe.2011.06.023
Safferman, S.I., Asce, M., Smith, J.S., Dong, Y., Saffron, C.M., Wallace, J.M., Binkley, D., Thomas, M.R., Miller, S.A., Bissel, E., Booth, J., Lenz, J.: Resources from wastes: benefits and complexity. J. Environ. Eng. 143(11), 1–15 (2017). https://doi.org/10.1061/(ASCE)EE.1943-7870.0001259
Bergmann, C.A.P., Kiel, H.A.J.: Torrefaction for biomass upgrading. In: 14th European Biomass Conference & Exhibition. Paris, France (2005)
Ren, X., Rokni, E., Liu, Y., Levendis, Y.A.: Reduction of HCl emissions from combustion of biomass by alkali carbonate sorbents or by thermal pretreatment. J. Energy Eng. 144(4), 1–9 (2018). https://doi.org/10.1061/(ASCE)EY.1943-7897.0000561
Santibanez, V.C., Vargas, U.M.: Power from agripellets. Frontiers Bioenergy Biofuels (2017). https://doi.org/10.5772/66186
Ibrahim, R.H.H., Darvell, L.I., Jones, J.M., Williams, A.: Physicochemical characterisation of torrefied biomass. J. Anal. Appl. Pyrol. 103, 21–30 (2013). https://doi.org/10.1016/j.jaap.2012.10.004
Singh, R.K., Sarkar, A., Chakraborty, J.P.: Effect of torrefaction on the physicochemical properties of eucalyptus derived biofuels: estimation of kinetic parameters and optimizing torrefaction using response surface methodology (RSM). Energy. 198, 117369 (2020). https://doi.org/10.1016/j.energy.2020.117369
Singh, S., Chakraborty, J.P., Mondal, M.K.: Optimization of process parameters for torrefaction of Acacia nilotica using response surface methodology and characteristics of torrefied biomass as upgraded fuel. Energy. 186, 115865 (2019). https://doi.org/10.1016/j.energy.2019.115865
Singh, R.K., Sarkar, A., Chakraborty, J.P.: Effect of torrefaction on the physicochemical properties of pigeon pea stalk (Cajanus cajan) and estimation of kinetic parameters. Renew. Energy 138, 805–819 (2019). https://doi.org/10.1016/j.renene.2019.02.022
Kanwal, S., Chaudhry, N., Munir, S., Sana, H.: Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manage. 88, 280–290 (2019). https://doi.org/10.1016/j.wasman.2019.03.053
Moreno, M.A.P., Agugliaro, F.M., Moreno, A.J.P.: Sustainable energy based on sunflower seed husk boiler for residential buildings. Sustainability. 10, 3407 (2018). https://doi.org/10.3390/su10103407
Isemin, R., Mikhalev, A., Klimov, D., Grammelis, P., Margaritis, N., Kourkoumpas, D.S., Zaichenko, V.: Torrefaction and combustion of pellets made of a mixture of coal sludge and straw. Fuel 210, 859–865 (2017). https://doi.org/10.1016/j.fuel.2017.09.032
Cremers, M., Koppejan, J., Middelkamp, J., Witkamp, J., Sokhansanj, S., Melin, S., Madrali, S.: Status overview of torrefaction technologies. A review of the commercialisation status of biomass torrefaction. IEA Bioenergy, Enschede (2015)
Zaichenko, V.M., Shterenberg, V.Y.: Torrefaction of wood pellets: new solutions. Therm. Eng. 64(10), 729–737 (2017). https://doi.org/10.1134/S0040601517100111
Faleeva, M.J., Sinelshchikov, V., Sytchev, G., Zaichenko, V.: Exothermic effect during torrefaction. J. Phys. Conf. Ser. 946, 012033 (2018). https://doi.org/10.1088/1742-6596/946/1/012033
Kislitsyn, A.N.: Wood Pyrolysis: Chemistry, Kinetics, Products, New Processes. Forest Industry, Moscow (1990)
Mahari, W.A.W., Chong, C.T., Cheng, C.K., Lee, C.L., Hendrata, K., Yek, P.N.Y., Ma, N.L., Lam, S.L.: Production of value-added liquid fuel via microwave co-pyrolysis of used frying oil and plastic waste. Energy. 162, 309–317 (2018). https://doi.org/10.1016/j.energy.2018.08.002
Bilgic, E., Yaman, S., Haykiri-Acma, H., Kucukbayrak, S.: Is torrefaction of polysaccharides-rich biomass equivalent to carbonization of lignin-rich biomass? Biores. Technol. 200, 201–207 (2015). https://doi.org/10.1016/j.biortech.2015.10.032
Bilgic, E., Yaman, S., Haykiri-Acma, H., Kucukbayrak, S.: Limits of variations on the structure and the fuel characteristics of sunflower seed shell through torrefaction. Fuel Process. Technol. 144, 197–202 (2016). https://doi.org/10.1016/j.fuproc.2016.01.006
Shevchenko, A.L., Sytchev, G.A., Zaichenko, V.M.: Possibility of the use of exothermic-reactions heat from thermal destruction of biomass to increase the energy efficiency of the torrefaction process. J. Phys. 1147, 012093 (2019). https://doi.org/10.1088/1742-6596/1147/1/012093
Sommersacher, P., Brunner, T., Obernberger, I.: Fuel indexes: a novel method for the evaluation of relevant combustion properties of new biomass fuels. Energy Fuels 26(1), 380–390 (2012). https://doi.org/10.1021/ef201282y
Brunner, T., Biedermann, F., Kanzian, W., Evic, N., Obernberger, I.: Advanced biomass fuel characterisation based on tests with a specially designed lab-scale reactor. Energy Fuels 27(10), 5691–5698 (2013). https://doi.org/10.1021/ef400559j
Gölles, M.: Entwicklung mathematischer Modelle einer Biomasserostfeuerungsanlage als Grundlage für modellbasierte Regelungskonzepte. Graz University of Technology, PhD-Thesis (2008)
Hofbauer, H.: Verbrennungsrechung und adiabate Verbrennungs temperatur. In: Kaltschmitt, M., Hartmann, H., Hofbauer H. (Hrsg.), Energie aus Biomasse. Grundlagen, Techniken und Verfahren. 3. Auflage (S. 830–837). Berlin: Springer (2016)
Sandström, M.: Structural and solid state EMF studies of phases in the CaO–K2O–P2O5 system with relevance for biomass combustion. PhD-Thesis, University of Technology, Sweden (2006)
Makino, H., Tanno, K.: Coal combustion for power production In: Riazi, M.R., Gupta, R. (Eds.) Coal Prod. Process. Technol. (pp. 263–265). CRC Press, Taylor and Francis Group (2015)
Ohm, T.I., Chae, J.S., Kim, J.K., Oh, S.C.: Study on the characteristics of biomass for co-combustion in coal power plant. J. Mater. Cycles Waste Manage. 17, 249–257 (2015). https://doi.org/10.1007/s10163-014-0334-y
Magasiner, N., Van Alphen, C., Inkson, M., Misplon, B.: Characterising fuels for biomass—coal fired co-generation. Proc. Annu. Congr. 1, 282–291 (2001)
Bridgeman, T.G., Jones, J.M., Shield, I., Williams, P.T.: Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 87(6), 844–856 (2008). https://doi.org/10.1016/j.fuel.2007.05.041
Chen, W.H., Kuo, P.C.: A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy. 35(6), 2580–2586 (2010). https://doi.org/10.1016/j.energy.2010.02.054
Svoboda, K., Pohorely, M., Hartman, M., Martinec, J.: Pretreatment and feeding of biomass for pressurized entrained flow gasification. Fuel Process. Technol. 90(5), 629–635 (2009). https://doi.org/10.1016/j.fuproc.2008.12.005
Tumuluru, J.S., Sokhansanj, S., Hess, J.R., Wright, C.T., Boardman, R.D.: A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 7(5), 384–401 (2011). https://doi.org/10.1089/ind.2011.7.384
Sommersacher, P., Brunner, T., Obernberger, I., Kienzl, N., Kanzian, W.: Application of novel and advanced fuel characterization tools for the combustion related characterization of different Wood/Kaolin and Straw/Kaolin mixtures. Energy Fuels 27(9), 5192–5206 (2013). https://doi.org/10.1021/ef400400n
Legal Information System of the Republic of Austria. https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=10007873
Knudsen, J.N., Jensen, P.A., Dam-Johansen, K.: Transformationand release to the gas phase of Cl, K, and S during combustion of annual biomass. Energy Fuels 18, 1385–1399 (2004). https://doi.org/10.1021/ef049944q
Johansen, J.M., Aho, M., Paakkinen, K., Taipale, R., Egsgaard, H., Jakobsen, J.G., Jappe Frandsen, F., Glarborg, P.: Release of K, Cl, and S during combustion and co-combustion with wood of high-chlorine biomass in bench and pilot scale fuel beds. Proc. Combust. Inst. 34(2), 2363–2372 (2013). https://doi.org/10.1016/j.proci.2012.07.025
Sommersacher, P., Kienzl, N., Brunner, T., Obernberger, I.: Simultaneous online determination of S, Cl, K, Na, Zn, and Pb release from a single particle during biomass combustion. Part 2: results from test runs with spruce and straw pellets. Energy Fuels 30(4), 3428–3440 (2016). https://doi.org/10.1021/acs.energyfuels.5b02766
Directive (EU) 2015/2193 of the European Parliament and of the Council of 25 November 2015 on the limitation of emissions of certain pollutants into the air from medium combustion plants (Text with EEA relevance) OJ L 313, 28.11.2015, p. 1–19 (BG, ES, CS, DA, DE, ET, EL, EN, FR, HR, IT, LV, LT, HU, MT, NL, PL, PT, RO, SK, SL, FI, SV) ELI: http://data.europa.eu/eli/dir/2015/2193/oj
Phyllis2, database for (treated) biomass, algae, feedstocks for biogas production and biochar. https://phyllis.nl/ECN.TNO
Kułazynski, M., Jabłonski, S., Kaczmarczyk, J., Swiatek, L., Pstrowska, K., Łukaszewicz, M.: Technological aspects of sunflower biomass and brown coal co-firing. J. Energy Inst. (2017). https://doi.org/10.1016/j.joei.2017.06.003
Sorgona, A., Longo, L., Proto, A.R., Cavalletti, P., Cecchini, M., Salvati, L., Gallucci, F., Colantoni, A.: Characterization of biochar and syngas obtained from pellets of grape vine and sun flower husk using a pyrolysis system. Proc. Soc. Behav. Sci. 223, 871–878 (2016). https://doi.org/10.1016/j.sbspro.2016.05.297
Fusco, L.D., Boucquey, A., Blondeau, J., Jeanmart, H., Contino, F.: Fouling propensity of high-phosphorus solid fuels: predictive criteria and ash deposits characterisation of sunflower hulls with P/Ca-additives in a drop tube furnace. Fuel 170, 16–26 (2016). https://doi.org/10.1016/j.fuel.2015.12.017
Dhanavath, K.N., Shah, K., Bhargava, S.K., Bankupalli, S., Parthasarathy, R.: Oxygen–steam gasification of karanja press seed cake: fixed bed experiments, ASPEN Plus process model development and benchmarking with saw dust, rice husk and sunflower husk. J. Environ. Chem. Eng. 6, 3061–3069 (2018). https://doi.org/10.1016/j.jece.2018.04.046
Blondeau, J., Ryckmans, Y., Corbisier, D.: Characterization of sunflower husk ash deposition in a drop tube furnace. In: 22nd European Biomass Conference and Exhibition. Hamburg, Germany (2014)
Ooi, T.C., Aries, E., Ewan, B.C.R., Thompson, D., Anderson, D.R., Fisher, R., Fray, T., Tognarelli, D.: The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 21, 167–177 (2008). https://doi.org/10.1016/j.mineng.2007.09.005
Raclavska, H., Juchelkova, D., Roubicek, V., Matysek, D.: Energy utilisation of biowaste—Sunflower-seed hulls for co-firing with coal. Fuel Process. Technol. 92, 13–20 (2011). https://doi.org/10.1016/j.fuproc.2010.03.006
Acknowledgements
The authors gratefully acknowledge the support of the FFG—Austrian Research Promotion Agency, GSRT—Greek General Secretariat for Research and Technology and Foundation for Assistance to Small Innovative Enterprises in Science and Technology (Russia). The work was carried out within the ERA.Net RUS Plus project “TORRECOMB_ID#213_ERA.NET RUS Plus—A novel approach for the implementation of torrefaction in residential and communal heating boilers”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Emission Limits in Austria According to the “Feuerungsanlagenverordnung” (FAV)
Appendix: Emission Limits in Austria According to the “Feuerungsanlagenverordnung” (FAV)
Parameter | Fuel heat capacity (MW) | ||||
|---|---|---|---|---|---|
≤ 0.4 | 0.4 to < 1 | 1 to 2 | > 2 to 10 | > 10 | |
Dust (mg/m3) | 150 | 50* | 50 | 20 | 20 |
CO (mg/m3) | 800** | 250 | 250 | 250 | 100 |
HC (mg/m3) | 50 | 20 | 20 | 20 | 20 |
NOx (mg/m3) | 500 | 500 | 400 | 400 | 200 |
SO2*** (mg/m3) | 350 | 350 | 350 | 350 | 350 |
HCl*** (mg/m3) | 30 | 30 | 30 | 30 | 30 |
Rights and permissions
About this article
Cite this article
Kienzl, N., Margaritis, N., Isemin, R. et al. Applicability of Torrefied Sunflower Husk Pellets in Small and Medium Scale Furnaces. Waste Biomass Valor 12, 2579–2596 (2021). https://doi.org/10.1007/s12649-020-01170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01170-7