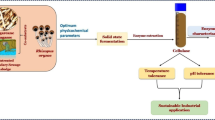
Abstract
Purpose
To avoid a negative environmental and economic impact of agricultural wastes, and following the principles of circular economy, the reuse of agricultural wastes is necessary. For this purpose, isolation of novel microorganisms with potential biotechnological application is recommended. The current researches in bioethanol production are aimed to reduce the production costs using low-cost substrates and in-house produced enzymes by novel isolated microorganisms. In line with this, in this study valorization of these agricultural by-products by novel isolate S. fulvissimus CKS7 to biotechnological value added products was done.
Methods
Standard microbiological methods were used for the isolation and characterization of strain. Enzymes activities were determinated using DNS method while, the ethanol concentration was determined based on the density of the alcohol distillate at 20 °C.
Results
The maximal enzymatic activities for amylase, cellulases (carboxymethyl cellulase and Avicelase), pectinase and xylanase were achieved using rye bran as a waste substrate for CKS7 growth. Obtained crude bacterial enzymes were used for enzymatic hydrolysis of lignocellulosic materials including horsetail waste, yellow gentian waste, corn stover, cotton material and corona pre-treated cotton material. The maximum yield of reducing sugars was obtained on horsetail waste and corona pre-treated cotton material. Waste brewer’s yeast Saccharomyces cerevisiae was successfully used for the production of bioethanol using horsetail waste hydrolysate and corona pre-treated cotton material hydrolysate.
Conclusion
The obtained results showed that bacterial strain CKS7 has a significant, still unexplored enzymatic potential that could be used to achieve a cleaner, environmental friendly and economically acceptable biofuel production.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This research was undertaken to isolate a novel microorganism, from natural habitat, with high biotechnological potential that could be used in industrial processes. Agricultural by-products are cheap waste substrates, rich in sugars and proteins that could be effectively used as substrate for bacterial growth and enzymes production. In this study, for the first time, enzymatic potential from the novel strain Streptomyces fulvissimus CKS7 was explored using agricultural by-products for bioethanol production.
In addition, for the first time, rye bran was proved to be a very potent substrate for S. fulvissimus CKS7 growth and various enzymes production. Obtained crude enzymes, could be used for enzymatic hydrolysis of horsetail waste, yellow gencian waste and waste cotton materials in order to obtain bioethanol.
Introduction
The microbial world is all around us. The diversity of the microbial world is still largely unknown and new microbial species are isolated continuously [1]. Detection and isolation of novel microorganisms in a natural environment (different types of soil, water, wood-feeding termite, etc.) is important for understanding and verifying their great biotechnological potential [2, 3]. The enormous diversity of the microbial world provides bases for many industrial processes [4, 5]. When searching for industrially important bioproducts (mainly biologically active metabolites), microorganisms from the soil play a dominant role. Soil microorganisms are crucial for the decomposition of organic matter in the soil, soil mineralization, nitrogen fixation and the promotion of plant growth [6]. Most of biological activities in a soil are processed through enzymatic processes [7]. In line with this, microorganisms isolated from the soil have proven ability to produce various enzymes. Enzymes with hydrolytic activity (including cellulases, xylanases and amylases) have several potential applications in different industries. Nowadays, enzymes that are used in the process of hydrolysis for the production of liquid biofuels are specifically requested.
Today, one of the great challenges of the modern society is reducing the amount of wastes. Considering that food and agricultural industries have a rapid economic growth, the resulting significant amounts of wastes have a negative environmental and economical impact on the environment and society. Most of the wastes that are disposed from food agricultural industries could be used for production of some biotechnological value added products [8].The production of microbial enzymes using agricultural by-products or wastes in solid state fermentation (SSF) resembles microbial growth in natural habitats, it is relatively simple to apply and is associated lower capital investment and lower operational costs [9,10,11,12].
This kind of waste contains fermentable sugars, proteins, minerals and fibers that could be used for the growth of microorganisms and the production of enzymes. Lignocellulosic waste materials are mainly composed of cellulose, hemicelulose and lignin and for efficient degradation of these polymers different processes that involved enzymes are required [13, 14].Thus, the use of crude enzymes obtained from SSF in the processes of enzymatic hydrolysis, compared with commercial enzymes is economically acceptable and justified.
Agricultural waste materials can be used for various purposes, and bioethanol production is among the most widely applied [15]. Bioethanol is so far the predominant biofuel in transportation sector that represents a great alternative to petroleum [16]. Recent trends in production of bioethanol include using different raw materials of lignocellulosic-starch structure or lignocellulosic waste biomass that could be hydrolyzed into simple sugars and converted by yeast fermentation into bioethanol. The most expensive part of bioethanol production is the cost of producing applied enzymes also as the pretreatment step [17, 18].
Among different soil microorganisms, the genus Streptomyces is exceptional due to its ability to produce bioactive secondary metabolites (mainly antibiotics) [19] and various enzymes [20, 21]. Recently, a new strain Streptomyces fulvissimus CKS7, isolated by our researchers group, showed very promising decolorization efficiency of a solution containing crystal violet dye using bacterial intracellular enzymes [22]. In this study we explore the hydrolytic potential of this strain using waste substrates and its potential application for hydrolysis process in bioethanol production. This represents the first study to report the production of extracellular hydrolases that include cellulases (Carboxymethyl cellulase-CMCase and Avicelase), amylase, pectinase and xylanase for S.fulvissimus species. The obtained enzymes were used in the process of hydrolysis of several waste lignocellulosic and cotton waste materials to study their potential for obtaining hydrolysates suitable for production ofbioethanol. For the first time, waste horsetail was used as substrate for hydrolysis and then for ethanol production.
Experimental Section (or Materials and Methods)
Sample Materials and Their Preparation
Lignocellulosic by-products wheat bran, barley bran and rye bran were provided by Klas, Sarajevo, Bosnia and Herzegovina. Raw waste materials were dried. The particle size of all tested bran was between 800 and 2000 µm. Sunflower and soybean meals were kindly donated by locally factory Bioprotein, Serbia. Lignocelluloic by-products, sunflower and soybean meal were used as substrates in SSF for enzymes production.
Equisetum arvense (horsetail) waste, Gentiana lutea (yellow gentian) waste was obtained from the Institute of Medicinal Plant Research “Dr Josif Pancic”, Belgrade, Serbia. The obtained horsetail and yellow gentian were dust waste produced during the industrial processing of the herbs. The particle size of the both dusts was in the range of 300–1000 μm. Corn waste or corn stover (particle size 500–2500 µm) was kindly donated by locally agricultural cooperative. Desized and bleached cotton woven fabric (117.5 g m−2, 52 picks cm−2, 27 ends cm−1, thickness of 0.26 mm) were cleaned from impurities in a manner described elsewhere [23]. Corona treatment of the cotton fabric was performed at atmospheric pressure using a commercial device Vetaphone CP-Lab MK II. The cotton samples were placed on the electrode roll covered with silicon coating, rotating at the minimum speed of 4 m min−1. The distance between electrodes was 2.3 mm. The power was 900 W and the number of passages was set on 30. Equisetum arvense (horsetail) waste, Gentiana lutea (yellow gentian) waste, corn waste and cotton fabric were used as substrates in enzymes hydrolysis.
Strain Isolation and Characterization
The strain CKS7 was isolated from the soil sample as described in our previous study [24] with some modifications. During isolation, instead of CMC agar plates in this study ISP1 (International Streptomyces Project 1) agar plates (casein hydrolysate 5.0 gl−1, yeast extract 3.0 g l−1, agar–agar 16 g l−1) were used. The plates were incubated at 30 °C for 3–5 days. Single colonies were isolated and purified by transferring them on the fresh agar plates. The purified colonies were preserved at + 4 °C for further identification.The isolated strain was identified as Streptomyces fulvissimus CKS7 based on the biochemical tests performed using API ZYM kit (bioMérieux, USA) according to the manufacturer’s instructions and the almost full-length 16S rRNA gene sequence. Growth at various temperatures and pH was performed by measuring the optical density at 540 nm using ISP1 broth and analyzing the modification of the pH of the ISP1 medium, respectively.
The enzymatic potential of the CKS7 strain was evaluated using selective agar medium plates (yeast extract 3.0 g l−1, agar 16.0 g l−1) with adding: CMC (CMC-carboxymethyl cellulose, 1.0 g l−1) and Avicel (microcrystalline cellulose, 1.0 g l−1) for cellulolytic activity; starch (soluble starch, 5.0 g l−1) for amylolytic activity; xylan (beechwood xylan, 1.0 g l−1) for xylanase activity and pectine (pectin from apple, 1.0 g l−1) for pectinase activity.
A loopfull of purified culture of the strain CKS7 was transferred from ISP1 agar medium on selective agar mediums and incubated for 3–5 days at 30 °C. After incubation agar plates were flooded with Gram’s iodine (2.0 g KI and 1.0 g I2 in 300 ml distilled water) for 5 min. Clear zones around colonies indicated cellulose, starch, xylan and pectin hydrolysis [25].
Inoculum Preparation and Solid State Fermentation (SSF)
The inoculum was prepared by growing the strain CKS7 in the ISP1 liquid medium with agitation (48 h, 30 °C, 120 rpm). SSF was performed using agricultural by-products: wheat bran, barley bran, rye bran, sunflower meal and soybean meal as substrates. Ten grams of each substrate was placed in 300 ml Erlenmeyer flasks and moistened with distilled water in a ratio 1:2 (w/v). After autoclaving (121 °C, 20 min), the flasks were inoculated with 10% inoculum. The contents of the flasks were then mixed thoroughly to ensure uniform distribution of the inoculum and incubated at 30 °C for 6 days. To extract the enzyme, entire flask that contained fermented medium was mixed with 50 ml 0.1 M acetate buffer pH 4.80 by shaking in a rotary shaker (190 rpm, 30 min, 25 °C); and after centrifugation (6000 rpm, 10 min, 4 °C) the supernatant was used as crude enzyme extract and was further analyzed for cellulases (CMCase and Avicelase), amylase, pectinase and xylanase activity.
Using the rye bran, the maximum of all tested enzymes activities was obtained, and this crude enzyme was utilized to optimize solid to moisture ratio (1:1, 1:1.5, 1:2, 1:2.5 and 1:3 (w/v)) during the production of enyzmes.
Enzymatic Activity Assay
Cellulases, amylase, xylanase and pectinase activities were measured by reduction of 3,5-dinitrosalicylic acid in the presence of glucose released by enzymatic hydrolysis of cellulose and starch, xylose released by enzymatic hydrolysis of xylan and d-galacturonic acid released by enzymatic hydrolysis of pectin, according to the method of Miller [26]. The release of reducing sugars was analyzed using modified DNS method with standard curve (glucose, xylose or d-galacturonic acid).
CMCase (endogluconase) and Avicelase (exogluconase) activity was performed by incubating 500 µl of crude enzyme extract with 500 µl of 1% solution of CMC or Avicel in acetate buffer (0.1 M, pH 4.8) 30 min at 50 °C. After incubation, 1 ml of DNS reagent was added, the reaction mixture was boiled for 15 min, cooled to room temperature and mixed with 5 ml of distilled water. The absorbance was read on the UV/visible spectrophotometer (Ultrospec 3300 pro, Amersham Bioscience, Sweden) at 540 nm (25 °C) against a blank (non incubated enzyme). One unit (IU) of CMCase or Avicelase activity was defined as amount of enzyme that released 1 μmol of glucose equivalents per minute [24].
Activity of other enzymes was measured by incubating 500 µl of crude enzyme extract with 500 µl of 1% solution of starch (for amylase), birch wood xylan (for xylanase) or pectin (apple pectin for pectinase) in acetate buffer (0.1 M, pH 4.8) for 15 min at 50 °C. After incubation, samples were mixed with DNS and the procedure describe above was followed. One unit of amylase, xylanase or pectinase activity was defined as the amount of enzyme that released 1 μmol of glucose, xylose or D-galacturonic acid equivalents per minute, respectively.
Enzymatic Hydrolysis
Different lignocellulosic waste materials including corn stover, Equisetum arvense (horsetail) waste, Gentiana lutea (yellow gentian) waste, cotton fabric and corona treated cotton fabric were used as substrates for enyzmatic hydrolysis, while the crude enzyme wasproduced by the strain CKS7 during SSF on rye bran. Prior the hydrolysis, these waste materials were autoclaved (121 °C, 1.2 bar, 20 min).The crude enzymes obtained during SSF were mixed with 0.1 M acetate buffer (1:1 v/v) for hydrolysis process. Hydrolysis was performed in three replications in 100 ml Erlenmeyer flasks at 50 °C, 4 days in a rotary shaker with 150 rpm. Hydrolysis of substrates was performed using 5% (w/v) of corn stover, 1% (w/v) of horsetail and yellow gentian and 1% (w/v) of cotton fiber and corona pre-treated treated cotton fiber. After the reaction, samples were centrifuged (6000×g, 10 min) to remove unhydrolyzed residue. Total reducing sugars in the supernatant was calculated as glucose equivalent that was estimated by DNS method [26]. The effects of used agricultural by-products (substrate) and solid to moisture ratio on enzyme activity during SSF were evaluated by a one-way ANOVA followed by the post-hoc Duncan test to consider the differences between individual parameters.
Waste Brewer’s Yeast Preparation
Waste brewer’s yeast was isolated using previously described procedure [27]. Inoculum was prepared by growing the yeast S. cerevisiae in malt extract broth (20 g l−1 malt extract) at 30 °C for 24 h in a rotary shaker at agitation speed of 120 rpm.
Ethanol Production
The horsetail hydrolysate and corona pre-treated cotton material hydrolysate, that contained the maximum amount of reducing sugars, were used for ethanol production. The obtained unfiltered hydrolysates were subjected to ethanol fermentation by waste brewer’s yeast S. cerevisiae in orbital shaker (48 h, 30 °C, 120 rpm. The yeast inoculum concentration was 2%. During fermentation the formation of ethanol and the number of viable yeast cells were determined. The ethanol concentration was determined based on the density of alcohol distillate at 20 °C and expressed in weight % (w/w) [28]. The number of viable yeast cells was obtained by indirect counting method-standard pour plate technique on malt extract agar plates. Serial dilutions of the samples were made, and after the incubation at 30 °C, grown colonies were counted. The changes in the viable cell number were calculated as:
where (CFU ml−1)1 is viable cell number after fermentation and (CFU ml−1)0 is viable cell number before fermentation.
Results and Discussion
Characterization of Isolated Microorganism and Screening for Enzymes
This study explores the enzymatic potential of a novel bacterial isolate Streptomyces fulvissimus CKS7, previously isolated from a soil sample (coniferous forest) from a foot of the Austrian Alps, for lignocellulosic hydrolysis and bioethanol production. Members of the genus Streptomyces are metabolically extremely active bacteria and many Streptomyces strains that produce enzymes and antimicrobial components have been reported earlier [19, 20, 29]. However, this is the first study that deals with the production of various enzymes on different agricultural by-products, their use in hydrolysis process, and the production of ethanol using the species S. fulvissimus. In additional, the results of this study indicate that members of the genus Streptomyces have a significant and currently neglected potential that could be used in the production of biofuels.
The isolated microorganism designated as the strain CKS7 is a Gram positive, rod shaped bacterium that belonged to the genus Streptomyces (familia Streptomycetaceae, class Actinobacteria) and formed filamentous mycelia (Online resource 1) which are characteristic for genus Streptomyces [30].
The strain could grow at temperatures between 25 and 37 °C with optimum at 30 °C and pH values from 6 to 8 with optimum at pH 7 (Table 1). Growth occurred on commercial nutrient agar medium/broth, tryptone soy (TSA) agar/broth and ISP1 agar/broth while the growth was the best in the ISP1 broth.
The API-ZIM test results showed the presence of enzymes from the groups: phosphatase, esterase, amino peptidase, protease, and glycosyl hydrolysis. Of the 19 tested enzymes, CKS7 produced 15 (Table 1), indicating that the strain had a very rich enzymatic profile. Based on the enzymatic profile and almost full-length 16S rRNA gene sequence the strain was designated as Streptomyces fulvissimus CKS7 (Fig. 1).
The 16S rRNA sequence has been submitted to NCBI GenBank with an accession number KP715854.1.
The strain was able to grow on cellulose, starch, xylan and pectin agar plate. The presence of halo zones on tested agar plates indicates areas of substrate hydrolysis (Fig. 2).
The strain CKS7 was able to hydrolyze both form of cellulose, CMC as soluble cellulose and Avicel as microcrystalline cellulose (Fig. 2a,b) proving to be a true cellulolytic bacterium [31].
Solid State Fermentation and Enzymes Production
In order to examine the enzymatic potential of the strain, the SSF process on agricultural waste materials was employed to obtain industrially important microbial enzymes. Several agricultural by-products, including wheat bran, barley bran, rye bran, sunflower meal and soybean meal were used as waste substrates for the growth of microorganism and the production of enzymes. These materials are cheap, abundant and reach in nutrients that support microorganisms growth [32]. Moreover, the problems of environmental pollution with conventional methods of disposal of biological wastes are reduced by this way [33].
SSF is a very similar to the natural growth of soil microorganism, which is accompanied by the production of enzymes required for the degradation of soils and twig wood. In the SSF, the selection of the suitable substrate is a crucial factor for the growth of microorganism and therefore production of enzyme [32]. Thus, in this study several agro-industrial wastes were used as substrates for potential enzymes production by the strain CKS7.
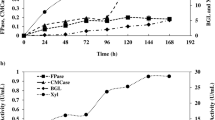
The production of cellulases (CMCase and Avicelse), amylase, pectinase and xylanase by strain CKS7 was measured (Table 2). According to the obtained data, all tested waste substrates were suitable for production of all tested, industrially important enzymes. Among them, rye bran facilitated synthesis of the maximum amount of all tested enzymes. According to the statistical analysis, all tested substrates affected CMC activity significantly (P < 0.001). There was no significant difference between substrates sunflower and soybean meal on Avicelase activity, barley bran and soybean meal on amylase activity, and wheat and barley bran on pectinase and xylanase activities (Table 2).
Literature data showed that cellulase activity could be obtained using wheat bran as a substrate for submerged fermentation using Streptomyces sp. [34, 35]. In contrast to this, only few reports about cellulase production on wheat bran as substrate for SSF fermentation using this genus were reported. More precisely, only Budihal [36] reported cellulase (CMCase and Avicelase) production on wheat bran byisolate Streptomyces DSK 29. Similary to this, rye bran was not previously used as substrate for microbial cellulase production. The strain CKS7 produced cellulolytic enzymes on both waste substrates, with higher activities on rye bran than on wheat bran. Due to the lack of literature data on cellulase production on rye bran, comparison with other publications is missing. Nevertheless, the S. fulvissimus CKS7 has a potential to grow on rye bran and to produce cellulase, CMCase and Avicelase. According to this, rye bran is appeared to be a potent substrate for cellulase production by this strain.
The higher content of arabinoxylans in a rye bran than in other cereals [37] facilitates stimulation of the higher xylanase activity in this bran. The content of starch in sunflower and soybean meal are poor and thus amylase activity is lower compared to other brans, as shown in our study and by others [38, 39]. Soybean meal with higher pectin content (~ 6%) than in other brans [40] was expected to be particularly stimulative for the production of pectinase. The pectinase activity was indeed the highest on soybean meal, but only slightly higher than that obtained on rye bran. Since all other tested enzymatic activities were the highest on rye bran, the crude enzyme mix obtained on this substrate was selected for further examinations.
During SSF with rye bran by S. fulvissimus CKS7, the optimum solid moisture ratio was 1:2.5 (w/v) (Table 3). The influence of solid moisture ratio on the enzyme activity was statistically significant (P < 0.001), although there was no significant difference between moisture content: 1:2 and 1:3 ratios and its effect on xylanase activities, 1:1.5 and 1:2 ratios on avicelase activity, and 1:1 and 1:3 ratios on pectinase activity (Table 3).
Moisture content is a critical factor for SSF processes because it directly affects the growth of microorganism through the secretion of nutrients. Since microorganisms grow on the surface of the substrate, the lower moisture content causes a reduction in the solubility of the nutrients, a low degree of swelling and a decrease in enzyme production [41]. On the other hand, the higher moisture content results in decreased of porosity, loss of particle structure and development of stickiness, which, in turn, prevents the penetration of oxygen and affects enzyme production.
Enzymatic Hydrolysis by Obtained Crude Enzymes
In order to further explore the enzymatic potential of the strain S. fulvissimus CKS7, the bacterial supernatant obtained during SSF on rye bran that contained mix of enzymes was used in the hydrolysis process of lignocellulosic waste substrates (corn stover, horsetail, yellow gentian, cotton and corona treated cotton). Our previous investigations (Online resource 2) showed that these lignocellulosic materials were not suitable for CKS7 growth and thus for enzymatic production. For this reason, in this study these waste materials were only used for enzymatic hydrolysis.
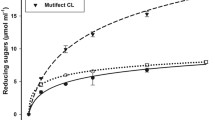
Five lignocellulosic waste materials including corn stover, horsetail, yellow gentian, cotton and corona treated cotton were used as substrates for enzymatic hydrolysis (Table 4).According to the obtained results all tested substrates were suitable for enzymatic treatment and significant amount of reducing sugars were obtained (Table 4). Maximum reducing sugars yield of 2.55 mg ml−1 was obtained at horsetail after 72 h of hydrolysis (Table 4). Slightly lower reducing sugars yield of 2.26 mg ml−1 was reported at corona treated cotton material.
Enzymatic hydrolysis is an important step in the production of biofuel. Among other enzymes, cellulases are used to hydrolyze cellulose from lignocellulosic biomass to produce reducing sugars including glucose, while xylanase can improve the hydrolysis of cellulose into fermentable sugars by degradation of xylan that restricts the cellulases from acting efficiently [42]. During the hydrolysis of lignocellulosic biomass, the presence of pectin can hinder the accessibility of cellulose [43] therefore, the presence of pectinase also could improve the release of fermentable sugars.
Apart from lignocellulosic structure, horsetail and yellow gentian contain other bioactive components (antimicrobial, anti-inflammatory, antioxidant activity etc.) [44, 45]. Some phenols naturally present in these plants can inhibit cellulose hydrolysis [46]. Apparently, such compounds in yellow gentian act as inhibitors of enzymatic hydrolysis and therefore lead to the reducing in sugar content compared to horesetail.
The corona pre-treatment of cotton materials caused an increase of reducing sugars upon hydrolysis (Table 4). Cotton fibers have a high cellulose content (~ 80%) but do not have lignin [47]. The surface area and roughness of corona treated cotton material were increased and this altered structure improved the fiber accessibility to enzymes and consequently the concentration of reducing sugars upon hydrolysis [48].
In many studies different pre-treatment methods for enhancing the digestibility of lignocellulosic biomass to improve enzymes hydrolysis have been employed [49]. However, in this study we tested the cleaner production where no chemical pre-treatment of lignocellulosic biomass was applied and therefore, comparison with literature data is difficult. Furthermore, some lignocellulosic substrates such as horsetail and yellow gentian have not previously used for the hydrolysis process. Enzymatic hydrolysis of corn stover to fermentable sugar is a very difficult and rate limiting process due to the recalcitrance of this lignocellulosic biomass [50, 51]. About 70% of the corn stover consists of lignocellulosic material that contains cellulose, hemicelluloses, pectin and lignin [52, 53]. Lignin and hemicelulose interfere with the cellulose hydrolysis because lignin acts as a barrier and prevents contact between cellulase and cellulose. Moreover, the hydrolysis of cellulose can hinder cellulose crystallinity [54].
It should be noted that, despite the relatively low content of reducing sugar obtained after enzymatic hydrolysis of corn stover (1.89 mg ml−1) compared to pre-treated lignocelulosic biomass of corn stover reported by Idrees [55] (36.65 g l−1) and Satarn [51] (24.96 g l−1), the enzymatic hydrolysis of lignocellulosic biomass is a very slow but very clean process without using additional chemicals.
Ethanol Production
The production of ethanol from the horsetail and corona pre-treated cotton hydrolysate was studied using a waste brewer’s yeast S. cerevisiae. After 48 h of yeast fermentation, the ethanol concentration was 0.46% in horsetail sample and 0.39% in corona pre-treated cotton material. Both tested samples showed an increase in the yeast cell yield after fermentation, 2.24 log10CFU ml−1 and 2.07 log10CFU ml−1 for horsetail and corona pre-treated cotton material, respectively.
Nikolic and coworkers [48] investigated the production of ethanol from corona pre-treated cotton materials which was previously hydrolyzed by commercial cellulase. They obtained a higher concentration of ethanol after yeast fermentation, than in our study (0.39% vs. 0.56%). Using the commercial cellulase a higher reducing sugars yield was obtained, therefore, the concentration of ethanol at the end of the process is higher. However, it should be kept in mind that crude enzymes produced by microorganisms are still much cheaper than commercial enzymes. Although not in the scope of the current study it could be expected that further optimization of the hydrolysis process and yeast fermentation could lead to an increased concentration of ethanol in the process using most promising waste materials.
Conclusions
The enzymatic potential of the bacterial strain Streptomyces fulvissimus CKS7 for the hydrolysis of lignocellulosic and the production of ethanol has been investigated. The strain CKS7 was able to grow on various agricultural by-products and produce several industrially important enzymes (cellulases, amylase, xylanase and pectinase). Rye bran appeared to be the most suitable waste substrate for bioconversion. Crude enzymatic mix could hydrolyze different lignocellulosic substrates to obtain reducing sugars. The obtained hydrolysates of horsetail and corona pre-tretaed cotton material were used for the production of ethanol. Although the resulting ethanol yield is modest, this study demonstrates that the strain CKS7 similar to other Streptomyces genus members is a promising candidate for biodegradation cellulosic wastes that can be used in biofuel production.
References
Fischer, C.N.: Exploring the diversity of the microbial world. Yale J. Biol. Med. 84(1), 55–58 (2011)
Alves, P.D.D., de Faria Siqueira, F., Facchin, S., Horta, C.C.R., Victória, J.M.N., Kalapothakis, E.: Survey of microbial enzymes in soil, water, and plant microenvironments. Open Microbiol. J. 8, 25–31 (2014)
Tsegaye, B., Balomajumder, C., Roy, P.: Isolation and characterization of novel lignolytic, cellulolytic, and hemicellulolytic bacteria from wood-feeding Termite Cryptotermes brevis. Int. Microbiol. 22, 29–39 (2019)
Méndez-Vilas, A.: Communicating Current Research and Educational Topics and Trends in Applied Microbiology, vol. 2. Formatex, Badajoz (2007)
Tsegaye, B., Balomajumder, C., Roy, P.: Biodelignification and hydrolysis of rice straw by novel bacteria isolated from wood feeding termite. 3 Biotech 8, 447–458 (2018)
Fendrihan, S.: Bioproducts with living microorganisms used in agriculture. Res. J. Pharm. Phytochem. 9, 10–14 (2016)
Skujiņš, J., Burns, R.: Extracellular enzymes in soil. CRC Crit. Rev. Microbiol. 4, 383–421 (1976)
Ravindran, R., Hassan, S., Williams, G., Jaiswal, A.: A review on bioconversion of agro-industrial wastes to industrially important enzymes. BIOEBG 5, 93–113 (2018)
Demir, H., Tari, C.: Bioconversion of wheat bran for polygalacturonase production by Aspergillus sojae in tray type solid-state fermentation. Int. Biodeter. Biodegr. 106, 60–66 (2016)
Jecu, L.: Solid state fermentation of agricultural wastes for endoglucanase production. Ind. Crop. Prod. 11, 1–5 (2000)
Pothiraj, C., Kanmani, P., Balaji, P.: Bioconversion of lignocellulose materials. Mycobiology 34, 159–165 (2006)
Pandey, A., Selvakumar, P., Soccol, C.R., Nigam, P.: Solid state fermentation for the production of industrial enzymes. Curr. Sci. 77, 149–162 (1999)
Auer, L., Lazuka, A., Sillam-Dussès, D., Miambi, E., O'Donohue, M., Hernandez-Raquet, G.: Uncovering the potential of termite gut microbiome for lignocellulose bioconversion in anaerobic batch bioreactors. Front. Microbiol. 8, 1637–2623 (2017)
Brune, A.: Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12(3), 168–180 (2014)
Razmovski, R., Vučurović, V.: Bioethanol production from sugar beet molasses and thick juice using Saccharomyces cerevisiae immobilized on maize stem ground tissue. Fuel 92, 1–8 (2012)
Domínguez-Bocanegra, A.R., Torres-Muñoz, J.A., López, R.A.: Production of bioethanol from agro-industrial wastes. Fuel 149, 85–89 (2015)
Johnson, E.: Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuel. Bioprod. Bior. 10, 164–174 (2016)
El-Naggar, N., Deraz, S., Khalil, A.: Bioethanol production from lignocellulosic feedstocks based on enzymatic hydrolysis: current status and recent developments. Biotechnology 13, 1–21 (2014)
de Lima Procópio, R.E., da Silva, I.R., Martins, M.K., de Azevedo, J.L., de Araújo, J.M.: Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 16, 466–471 (2012)
Sathya, R., Ushadevi, T.: Industrially important enzymes producing streptomyces species from mangrove sediments. Int. J. Pharm. Pharm. Sci. 6, 233–237 (2014)
Sinjaroonsak, S., Chaiyaso, T., Aran, H.: Optimization of cellulase and xylanase productions by Streptomyces thermocoprophilus TC13W using low cost pretreated oil palm empty fruit bunch. Waste Biomass Valori. (2019). https://doi.org/10.1007/s12649-019-00720-y
Buntić, A., Pavlović, M., Šiler-Marinković, S., Dimitrijević-Branković, S.: Biological treatment of colored wastewater by Streptomyces fulvissimus CKS 7. Water Sci. Technol. 73, 2231–2236 (2016)
Marković, D., Deeks, C., Nunney, T., Radovanović, Ž., Radoičić, M., Šaponjić, Z., Radetić, M.: Antibacterial activity of Cu-based nanoparticles synthesized on the cotton fabrics modified with polycarboxylic acids. Carbohydr. Polym. 200, 173–182 (2018)
Mihajlovski, K.R., Carević, M.B., Dević, M.L., Šiler-Marinković, S., Rajilić-Stojanović, M.D., Dimitrijević-Branković, S.: Lignocellulosic waste material as substrate for Avicelase production by a new strain of Paenibacillus chitinolyticus CKS1. Int. Biodeter. 104, 426–434 (2015)
Kasana, R.C., Salwan, R., Dhar, H., Dutt, S., Gulati, A.: A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr. Microbiol. 57(5), 503–507 (2008)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Mihajlovski, K., Radovanović, Ž., Carević, M., Dimitrijević-Branković, S.: Valorization of damaged rice grains: Valorization of damaged rice grains: Optimization of bioethanol production by waste brewer’s yeast using an amylolytic potential from the Paenibacillus chitinolyticus CKS1. Fuel 224, 591–599 (2018)
Horwitz, W., Chichilo, P., Reynolds, H.: Official Methods of Analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists, Washington (1970)
Sanjivkumar, M., Brindhashini, A., Deivakumari, M., Palavesam, A., Immanuel, G.: Investigation on saccharification and bioethanol production from pretreated agro-residues using a mangrove associated actinobacterium Streptomyces variabilis (MAB3). Waste Biomass Valori. 9, 969–984 (2018)
Ikeda, H., Ishikawa, J., Hanamoto, A., Shinose, M., Kikuchi, H., Shiba, T., Sakaki, Y., Hattori, M., Ōmura, S.: Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21, 526–531 (2003)
Lynd, L.R., Weimer, P.J., Van Zyl, W.H., Pretorius, I.S.: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002)
Lizardi-Jiménez, M., Hernández-Martínez, R.: Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech 7, 44–53 (2017)
Rodríguez Couto, S.: Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J. 3, 859–870 (2008)
Azzeddine, B., Abdelaziz, M., Estelle, C., Mouloud, K., Nawel, B., Nabila, B., Francis, D., Said, B.: Optimization and partial characterization of endoglucanase produced by Streptomyces sp. B-PNG23. Arch. Biol. Sci. 65, 549–558 (2013)
de Lima, A.L.G., do Nascimento, R.P., da Silva Bon, E.P., Coelho, R.R.R.: Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme Microb. Technol. 37, 272–277 (2005)
Budihal, S., Agsar, D.: Exploration of agrowastes for the production of cellulase by Streptomyces DSK29 under submerged and solid state systems. Int. J. Curr. Microbiol. Appl. Sci 4, 681–689 (2015)
Izydorczyk, M.: Arabinoxylans. In: Philips, G.O., Williams, P.A. (eds.) Handbook of Hydrocoloids, pp. 653–692. Elsevier, New Delhi (2009)
Banaszkiewicz, T.: Nutritional value of soybean meal. In: El-Shemy, H. (ed.) Soybean and Nutrition, pp. 1–23. InTech, Rijeka (2011)
Sredanović, S., Lević, J., Đuragić, O.: Enzyme enhancement of the nutritional value of sunflower meal. Enzyme Microb. Technol. 21, 197–202 (2005)
Malathi, V., Devegowda, G.: In vitro evaluation of nonstarch polysaccharide digestibility of feed ingredients by enzymes. Poult. Sci. 80, 302–305 (2001)
Patil, N.S., Jadhav, J.P.: Enzymatic production of N-acetyl-D-glucosamine by solid state fermentation of chitinase by Penicillium ochrochloron MTCC 517 using agricultural residues. Int. Biodeter. Biodegr. 91, 9–17 (2014)
de Queiroz Brito-Cunha, C.C., de Campos, I.T.N., de Faria, F.P., Bataus, L.A.M.: Screening and xylanase production by Streptomyces sp. grown on lignocellulosic wastes. Appl. Biochem. Biotechnol. 170, 598–608 (2013)
Zheng, Y.-X., Wang, Y.-L., Pan, J., Zhang, J.-R., Dai, Y., Chen, K.-Y.: Semi-continuous production of high-activity pectinases by immobilized Rhizopus oryzae using tobacco wastewater as substrate and their utilization in the hydrolysis of pectin-containing lignocellulosic biomass at high solid content. Bioresour. Technol. 241, 1138–1144 (2017)
Mihailović, V., Mišić, D., Matić, S., Mihailović, M., Stanić, S., Vrvić, M.M., Katanić, J., Mladenović, M., Stanković, N., Boroja, T.: Comparative phytochemical analysis of Gentiana cruciata L. roots and aerial parts, and their biological activities. Ind. Crop. Prod. 73, 49–62 (2015)
Milutinović, M., Radovanović, N., Rajilić-Stojanović, M., Šiler-Marinković, S., Dimitrijević, S., Dimitrijević-Branković, S.: Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense. Ind. Crop. Prod. 61, 388–397 (2014)
Ximenes, E., Kim, Y., Mosier, N., Dien, B., Ladisch, M.: Inhibition of cellulases by phenols. Enzyme Microb. Technol. 46, 170–176 (2010)
Gupta, A., Verma, J.P.: Sustainable bio-ethanol production from agro-residues: a review. Renew. Sust. Energ. Rev. 41, 550–567 (2015)
Nikolić, S., Lazić, V., Veljović, Đ., Mojović, L.: Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr. Polym. 164, 136–144 (2017)
Sun, S., Sun, S., Cao, X., Sun, R.: The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 199, 49–58 (2016)
Lu, X., Zhang, Y., Yang, J., Liang, Y.: Enzymatic hydrolysis of corn stover after pretreatment with dilute sulfuric acid. Chem. Eng. Technol. 30, 938–944 (2007)
Satarn, J., Lamamorphanth, W., Kamwilaisak, K.: Acid hydrolysis from corn stover for reducing sugar. Adv. Mater. Resour. 931, 1608–1613 (2014)
Panagiotou, G., Kekos, D., Macris, B.J., Christakopoulos, P.: Production of cellulolytic and xylanolytic enzymes by Fusarium oxysporum grown on corn stover in solid state fermentation. Ind. Crop. Prod. 18, 37–45 (2003)
Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K.: Waste to Wealth. Springer, Singapore (2018)
Umamaheswari, M., Jayakumari, M., Maheswari, K., Subashree, M., Mala, P., Sevanthi, T., Manikandan, T.: Bioethanol production from cellulosic materials. Int. J. Curr. Res. 1, 005–011 (2010)
Idrees, M., Adnan, A., Bokhari, S.A., Qureshi, F.A.: Production of fermentable sugars by combined chemo-enzymatic hydrolysis of cellulosic material for bioethanol production. Braz. J. Chem. Eng. 31, 355–363 (2014)
Acknowledgements
The financial support for this investigation given by the Ministry of Education, Science and Technological Development of the Republic of Serbia under the project TR 31035 is gratefully acknowledged. The authors would like to thank the Dr Darka Marković for providing cotton and corona pre-treated cotton material. Also, the authors would like to thank agricultural cooperative “Mrkšićevi salaši” for obtaining corn waste.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain studies with human participants or animals.
Informed Consent
Informed consent was obtained from all the individual participants included in the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mihajlovski, K., Buntić, A., Milić, M. et al. From Agricultural Waste to Biofuel: Enzymatic Potential of a Bacterial Isolate Streptomyces fulvissimus CKS7 for Bioethanol Production. Waste Biomass Valor 12, 165–174 (2021). https://doi.org/10.1007/s12649-020-00960-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00960-3