Abstract
The present study was undertaken to evaluate the saccharification, followed by bioethanol production by an actinobacterium Streptomyces variabilis (MAB3) isolated from the sediment sample of mangrove environment using Modified Yeast extract—Peptone medium substituted with different concentrations of xylanase (50 to 400 IU) pretreated various agro-residues like vegetable, banana, mango, sugarcane bagasse and sugarcane juice through fermentation process. Among the tested residues, sugarcane juice substituted medium pretreated with 200.0 IU concentration of xylanase exhibited maximum level of reducing sugar (50.19 mg/g), degree of saccharification (59.49%) as well as bioethanol (4.69 g/L) production. Further the organism expressed highest saccharification yield and bioethanol production at the optimized cultural conditions of pH 6, temperature 30 °C, inoculum size 2.5% and incubation time 72 h substituted with 2.5% dextrose and 2.0% urea, respectively as carbon and nitrogen sources. The concentration of produced bioethanol (82.26%) from the optimized medium was determined through HPLC analysis. Finally the bioethanol was evaluated through FT-IR and GC-MS analysis. The basic functional ethanol groups (O–H and C–O) from the optimized medium were denoted in both FT-IR and GC-MS analysis at the wavenumber of 3272.22 and 1077.15 cm−1 with the retention time of 2.60 min, respectively. Based on the results, it could be confirmed that the selected isolate is a potent strain and it can able to hydrolyze the pretreated agro-residues and also it can able to convert the pretreated agro residues into economically important byproduct like bioethanol.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ethanol is an important alternative energy source, which is being used in many countries to lower the dependency on fossil fuels [1]. Efficient conversion of lignocellulosic materials into fuel ethanol has become a world priority due to the depletion of the fossil fuels and the increasing demand of fuel in the transportation sector [2]. Lignocellulosic (LCC) materials such as agricultural (wheat bran, sugarcane bagasse, soybean, rice straw, corncob and orange peel) and forest residues, crops and herbaceous materials in large quantities are available in many countries with various climatic conditions, making them suitable and potentially cheap feedstocks for sustainable production of economically important products like ethanol [3]. In beverage industries, lignocellulosic biomasses are used as raw material for the production of alcoholic derivatives and it constitutes cellulose, hemicellulose (carbohydrate polymers) and lignin [2, 4]. However, these wastes are biodegradable and can be converted into valuable products such as biofuels, chemicals and cheap energy sources for fermentation and ameliorated animal feeds [5]. Biomass conversion to ethanol proceeds in a step wise manner, where pretreatment and saccharification are the two most important steps which determine the overall cost and efficiency. The hydrolysis of these polysaccharides is hampered by the presence of lignin and the compact architecture of the cell-wall which makes them much more difficult than starch to be enzymatically degraded to fermentable sugars. Therefore, a pretreatment of this material is necessary to remove or alter the lignin in order to improve the rate of enzymatic hydrolysis of cellulose or hemicellulose and increase the yield of fermentable sugars [6]. The purpose of the pretreatment is to remove lignin and hemicellulose, reduce cellulose crystallinity, and increase the porosity of the materials. Biomass-pretreatment is needed to make the cellulose present in plant cell walls accessible for enzymatic hydrolysis [7].
Both the pretreatment and enzymatic hydrolysis, yield fermentable sugars like glucose, proceeds the final fermentation step in which bioethanol is obtained. However, the yield of fermentable sugars by using enzymatic hydrolysis is highly dependent on the type and severity of the biomass-pretreatment [8]. So far, several hydrolytic enzymes were used for pretreatment, namely cellulases and xylanases which have been isolated from different microorganisms, including fungi, yeasts and bacteria especially actinomycetes [9]. Among the various genera of actinomycetes identified so far, Streptomycetes are quite significant as they bring about decomposition of various plant based polysaccharides by the production of wood hydrolytic enzymes, such as cellulases and hemicellulases (xylanases) [10]. Xylanases from Actinomadura sp. [11], Streptomyces lividans [12], Streptomyces sp. [13], Streptomyces cyaneus SN32 [14] and Streptomyces sp [15]. have also been reported. These enzymes have received worldwide attention due to their potential applications especially in the biodegradation of agronomic wastes and are being increasingly used in food and beverage industries. Considering the importance of enzymatic treatment, the present study was focused on the saccharification, production, optimization, estimation and characterization of bioethanol from xylanase pretreated agro-residues through fermentation process using mangrove associated actinobacterium isolated from the rhizosphere sediment of Manakudy estuary, South west coast of India.
Materials and Methods
Collection and Analysis of Sediment Samples
The rhizosphere sediment soil samples were collected from five regions (RS1 to RS5) of mangrove environment of Manakudy estuary, South India, using sterile plastic containers and the samples were transported aseptically to the laboratory. In the laboratory, the samples were air dried under room temperature for 2–3 days. The physico-chemical properties of the collected rhizosphere sediment samples were performed using standard procedures described in APHA [16] and ASTM [17].
Isolation of Actinobacterial Strains
The actinobacterial strains from each soil samples (RS1 to RS5) were isolated according to the method of Radhakrishnan et al. [18] through serial dilution and spread plate technique using sterile Starch casein agar (SCA) medium at pH 8. Totally 14 different actinobacterial colonies based on different characteristics (morphology, appearance and reverse-side pigmentation) were picked up and inoculated individually in International Streptomyces Project Medium 2 (ISP2) agar at pH 8 and incubated at 28 °C for 5–7 days. They were designated as MAB1 to MAB14. These actinobacterial colonies were sub-cultured on Yeast extract—Malt extract agar (ISP2-agar) slants and preserved at 4 °C for further studies.
Preparation of Actinobacterial Inoculum
In different 250 mL conical flasks, 50 mL of sterile ISP2 broth were taken, into which all the isolated actinobacterial strains were inoculated separately. The inoculated flasks were incubated at 30 °C for 24–48 h. After incubation 5 mL of culture broth of individual strain was used as an inoculum for bioethanol production.
Screening for Bioethanol Production
5.0% each of all the 14 (MAB1 to MAB14) actinobacterial strains were individually inoculated in 100mL sterile modified YEPX medium at pH 6.5 and the flasks were incubated in rotary shaker at 150 rpm for 5–7days [19]. After incubation, the production medium from the individual flasks were centrifuged at 5000×g for 15 min. 0.5 mL each of individual supernatant and 4.5 mL of distilled water were taken in 15 mL test tubes. 1 ml of (0.1 M) potassium dichromate and few drops of H2SO4 (0.1 N) were continuously added to the test tubes. Then the reaction tubes were kept in a water bath for 10 min. The colour of the samples was turned from yellowish to green, which indicated the presence of bioethanol. Based on the result of this study, the strain MAB3 was identified as potent strain, which produced maximum amount of bioethanol and it was confirmed based on colour intensity by using UV–Vis spectrophotometer (UV2301II model) at 600 nm. Therefore this particular strain was treated as potent candidate strain and it was further characterized.
Taxonomical Characterization and Identification of Actinobacterium
The cultural characteristics of the selected potent actinobacterial strain (MAB3) were studied by using different culture media (ISP1 to ISP7) and the micromorphology of the potent strain was examined under bright field microscope and scanning electron microscope. Utilization of carbon sources by the candidate strain was carried out according to the method of Shirling and Gottileb [20] and physiological characteristics (using ISP2 medium with different pH (5 to 12), temperature (20 to 90 °C) and NaCl (0 to 7%) tolerance studies) of the potential strain were studied using standard procedures. In molecular characterization, the total genomic DNA of the potent strain (MAB3) was isolated using the phenol chloroform method of Nathan et al. [21] with slight modification. The 16S rRNA gene was amplified using the forward primer: 5′-CCAGCAGCCGCGGTAATA CG-3′ and reverse primer: 5′-TACCAGGGTATCTAATCC-3′. The 16S rRNA gene sequence data was analyzed using BLAST search. The sequences were aligned using the CLUSTAL NW program [22]. The evolutionary distance was calculated by Kimura 2 parameter, a phylogenetic tree was constructed by the neighbor-joining method. The 16S rRNA gene sequence determined in this study was deposited in GenBank of NCBI data base library under the accession number KM435070.
Collection of Enzyme Sample
The recommended xylanase enzyme for pretreatment process was obtained from MNP laboratory, Centre for Marine Science and Technology, M.S. University, Rajakkamangalam, India, which was the purified enzyme already produced from Streptomyces olivaceus (MSU3) [23].
Pretreatment of Agro-Residues
The agro-residues such as vegetables, banana, mango, sugarcane bagasse, and sugarcane juice were collected from a local market at Nagercoil, Kanyakumari district, India. Samples were aseptically transported to the laboratory. Then, the pretreatment process of individual residues was performed by a modified method of Detroy et al. [24] using different concentrations (50, 100, 200, 300 and 400 IU) of xylanase enzyme.
Determination of Total Sugar Content in the Collected Agro-Residues
The total sugar content in the collected agro-residues was determined by the method proposed by Saritha et al. [25] using anthrone reagent. The concentration of total sugar was calculated by using glucose as a standard.
Saccharification of Pretreated Agro-Residues
In enzymatic hydrolysis or saccharification, a suspension of individual pretreated agro-residues (10 g/L) were prepared in 50 mM acetate buffer (pH 7.0) and autoclaved at 121 °C for 20 min. Exactly 15 mL each of substrate suspensions were taken in 100 mL Erlenmeyer flasks and 5 mL of culture broth was inoculated and kept in a rotary shaker (150 rpm) at 30 ± 2 °C for 48 h [26]. The resultant supernatants, following centrifugation (5000×g for 15 min) were assayed for total reducing sugars using DNS method [27]. The release of sugar was expressed as equivalent to glucose. The degree of saccharification was calculated as
Bioethanol Production
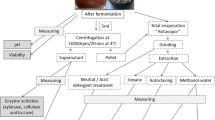
Ethanol production was performed by using modified YEPX medium containing : d-xylose 40 g/L, yeast extract 5 g/L, peptone 20 g/L, KH2PO4 0.5 g/L, (NH4)2SO4 0.5 g/L and MgSO4·7H2O 0.5 g/L. Instead of d-xylose, various pretreated agro-residues were used individually as carbon source for ethanol production. The pH of the medium was adjusted to 6.5 and the medium was sterilized at 121 °C for 15 min. After sterilization 5 × 106 CFU/mL of actinobacterial culture was inoculated and the flasks were incubated at 28 °C on a rotary shaker at 150 rpm [28]. After 5–7 days of incubation, the cultures were centrifuged at 5000×g for 15 min at 4 °C. The supernatants were filtered using a 0.2 µm filter paper (Milipore) and analyzed for ethanol production through spectrophotometric method at 600 nm. Ethanol concentration was determined by standard curve using different concentrations (1–10% v/v) of absolute ethanol as a standard [29]. Based on the results, among the tested concentrations of enzyme pretreated residues, sugarcane juice (40% v/v) pretreated with 200.0 IU xylanase rendered maximum degree of saccharification as well as maximum bioethanol production. Therefore this particular agro-residue (sugarcane juice treated with 200.0 IU enzyme) alone was further selected for optimization study.
Optimization of Culture Conditions on Saccharification and Bioethanol Production
The saccharification and bioethanol production were optimized according to the modified method of Hossain et al. [30] by various parameters like different pH (4, 5, 6, 7, 8, 9, 10, 11 and 12), temperature (20, 30, 40, 50 and 70 °C), carbon source (xylose, dextrose, sucrose, fructose, lactose and mannitol), nitrogen source (urea, beef extract, yeast extract, tryptone, peptone and ammonium sulphate), inoculum concentrations (0.5 to 5.0%) and incubation time (24 to 120 h) using xylanase (200 IU) pretreated sugarcane juice medium. 40 mL each of sugarcane juice medium were taken in different 250 mL conical flasks. Then, the flasks were autoclaved at 121 °C for 30 min. After sterilization, culture inoculum was added and kept in a rotary shaker at 150 rpm for 5–7 days. After incubation, the medium from the individual flasks were centrifuged at 5000×g for 15 min at 4 °C. The degree of saccharification and bioethanol from the culture free filtrates were determined by spectrophotometery (UV2301II model) at 540 and 600 nm respectively using glucose and absolute ethanol as standards.
Determination of Bioethanol Using HPLC
The production of bioethanol from the optimized medium (Sugarcane juice medium) was determined through high performance liquid chromatography (HPLC) analysis using an analytical column (Aminex-HPX-87H). 0.5 µL of bioethanol sample was used. In the column, the sample was eluted with 5mM sulfuric acid and HPLC grade water at 60 °C with the flow rate of 0.6 mL/min.
Characterization of Bioethanol
The produced bioethanol from the optimized medium was characterized by using FT-IR and GC-MS analysis.
Characterization of Bioethanol Using FT-IR-Analysis
The FT-IR analysis of the bioethanol sample was performed by preparing KBr pellets and correspondingly subjected in Perkin Elmer spectrophotometer (Perkin Elmer model spectrum-I Pc) in the absorption range between 4000 and 400 cm−1 with a detector at the resolution of 4 cm−1. The number of scans taken per sample was 20. The peak values of the FT-IR were recorded and were repeated twice for the spectrum confirmation [31].
Characterization of Bioethanol Using GC-MS Analysis
The GC-MS analysis of the bioethanol was performed according to the method of Dizhbite et al. [32] using Perkin Elmer Clarus-680 model with capillary column Elite-5MS, 30 m × 0.25 mm ID, 250 µm df. The injector temperature of 260 °C, ion source of 260 °C with EI of 70 eV, the MS scan range of 50–60 Da, carrier gas (helium) at the flow rate of 1 mL/min and the split ratio of 10:1. The identification of the individual compounds was performed on the basis of GC/MS chromatogram using Library NIST-2008, whereas the relative area of the peak of individual compounds was calculated using the Turbo mass ver. 5.4.2 software on the basis of GC/MS data.
Kinetic Parameters
The kinetic studies of bioethanol production were carried out at a constant actinobacterial inoculum concentration (2.5%) with different concentrations of sugarcane juice (10–100% v/v) as substrate. A typical Lineweaver–Burk plot was obtained when 1/µ was plotted against 1/s. Kinetic parameters (K s and µ max ) were estimated by linear regression from Lineweaver–Burk plot [33].
Statistical Analysis
The data obtained in the present study were expressed as Mean ± SD and were analyzed using ANOVA test and subsequently conducted post hoc multiple comparison with SNK test at 5% level of significance using computer software STATISTICA 6.0 (Statosoft, Bedford, UK).
Results
Analysis and Isolation of Actinobacteria from Rhizosphere Sediments
There were five rhizosphere sediment samples (RS1 to RS5) collected near the mangrove plant Avicennia marina at Manakudy estuary. The physico-chemical characteristics of respective soil samples were analyzed and the results obtained are presented in Table 1. It showed that the highest volume of minerals like nitrogen (72.0%), iron (1.30 ppm) and copper (1.04 ppm) were recorded in RS3 soil than other collected samples and which was black in colour, granular with low lime status. The pH value of RS3 soil was 8.2. But the remaining macro and micro nutrients were relatively lower than other samples like RS1, RS4 and RS5. The highest pH (9.8) was recorded in RS2 soil; it was noticed as pale grey and sandy in nature. Among these five soil samples, RS3 represented maximum (1.9 × 103 CFU/g) mycelial growth of actinobacteria in Starch Casein agar medium, whereas the other soil samples such as RS1, RS2, RS4 and RS5 represented the mycelial growth of actinobacteria in the order of 1.1 × 102, 1.4 × 102, 1.6 × 102 and 1.2 × 102 CFU/g, respectively. In all these five soil samples, totally 14 different actinobacterial strains were isolated based on their morphological characteristics such as appearance, aerial mass colour, aerial mycelium, substrate mycelium and pigmentation. All these 14 strains were designated as MAB1 to MAB14. Appearance of all the strains represented powdery and only two strains (MAB4 and MAB13) represented leathery appearance. Aerial mass colour of majority of the strains represented white colour, followed by grey, purple, dark red, whitish grey, pink colonies etc. All the 14 strains have aerial mycelium and substrate mycelium. The pigmentation on reverse side of actinobacterial strains exhibited different colours with dark brown, brown, yellow, pale yellow, black, blue, pink etc. However, only few strains like MAB3, MAB6 and MAB8 represented brown, black and pink colours soluble pigments (Table 2).
Screening of Bioethanol Production
All the 14 strains were screened for bioethanol production. Among them only three isolates such as MAB3 (0.3449 OD), MAB7 (0.1672 OD) and MAB8 (0.098 OD) showed positive result on bioethanol production. Among these three isolates, only one isolate MAB3 showed highest production of bioethanol. Hence this particular strain was further subjected to fermentation process.
Taxonomical Identification of Selected Actinobacterial Strain
The selected potent actinobacterial strain (MAB3) (Fig. 1a) which showed maximum production of bioethanol during screening study was further identified by both cultural and physiological characteristics using standard procedure and the results are given below.
Cultural Characterization of Selected Actinobacterial Strain
The selected actinobacterial strain (MAB3) was identified by using various defined culture media ISP1 to ISP7 and the results on morphological appearance on the cultural media are given in Table 3. Of the various ISP media tested, strain MAB3 showed good growth on all ISP media, except ISP3 and ISP5 media and in these two media, it showed moderate growth. Reverse side pigmentation was observed in the strain grown in all the media. It was noted that the strain grown in ISP1, ISP4 and ISP6 media represented pale brown in colour, whereas the strain grown in ISP3, ISP5 and ISP7 media, represented yellow and brown colours. The appearance of this strain in different media showed powdery appearance. The aerial mass colour of this strain grown in different media represented different colours with whitish-grey, grey, white, pale-white, pale-grey, etc. The Micromorphology of this strain was examined through bright field microscope as well as scanning electron microscope (SEM) (Fig. 1b). Under this observation, both substrate and aerial mycelia were observed. The spore chain morphology was identified and it appeared as smooth and spiral shape.
Physiological Characteristics of Selected Actinobacterial Strain
The result on physiological characteristics of the actinobacterial strain (MAB3) is represented in Table 4. In carbon utilization study, the strain MAB3 showed good mycelial growth in all the tested sugars, except in mannitol and arabinose. In tolerance studies, ISP2 medium with different pH (6 to 11), temperature (20 to 40 °C) and NaCl concentrations (0 to 2%) showed good mycelial growth. Moderate growth of the strain MAB3 was observed at pH 12, temperature 50 °C and at the NaCl concentration 3%, in ISP2 medium. However, no growth of mycelia was noticed in ISP2 medium with pH 5, temperature 60 °C and NaCl concentration above 3%.
16S rRNA Sequencing
The active selected actinobacterial strain (MAB3) was identified by 16S rRNA sequencing and the result obtained was deposited in GenBank of NCBI data library under accession number KM435070. The phylogenetic tree was constructed by the neighbor-joining method (Fig. 2). Based on the studied phenotypic characteristics and 16 S rRNA sequencing, the potent actinobacterium MAB3 was identified as Streptomyces variabilis.
Determination of Total Sugar Content in the Collected Agro-Residues
The total sugar content in the collected agro-residues was analyzed through anthrone method and the result obtained is summarized in Table 5. It revealed that the maximum sugar content (60.22 mg/g) was found in sugarcane juice than other respective agro-residues such as sugarcane bagasse (51.00 mg/g), vegetable residue (38.16 mg/g), banana residue (42.0 mg/g) and mango peel (46.82 mg/g).
Pretreatment Process
The collected agro-residues were individually pretreated with xylanase enzyme at different concentrations (50, 100, 200, 300 and 400 IU). Then these pretreated residues were used as carbon source for saccharification and bioethanol production (Tables 6, 7). The results obtained are given below.
Xylanolytic Hydrolysis of Pretreated Agro-Residues
After incubation of the individual pretreated agro residues along with the candidate strain S. variabilis, the culture suspensions were individually centrifuged, followed by, the supernatants were collected and assayed. The degree of saccharification was calculated and it is recorded in Table 6. Among the tested samples, mango residue showed lowest saccharification yield (22.67 to 35.36%) at the respective enzyme concentrations (50 to 400.0 IU), whereas the degree of saccharification of sugarcane juice was comparatively high (30.29 to 59.49%), followed by sugarcane bagasse (26.07 to 49.83%). When compared to the different concentrations of enzyme treatment, maximum degree (35.36 to 59.49%) of saccharification was observed at 200.0 IU concentration in all the tested residues with maximum at sugarcane juice. The statistical two-way ANOVA result on saccharification revealed that the differences between substrates as well as differences between enzyme concentrations were highly significant (P < 0.001).
Bioethanol Production
Bioethanol production was performed by using various pretreated agro residues. After incubation, the samples were centrifuged and the clear supernatant solutions were treated with (0.1 M) potassium dichromate and allowed to stand for few minutes, followed by the results were observed (Table 7). Among the five pretreated agro residues, the production of bioethanol from sugarcane juice was higher (3.10 to 4.69 g/L) at the respective concentrations (50 to 400.0 IU) of enzyme treatment, than others, but mango residue showed least bioethanol production (1.36 to 1.63 g/L).
Estimation of Reducing Sugar Level in Different Untreated and Pretreated Agro-Residues
After bioconversion, all the tested agro-residues pretreated with 200 IU enzyme, exhibited maximum degree of saccharification (35.36 to 59.49%) as well as bioethanol production (1.63 to 4.69 g/L). Hence, this particular concentration of xylanase treated agro-residues were selected for estimation of reducing sugar respectively compared with untreated agro-residues and the results are given in Table 5. The formation of reducing sugar (glucose) level in both untreated (43.02 mg/g) and pretreated (50.19 mg/g) sugarcane juice was observed maximum, when compared to other agro-residues such as sugarcane bagasse (35.20 and 42.56 mg/g), banana residue (27.54 and 33.72 mg/g) and mango peel (30.72 and 39.20 mg/g). Meanwhile, the highest value of 19.09 and 16.15 mg/g of non-reducing sugar level was recorded in both untreated and pretreated vegetable residue respectively. But the remaining agro-residues with the non-reducing sugar level such as banana residue (untreated: 14.46 mg/g and pretreated: 8.28 mg/g), mango peel (untreated: 16.10 mg/g and pretreated: 7.62 mg/g), sugarcane juice (untreated: 17.20 mg/g and pretreated: 10.03 mg/g) and sugarcane bagasse (untreated: 15.80 mg/g and pretreated: 8.44 mg/g) were observed and recorded.
Optimization of Saccharification and Bioethanol Production
In the preliminary screening study, the degree of saccharification (35.36 to 59.49%) and bioethanol production (1.63 to 4.69 g/L) were obtained maximum at the basal medium substituted with pretreated sugarcane juice at 200.0 IU enzyme concentration. Therefore this pretreated sugarcane juice was selected for further optimization studies. The saccharification and bioethanol production were optimized by varying factors including different pH, temperature, carbon sources, nitrogen sources, incubation time and different innoculum size of the candidate organism S. variabilis (MAB3). Among the tested pH, the maximum degree of saccharification (43.50%) and bioethanol production (4.38 g/L) were recorded at pH 6. With a further increase in medium pH of 7 to 12, a gradual decrease of bioethanol from 3.41 to 1.75 g/L and decrease of saccharification from 38.5 to 21.54% respectively were observed and recorded. In temperature optimization study, the maximum degree of saccharification (40.17%) and bioethanol production (2.73 g/L) were recorded at 30 °C. Beyond 30 °C, the degree of saccharification (38.08 to 20.0%) and bioethanol production (2.67 to 1.60 g/L) were gradually decreased at 40–70 °C, respectively (Fig. 3).
Effect of different carbon (a), nitrogen (b) sources, pH (c), temperature (d), inoculum size (e) and incubation time (f) on saccharification (%) and bioethanol production (g/L) by S. variabilis (MAB3) in pretreated sugar cane juice substituted medium. Each value is the Mean ± SD of triplicate analysis, bars represented on saccharification yield with different superscript letters are statistically significant (One-way ANOVA test: P < 0.05; further post hoc multiple comparison with SNK test)
The study on effect of nutritional sources on saccharification and bioethanol production indicated that among the tested carbon sources, dextrose substituted medium displayed maximum degree of saccharification (45.72%) and bioethanol production (3.14 g/L), whereas fructose substituted medium displayed minimum degree (26.00%) of saccharification and 1.20 g/L of bioethanol production. Similarly, among the tested nitrogen sources, urea substituted medium showed maximum degree of saccharification (55.63%) and production of bioethanol (3.27 g/L), followed by tryptone (46.18% and 3.18 g/L), peptone (41.26% and 3.02 g/L), beef extract (37.21% and 2.76 g/L) etc., but the yeast extract substituted medium resulted in least degree of saccharification (35.00%) and also bioethanol production (2.56 g/L). In the case of inoculum size effect on saccharification and bioethanol production, it revealed that at the least level of inoculum size (0.5%), the saccharification and ethanol production were recorded as 22.03% and 1.22 g/L, respectively. However, when the size of inoculum increased, the saccharification yield and bioethanol production were also simultaneously increased and attained their maximum (50.82% saccharification and 2.83 g/L bioethanol production) at 2.5% of inoculum size. Further increase in inoculum size showed decrease in level of saccharification and ethanol production. The effect of incubation time on saccharification and bioethanol production displayed a maximum of 56.17% saccharification and 3.47 g/L ethanol production at 72 h of incubation, whereas least level of saccharification (37.39%) and ethanol production (2.17 g/L) were observed at 24 h of incubation (Fig. 3).
Determination of Bioethanol by HPLC Analysis
The bioethanol obtained (4.38 g/L) from the optimized pretreated medium during 72 h of incubation was centrifuged at 5000×g for 10 min. After centrifugation, the supernatant solution was distilled and tested for bioethanol concentration. The concentration of bioethanol was determined by HPLC analysis; here the bioethanol from the supernatant sample showed a steep peak with the retention time of 5.50 min, and percentage areas of 900,030 μV/sec. The concentration of bioethanol from the potent actinobacterium was determined as 82.26%.
Characterization of Bioethanol
Characterization by FT-IR Analysis
The produced bioethanol was characterized by FT-IR analysis and its spectrum is represented in Fig. 4. Totally five functional groups were found in between the wavenumber of 3272.22–1077.15 cm−1. The alcohol groups (O–H and C–O) were found in 3272.67 and 1077.15 cm−1 wavenumber respectively with strong intensity. The remaining functional groups of –C=C– (alkyne), C=O (carbonyl) and C=C (aromatic) were respectively denoted at 2109.67, 1687.40 and 1450.68 cm−1 wavenumber with variable and weak intensity.
Characterization by GC-MS Analysis
In GC-MS analysis, the chromatogram of bioethanol expressed 4 fuctional groups found in between the retention time of 2.60 to 24.38 min (Fig. 5). The ethanol or alcohol content was seen in the chromatogram with retention time of 2.60 min. Remaining respective peaks were denoted as acetic acid/benzoic acid (14.67 min), methyl-2-pentene (23.20 min) and propionic acid (24.38 min).
Kinetic Parameters
The kinetic parameters of the bioethanol production from S. variabilis (MAB3) were analyzed (Fig. 6). The µ max and K s values of the bioethanol production were recorded as 0.527 h−1 and 180.0 g/L respectively.
Discussion
Actinobacteria are indeed well adapted and are functional members of the aquatic, soil and marine sediment microbial communities [34]. Actinobacteria are constantly hold a special significance in the research area during the last five decades and used as a tool by fermentation biotechnologists for the production of value added products [35]. The present study was held for saccharification, followed by the production of value added product like bioethanol from xylanase pretreated agro-residues by using a potent actinobacterium Streptomyces variabilis (MAB3), isolated from rhizosphere sediment samples of Manakudy estuary, South west coast of India. Initially 14 actinobacterial strains were enumerated from the sediment samples, which were screened for saccharification and bioethanol production using pretreated various agro-residues as carbon sources. In an another study, totally, 238 morphologically different marine and terrestrial actinobacterial strains from 185 sediments/rhizosphere soil samples of different sites of Manakudy estuary, South-west coastal regions and Yercaud hills of Eastern Ghats of Tamilnadu, India, were isolated by Nithya et al. [36]. They also reported that 88 strains, out of 238 isolates showed cellulase as well as xylanase activity converting lignocelluloses into simple sugars. Similarly, Remya and Vijayakumar [37] enumerated 173 actinobacterial colonies from sediment samples collected near shore marine environment and mangrove ecosystem at eight different locations of Kerala, West coast of India for the production of value added products.
In an another study, Latif and Rajoka [38] examined the actinobacteria (62 isolates) from rhizosphere soil samples from Serdang and Bangi, which have the ability to degrade cellulose, mannan and xylan with the production of bioethanol. Likewise, in the present investigation all the 14 actinobacterial strains were screened for bioethanol production, simultaneously by saccharification process using various agro-residues such as vegetables, banana, mango, sugarcane bagasse and sugarcane juice pretreated with xylanase enzyme at different concentrations (50, 100, 200, 300 and 400 IU). Among the 14 strains, only three actinobacterial strains showed positive results on saccharification as well as bioethanol production. From these three positive strains, only one highly active strain (MAB3) was selected and further identified up to species level as Streptomyces variabilis (MAB3) by using International Streptomyces Project (ISP) medium with standard procedures as well as 16 S rRNA sequencing method. Many studies have been carried out, where the actinomycete isolates were identified as species of Streptomyces based on phenotypic and biochemical properties [39, 40]. The members of Streptomyces can be distinguished from other spore forming actinomycetes based on morphology and hence morphology plays an important role in the characterization of Streptomyces species [41].
Latif and Rajoka [38] also expressed the production of ethanol and xylitol from enzyme broth of Cheatomium thermophile using S. cerevisiae and C. tropicalis by simultaneous saccharification (50.0%) and fermentation (SSF) using pretreated dry corn cobs at 96 h of fermentation. Clarkin and Clesceri [42] evaluated the enzymatic (50 IU cellulase) pretreatment of commercial cellulose showed maximum level of reducing sugar (135.37 mg/day) with the saccharification yield of 88.19%. In the present investigation, the degree of saccharification and bioethanol production through fermentation from pretreated agro-residues using selected S. variabilis (MAB3) strain was noted. Among the tested pretreated residues, the sugarcane juice substituted medium pretreated at 200.0 IU of enzyme concentration, showed highest level of reducing sugar (50.19 mg/g), degree of saccharification (59.49%), followed by bioethanol production (4.69 g/L). After bioconversion, the release of non-reducing sugar level from the sugarcane juice substituted medium with 200.0 IU enzyme was observed as 10.03 mg/g. Similarly, Zhang et al. [43] observed the maximum reducing sugar level of 42.86 mg/g and saccharification yield of 37% (w/w) in pretreated bamboo residues by Coriolus versicolor. In an another study, Bhattacharya et al. [44] portrayed the enzymatic (cellulase and xylanase) hydrolysate of water hyacinth biomass (WHB) containing reducing sugars such as xylose was used for ethanol fermentation and the maximum ethanol yield was obtained as 2.217 g/L, when Pichia stipitis was used, followed by 2.163 and 1.927 g/L for Candida shehatae and S. cerevisae, respectively. Likewise, Zhu et al. [45] achieved 37% of reducing sugar from sulfite treated spruce chips and red pine processed with 8 to 10% bisulphate at 180 °C for 30 min.
Kshirsagar et al. [46] studied the maximum level of reducing sugar (390 mg/g), degree of saccharification (60.0%) and bioethanol production (17.0 g/L) from xylanase (110 IU) pretreated wheat straw using Amycolatopsis sp GDS under fermentation condition. Ali et al. [47] performed bioethanol production from agricultural cellulosic wastes such as groundnut hull and rice husk using two strains namely A. niger for saccharification and S. cerevisiae for ethanol production, by adopting stationary and shaking fermentation method. During ethanol production, the strain S. cerevisiae produced high yield of ethanol from groundnut hull (6.2 g/100 g) in stationary fermentation method. Wan and Li [48] examined the 35 days pretreated corn stover with a fungi Ceriporiopsis subvermispora for 18 days incubation at 28 °C with 75% moisture content. The overall glucose yield of 57.67% and the highest overall ethanol yield of 57.80% were obtained. Patel et al. [49] studied the pretreatment of wheat straw and rice straw with A. niger and A. awamori, followed by the bioethanol production using S. cereviseae (NCIM 3095) and the yielded amount of ethanol was observed as 2.5 and 2.2 g/L respectively. Ahmed et al. [26] performed saccharification process, followed by bioethanol production from enzymatic treated sugarcane bagasse using S. cerevisiae, which showed the maximum degree of saccharification (42.70%) in 24 h of incubation and the concentration of ethanol were observed as 1.15%.
Stalin et al. [50] estimated the biogas production from enzyme (cellulase and xylanase) pretreated rice straw and wheat straw wastes using marine actinobacterial strain Streptomyces caluvligerus at pH 8 and temperature 55 °C. Belkacemi and Hamoudi [51] reported the cellulolytic hydrolysis of corn stalk hemi-cellulose at pH 5 and temperature 30 °C, expressed with 90% yield of saccharification. In accordance with this, in the present study, the saccharification and bioethanol production were optimized for various factors and an overall maximum yield of saccharification and bioethanol production were obtained at a pH 6 (43.50% and 4.38 g/L), temperature 30 °C (40.17% and 2.73 g/L), inoculum size 2.5% (50.82% and 2.83 g/L), incubation time 72 h (56.17% and 3.47 g/L). 3.0% of dextrose (45.72% and 3.14 g/L) and 2% of urea (55.63% and 3.27 g/L) were determined as best carbon and nitrogen sources respectively. Likewise Ray [52] studied the maximum degree of saccharification of pretreated sugarcane bagasse with (180 IU) endo-xylanase from Streptomyces sp. OM09 at pH 6 (22.75%), temperature 60 °C (20.77%) and the incubation time of 30 min (26.02%). Huang et al. [53] reported the xylanolytic (150.0 IU/g) hydrolysis of bamboo residue yielded the maximum degree of saccharification (83.15%) at pH 6, temperature 40 °C and 72 h of incubation. In an another report, Saritha et al. [25] documented highest saccharification yield (97.8%) from accelerase pretreated paddy straw using S. griseorubens at pH 6, temperature 30 °C, inoculum size 1.5% and 48 h of incubation time. Ali et al. [47] studied the production of ethanol from Czapek-Dox (CD) medium containing dry corn cobs at pH 6, incubation temperature 40 °C and 48 h of fermentation process, that showed the highest bioethanol production of 22.48 g/L.
In an another study, Hossain et al. [30] performed bioethanol production using untreated, lime pretreated and diluted alkaline peroxide pretreated wheat straw by simultaneous saccharification and fermentation (SSF) process in a continuous stirred batch bioreactor (CSBR) using a fungi F. oxysporum. The optimum parameters used for the bioethanol fermentation were incubation time 48 h, pH 6, temperature 50 °C, stirring speed of 35 rpm and wheat straw 35.0 g/l. Here the maximum yeild of ethanol was found to be 0.756, 0.796 and 0.810 g/g under optimum conditions for untreated, lime pretreated and diluted alkaline peroxide pretreated wheat straw, respectively. Christakopoulos et al. [54] stated that the optimum condition for the direct conversion of cellulose to ethanol by F. oxysporum is at the temperature 34 °C, inoculum size of 3.5% and the pH of 5.5–6.0, respectively. In an another study, the maximum amount of ethanol (7.50%) was obtained by using S. cerevisiae through fermentation process with the fermentation medium containing 15% of sugar, initial pH of 4.5, temperature of 30 °C, inoculum size 3.5% under the incubation time of 120 h was stated by Mariam et al. [55].
Sindhu et al. [56] studied the ethanol production using pretreated rice straw as a carbon source by S. cerevisae, who also reported the amount of ethanol production (14%) was calculated through HPLC analysis with a peak at the time of 2.582 min and the percentage area of 229,635 μV/sec. In accordance with this, in the present study, after optimization, the concentration of bioethanol was estimated from the optimized sugarcane juice medium by HPLC analytical method. Here the bioethanol from the sample showed steep peak with the retention time of 5.50 min and the concentration was calculated as 82.26%. In an another study, 63% (v/w) ethanol yield from rice straw pretreated by a fugal strain Phanerochaete chrysoporium under submerged cultivation was achieved by Bak et al. [57]. Further in the present investigation, the ethanol was characterized using FT-IR analysis and the functional groups O–H and C–O stretch were found respectively at the wavenumber of 3272.22 and 1077.15 cm−1. In GC-MS analysis, the ethanol or alcoholic groups were found in the chromatogram with retention time of 2.60 min. Similarly, Bhalt et al. [58] studied the ethanol production from the saccharified sugars of agricultural waste pearl millet bran through solid state fermentation by A. flavus FPDN1 and the concentration of resulted ethanol was noted as 88.0% with 3.31 min retention time. Du et al. [59] portrayed the chromatogram of GC-MS (RT) analysis of ethanol from Tuber formosanum.The functional ethanol groups were noted at the retention time of 2.58 min with the peak area of 7.84%. The kinetic parameters of the study were analyzed and the µ max and K s values of the bioethanol production were recorded as 0.527 h−1 and 180.0 g/L respectively. Similarly, Ahmad et al. [60] reported the kinetic parameters (µ max and K s ) of the ethanol production from S. cerevisiae were exhibited as 0.084 h−1 and 213.6 g/L respectively. In an another study, Shafaghat et al. [61] studied the kinetic parameters such as µ max (2.0 h−1) and K s (30.5 g/L) of bioethanol production using sugarcane syrup as substrate. Imamoglu and Sukan [62] performed the kinetic modeling for bioethanol production using recombinant E. coli KO11 and the parameters were exhibited as µ max = 0.57 h−1 and K s = 13.60 g/L.
From this study, it could be concluded that the isolated actinobacterial strain S.variabilis (MAB3) from rhizosphere sediment soil samples of Manakudy estuary actively saccharified various pretreated agro-residues, followed by the production of bioethanol. The isolated potent S. variabilis (MAB3) exhibited maximum degree of saccharification (59.49%) and bioethanol production (4.69 g/L) by utilising pretreated sugarcane juice medium with 200 IU enzyme. Further they were optimized by various factors. The highest saccharification yield and bioethanol production were attained in 200 IU treated optimized sugarcane juice medium at pH 6 (43.50% and 4.38 g/L), temperature 30 °C (40.17% and 2.73 g/L), incubation time 72 h (56.17% and 3.47 g/L) and the medium substituted with 3% dextrose (45.72% and 3.14 g/L) and 2% urea (55.63% and 3.27 g/L) respectively as carbon and nitrogen sources with the innoculum concentration of 2.5% (50.82% and 2.83 g/L). After bioconversion, the maximum level of reducing sugar (50.19 mg/g) was observed in 200 IU xylanase pretreated sugarcane juice medium with the level of non-reducing sugar of 10.03 mg/g respectively compared with untreated medium (reducing sugar level: 43.02 mg/g and non-reducing sugar level: 17.20 mg/g). The concentration of produced bioethanol was estimated using HPLC analysis, predicted as 82.26% with the retention time of 5.50 min, and the percentage area of 900,030 μV/sec. Finally, the produced bioethanol was characterized by FT-IR and GC-MS analysis. It expressed the ethanol functional groups were found in the absorption range of 3272.22 cm−1 with the retention time of 2.60 min. Hence, the investigation of this research indicates that the experimental approach has not been declared so far for enhancing the actinobacterium Streptomyces variabilis (MAB3) for bioethanol production. These bioethanol study can be renewable and potentially displace hydrocarbon liquid fuels in future. Ultimately, this study was the first report for production and optimization of bioethanol from agro-residues using S. variabilis (MAB3).
References
Buaban, B., Inoue, H., Yano, S., Tanapongpipat, S., Rengpipat, S., Eurwilaichitr, L.: Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J. Biosci. Bioeng. 110, 18–25 (2010)
Girio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Lukasik, R.: Hemicelluloses for fuel ethanol: a review. Bioresour. Technol. 101, 4775–4800 (2010)
Chandrakant, P., Bisaria, V.S.: Simultaneous bioconversion of cellulose and hemicelluloses to ethanol. Critc. Rev. Biotechnol. 18, 295–331 (1998)
Lee, S.J., Kim, S.B., Kang, S.W., Han, S.O., Park, C., Kim, S.W.: Effect of crude glycerol-derived inhibitors on ethanol production by Enterobacter aerogenes. Bioprocess Biosys. Eng. 35, 85–92 (2010)
Howard, R.L., Abotsi, E., Jansen van Rensburg, E.L., Howard, S.: Cellulase production from fungal isolate Fusarium sp. Afr. J. Biotechnol. 2, 602–619 (2003)
Wyman, C.E., Dale, B.E., Elander, R.T., Holtzapple, M., Ladisch, M.R., Lee, Y.Y.: Coordinated development of leading biomass pretreatment technologies. Biotechnol. Prog. 96, 1959–1966 (2005)
Lynd, L.R., Weimer, P.J., Van Zyl, W.H., Pretorius, I.S.: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002)
Chang, V.C., Holzapple, M.T.: Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 5, 37–47 (2000)
Walia, B.S., Tan, L.U., Saddler, J.N.: Purification and characterization of an alkaline xylanase from Streptomyces viridosporus T7A. Microbiol. Rev. 52, 305–317 (2012)
Beg, Q.K., Bhushan, B., Kapoor, M., Moondal, G.S.: Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in bioleaching of eucalyptus kraft pulp. Enzy. Microbial. Technol. 27, 459–466 (2000)
Sriyapai, D.H., Alves, L., Ribeiro, S., Amaral-Collac, O.M.T: Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie Van Leeuwenhoek. 102, 719–734 (2011)
Ragauskas, L.R., Gomes, R.C., Manfio, G.P., Alviano, C.S., Linhares, L.F.: Production and partial characterization of xylanase from Streptomyces sp. strain AMT-3 isolated from Brazilian cerrado soil. Enzy. Microbial Technol. 31, 549–555 (1994)
Zhou, S., Abotsi, E., Ingram, L.O.: Synergistic hydrolysis of carboxymethyl cellulose and acid swollen cellulose by two endoglucanase (Cel Z and Cel Y) from Erwinia chrysantheme. J. Bacteriol. 182, 5676–5682 (2010)
Ninawe, S., Kuhad, R.C.: Use of xylan-rich cost effective agro-residues in the production of xylanase by Streptomyces cyaneus SN32. J. Appl. Microbiol. 99, 1141–1148 (2006)
Bajaj, B.K., Singh, S.M.: Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28. Biotechnology 1, 161–171 (2010)
APHA: Standard Methods for the Examination of Water/Waste Water. APHAAWWA-WPCF, Washington, DC (1995)
ASTM: Annual Book of American Society for Testing and Materials Standard, vol. 1527. ASTM, Philadelphia (1979)
RadhaKrishnan, M., Balaji, S., Balagurunadhan, R.: Thermotolerant actinomycetes from the Himalayan Mountain antagonistic potential characterization and identification of selected strains. Malay. J. Appl. Biol. 36, 59–65 (2007)
Thenmozhi, R., Victoria, J.: Optimization and improvement of ethanol production by the microporation of organic wastes. Pelagia research library. Adv. Appl. Sci. Res. 4, 119–123 (2013)
Shiling, E.B., Gottlieb, D.: Methods for characterization of Streptomyces species. Int. J. Sys. Bacteriol. 16, 312–340 (1966)
Nathan, A., Jessica, M., Bernan, V., Dworkin, M., Sherman, D.H.: Isolation and characterization of novel marine derived actinomycete taxa rich in bioactive metabolites. Appl. Environ. Microbiol. 70, 7520–7529 (2004)
Thompson, J.D., Gibson, J.J., Plewniak Jeanmougin, F., Higgins, D.G.: The clustal windows interface: flexible strategies for multiple sequence alignment aided by quality analysis stools. Nucleic Acid Res. 24, 4876–4882 (1997)
Sanjivkumar, M., Silambarasan, T., Palavesam, A., Immanuel, G.: Biosynthesis, purification and characterization of β-1,4-xylanase from a novel mangrove associated actinobacterium Streptomyces olivaceus (MSU3) and its applications. Protein Expr. Purif. 130, 1–12 (2017)
Detroy, R.W., Cunningham, R.L., Bothast, R.J., Bagby, M.O.: Bioconversion of wheat straw cellulose/hemicelluloses to ethanol by Saccharomyces uvarum and Pachysolen tannophilus. Biotechnol. Bioeng. 24, 1105–1113 (1982)
Saritha, M., Arora, A., Singh, S., Nain, L.: Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour. Technol. 135, 12–17 (2013)
Ahmed, F., Rahmam, S.R., Gomes, D.J.: Saccharification of sugarcane bagasse by enzymatic treatment for bioethanol production. Malay. J. Microbiol. 8, 97–103 (2012)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Rao, R.S., Bhadra, B., Shivaji, S.: Isolation and characterization of ethanol producing yeasts from characterization of ethanol producing yeasts from fruits and tree barks. Lett. Appl. Microbiol. 47, 19–24 (2008)
Caputi, A., Ueda, M., Brown, T.: Spectrophotometric determination of ethanol in wine. Am. J. Environ. Vitic. 19, 160–165 (1968)
Hossain, M., Anantharaman, N., Das, M.: Bioethanol fermentation from untreated and pretreated lignocellulosic wheat straw using fungi Fusarium oxysporum. Ind. J. Chem. Technol. 19, 63–70 (2012)
Elumalai, S., Sakthivel, R.: GC-MS and FT-IR spectroscopic determination of fatty acid methyl ester of 16 fresh water microalgae, isolated from cement industries of TamilNadu, India. J. Algal Biomass Utn. 4, 50–69 (2013)
Dizhbite, T., Telysheva, G., Dobele, G., Arshanitsa, A., Kampars, V.: Py-GC/MS for characterization of non-hydrolyzed residues from bioethanol production from softwood. J. Analy. Appl. Pyrol. 90, 126–132 (2011)
Lineweaver, H., Burk, D.: The determination of enzyme dissociation constants. J. Am. Chem. Soc. 57, 685 (1934)
Jensen, P., Dwight, R., Fenical, A.: The distribution of actinomycetes in near-shore tropical marine sediments. Appl. Environ. Microbiol. 57, 1102–1108 (1991)
Williams, P.G.: Planning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol. 27, 45–52 (2009)
Nithya, B., Ponmurugan, P., Fredimoses, M.: 16 S rRNA phylogenetic analysis of actinomycetes isolated from Eastern Ghats and marine mangrove associated with antibacterial and anticancerous activities. Afr. J. Biotechnol. 11, 12379–12388 (2012)
Remya, M., Vijayakumar, R.: Isolation and characterization of marine antagonistic actinomycetes from west coast of india. Medicine Biol. 15, 13–19 (2008)
Latif, F., Rajoka, M.: Production of ethanol and xylitol from corn cobs by yeasts. Bioresour. Technol. 77, 57–63 (2001)
Goodfellow, M., Willam, S.T., Mordarski, M.: Actinomycetes in biotechnology, Academic press, London (1988)
Rifaat, H.M., Awad, A.H., Gebreel, H.M.: Production of xylanase by Streptomyces sp. and their bleaching effect on rice straw pulp. Appl. Ecol. Environ. Res. 2, 45–51 (2004)
Anderson, A.S., Wellington, E.M.H: The taxonomy of Streptomyces and related genera. Int. J. Sys. Evol. Microbiol. 51, 797–814 (2001)
Clarkin, S.D., Clesceri, L.S.: Enzymatic hydrolysis and cellulose based ion-exchange powdered mixed resins. Appl. Microbiol. Biotechnol. 60, 485–488 (2002)
Zhang, X., Xu, C., Wang, H.: Pretreatment of bamboo residues with Coriolus versicolor for enzymatic hydrolysis. J. Biosci. Bioeng. 104, 149–151 (2007)
Bhattacharya, A., Ganguly, A., Subhabrata, D., Chatterjee, P., Apurba, D.: Fungal isolates from local environment: isolation, screening and application for the production of ethanol from water hyacinth. Int. J. Emer. Technol. Adv. Eng. 3, 58–65 (2013)
Zhu, J.Y., Pan, X.J., Wang, G.S., Gleisner, R.: Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 100, 2411–2417 (2009)
Kshirsagar, S.D., Saratale, G.D., Saratale, R.G., Oh, M.K.: An isolated Amycolatopsis sp. GDS for cellulase and xylanase production using agricultural waste biomass. J. Appl. Miocrobiol. 11, 136–141 (2016)
Ali, U.F., Ibrahim, Z.M., Isaac, G.S.: Ethanol and xylitol production from xylanase broth of Thermomyces lanuginosus grown on some lignocellulosic wastes using Candida tropicalis EMCC2. Life Sci. J. 10, 968–978 (2013)
Wan, C., Li, Y: Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 101, 6398–6403 (2010)
Patel, J., Onkarappa, R., Shobha, K.S.J: Comparative study of ethanol production from microbial pretreated agricultural residues. Appl. Sci. Environ. Manag. 11, 137–141 (2007)
Stalin, T., Sathya priya, B., Selvam, K.: Ecofriendly application of cellulase and xylanase producing marine Streptomyces clavuligerus as enhancer in biogas production from waste. Afr. J. Environ. Sci. Technol. 6, 258–262 (2012)
Belkacemi, K., Hamoudi, S.: Enzymatic hydrolysis of dissolved corn stalk hemicelluloses: reaction kinetics and modeling. J. Chem. Technol. Biotechnol. 78, 802–808 (2003)
Ray, R.R.: Saccharification of agro-wastes by endo-xylanase from Streptomyces sp OM09. Int. J. Life Sci. Biotechnol. Pharm. Res. 2, 165–170 (2013)
Huang, C., He, J., Li, X., Min, D., Yong, Q.: Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin carbohydrate complexes. Bioresour. Technol. 192, 471–477 (2015)
Christakopoulos, P.F., Koullas, D.P., Kekos, D., Koukios, E.G., Macris, B.J.: Direct ethanol conversion of pretreated straw by Fusarium oxysporum. Bioresour. Technol. 35, 297–300 (1989)
Mariam, I., Manzoor, K., Ali, S., Ikram-UI-Haq H.: Enhanced production of ethanol from free and immobilized Saccharomyces cerevisiae under stationary culture. Pak. J. Biotechnol. 41, 821–833 (2009)
Sindhu, V., Kanchana, C.N., Vasanthi, N.S., Ravikumar, R.: Design and development of novel bioreactor for the production of ethanol from low cost pretreated rice straw. IOSR J. Eng. 2, 1424–1428 (2012)
Bak, J.S., Ko, J.K., Choi, I.G., Park, Y.C.: Fungal pretreatment of lignocelluloses by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnol. Bioeng. 104, 471–482 (2009)
Bhalt, N., Dharmesh, A., Thakor, P.: Production of xylanase by Aspergillus flavus (FPDN1) on pearl millet bran: optimization of culture conditions and application in bioethanol production. Int. J. Res. Chem. Environ. 2, 204–210 (2012)
Du, M., Huang, S., Wang, J.: The volatiles from fermentation product of Tuber formosanum. J. Forest. 4, 426–429 (2014)
Ahmad, F., Jameel, A.T., Kamarudin, M.H., Mel, M.: Study of growth kinetic and modeling of ethanol production by Saccharomyces cerevisiae. Afr. J. Biotechnol. 16, 18842–18846 (2011)
Shafaghat, H., Najafpour, G.D., Rezaei, P.S., Baei, M.S.: Ethanol production with natural carbon sources in batch and continuous fermentation using free and immobilized Saccharomyces cerevisiae. J. Sci. Ind. Res. 70, 162–164 (2011)
Imamoglu, E., Sukan, F.V.: Scale-up and kinetic modeling for bioethanol production. Bioresour. Technol. 144, 311–320 (2013)
Acknowledgements
The authors gratefully acknowledge the University Grants Commission (UGC), New Delhi, Govt. of India, for its financial support in the form of Special Assistance Programme (SAP) [UGC No. F.3–24/2012(SAPII) dtd October 2012].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanjivkumar, M., Brindhashini, A., Deivakumari, M. et al. Investigation on Saccharification and Bioethanol Production from Pretreated Agro-Residues Using a Mangrove Associated Actinobacterium Streptomyces variabilis (MAB3). Waste Biomass Valor 9, 969–984 (2018). https://doi.org/10.1007/s12649-017-9886-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-9886-0