Abstract
This paper examines the main results of an accelerated carbonation treatment applied to different types and size fractions of stainless steel slag. The objectives of this work were essentially to assess the CO2 uptake achievable by each type of slag under mild operating conditions and to investigate the effects of carbonation on the mineralogy and leaching behaviour of the residues. The following types of materials were tested: different size fractions of commingled slag, milled electric arc furnace (EAF) slag and argon oxygen decarburization (AOD) slag. Each material was thoroughly characterized in terms of elemental composition, mineralogy and leaching behaviour. Accelerated carbonation batch experiments were performed exposing humidified (with liquid to solid ratios <0.6 l/kg) slag to 100% CO2 for operating times between 0.5 and 24 h, at controlled temperature and pressure. Maximum CO2 uptakes of 130, 180 and 300 g CO2/kg slag were achieved (at 50°C and 3 bar) for the finest fraction of the mixture, the milled EAF slag and the AOD slag, respectively. The mineralogy of each type of residue showed to be affected by the treatment, exhibiting an increase in calcite concentration and a decrease in the content of specific silicate and oxide phases. The leaching behaviour of all types of carbonated slag was also modified, exhibiting a reduction by 1–2 units of the natural pH of the materials, accompanied by a decrease of Ca release and an increase of Si leaching, as a result of modified leaching-controlling phases. In conclusion, at the tested operating conditions, AOD slag was the most reactive material with CO2. Milling, however, proved effective in increasing the carbonation yield of the EAF slag compared to that measured for the different size fractions of the commingled slag mixture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The energy sector and specific industrial process activities, such as cement, iron and steel manufacturing, account for most of the anthropogenic emissions of CO2 from stationary sources. In particular, the steel industry is globally the largest energy consuming manufacturing sector, and accounts for 7–12% of anthropogenic greenhouse gas (GHG) emissions [1]. Several measures, such as scrap steel recycling, are hence being developed to improve energy efficiency and cut CO2 emissions [2], but more drastic measures are needed if the demanding reduction targets established by many countries, such as e.g. the European Union’s 20–20–20 climate and energy targets, are to be attained.
Besides GHGs emissions, the above mentioned industrial processes also generate considerable amounts of solid residues, which are either reused in different applications or finally landfilled; in both cases, the predominantly inorganic constituents (heavy metals, metalloids, alkalies and anions) of these residues may be released into the environment, with potential adverse environmental impacts on terrestrial ecosystems and human health. Within this framework, hence, a combined approach aimed at reducing CO2 emissions, at the same time improving the environmental behaviour of process by-products, appears to be highly desirable in view of increasing the environmental sustainability of a given industrial process.
During steel manufacturing, a significant amount (10–15% by weight of the produced steel [3]) of various types of slag is generated. Based on current steel production data [4], it can be estimated that roughly 60 Mt of commingled slag was generated globally in 2009 (2 Mt of which in Italy) by steel manufacturing plants employing the electric arc furnace (EAF) technology. The main type of slag produced by these plants is that resulting from the EAF furnace (EAF slag) which makes up about 60% of the solid residues; the remaining slag is generated during steel refining processes, such as e.g. argon oxygen decarburization and desulphurization, in percentages that vary depending on processing conditions.
Steelmaking slags in general are alkaline materials consisting principally of Ca, Mg and Al silicates, which may contain also significant amounts of Fe, Mn and other elements such as Cr, Ni or Ti [5]. The percentage of each constituent varies depending on the feedstock and processing conditions; alloy steel slags, such as stainless steel slag, generally display a lower Fe and a higher Si content, high Cr concentrations (2–5%) as well as the presence of some Ni (0.02–0.55%). Steel slag is usually regarded as a non-hazardous waste owing to its limited release of trace contaminants, as mirrored by compliance with standard leaching criteria [3]; however, some types of stainless steel slag, such as that produced during steel refining, may release non negligible amounts of Cr [6]. Depending on its composition and particle size, steel slag can be employed for various uses, e.g. as an aggregate or as a filler in cement production [5]. One of the most important factors for the utilization of steel slag in engineering applications is its content of free CaO and MgO, which must fall below a certain threshold to limit hydration and natural carbonation phenomena resulting in increases in slag volume and hence strength losses of the final construction material [5].
Accelerated carbonation has been shown to improve the chemical stability and leaching behaviour of several types of industrial residues, and to enhance the mechanical properties of materials (including steel slag) for reuse as aggregates in civil works [7–10]. Moreover, accelerated carbonation of industrial alkaline residues is also being currently investigated as a process for storing point-source emissions of CO2 in a solid and thermodynamically stable form. This process, commonly referred to as mineral carbonation, involves the neutralization of an acid (CO2(g) or H2CO3/H2O) with a solid base (Ca and Mg-containing oxides and/or silicates) leading to the formation of a carbonate phase (see e.g. [11–13]).
Specifically, accelerated carbonation has been applied to the following solid waste streams: residues from coal-fired power stations [14–19], oil shale combustion by-products [14, 20, 21], as well as lignite and wood combustion fly ash [19, 22]; other types of combustion residues including deinking ash, paper mill ash [17, 19, 23], cement kiln/bypass dust [19, 24] and ashes from incineration of municipal solid waste, special waste and biomass [19, 25–28]; cement-based materials [29, 30] and different types of steel slag [17, 31–36]; other industrial residues including by-products from bauxite processing [19].
The above mentioned residues have been typically subjected to direct aqueous carbonation (see e.g. [14, 17, 22–25]), which involves the dissolution of the reacting phases and the reaction of dissolved Ca or Mg ions with CO2 in a single stage. To a lower extent, for materials characterized by a high lime and portlandite content, experiments have also been conducted using the direct gas–solid reaction, in which carbonation occurs directly at the gas–solid interface for temperatures above 400°C [16, 26]. For waste residues characterized by high contents of silicate phases, such as steel slag and cement waste, the indirect aqueous route, in which the dissolution and precipitation processes are carried out in separated steps, has also been tested to enhance Ca dissolution from the solid matrix and to produce a pure calcium carbonate reaction product with specific properties [32–34]. Direct aqueous carbonation studies have been performed in two different modes, either in slurry phase at Liquid to Solid (L/S) ratios between 5 and 50 w/w [18, 22, 23, 35, 36] (in particular for waste materials with high silicate contents), or at lower water contents (L/S ratios < 1.5 w/w) [14, 15, 17, 19–21, 24, 25, 27, 28, 30]. This latter type of treatment, indicated also as wet route process, in which CO2, Ca and Mg ions dissolution, as well as carbonation reactions, occur in the thin liquid film in direct contact with the solid residues, was originally applied for cement curing processes (see e.g. [37, 38]). It has hence been applied as a carbonation route also for industrial residues with high contents of soluble elements to avoid the treatment and disposal of the processing liquid, as well as to favour dissolution kinetics at moderate operating conditions.
Regarding steel slag carbonation, the highest CO2 uptakes obtained applying the direct slurry-phase route were achieved in 30 min at 19 bar CO2 and 100°C for finely milled (particle size < 38 μm) slag [31]; at these operating conditions a CO2 uptake of 180 g CO2/kg, corresponding to 74% conversion of the Ca content of the slag, was reported. The carbonation of steelmaking slag in slurry phase at milder operating conditions was found to be slow and relevant CO2 uptakes were measured only for ladle slag, due to its higher content of reactive phases, such as portlandite [36]; at ambient temperature, atmospheric pressure and 100% CO2, an uptake of 120 g CO2/kg was achieved in about 22 h for the 150–250 μm particle size fraction of ladle slag and a L/S ratio of 10 l/kg [36]. On the other hand, experiments carried out on partially humidified steelmaking slag indicated a faster carbonation reaction for milled (<125 μm) stainless steel slag, with a maximum CO2 uptake of 180 g/kg in 1 h using 100% CO2 at 3 bar, ambient temperature and a L/S ratio of 0.125 l/kg [9, 17]. The above discussed data thus indicate that the influence of process parameters on carbonation kinetics may differ depending on the type of carbonation route (slurry or wet) adopted. For the wet route, the lack of a systematic study on the effect of the operating conditions on reaction kinetics and on the environmental behaviour of the carbonated material, as the one developed for the slurry-phase process [31, 39], can be noted.
In a recent work, we investigated the effects of accelerated carbonation applied via the wet route under mild operating conditions on different particle size fractions of a mixture of stainless steel slag [40]. The aims were to assess the CO2 uptake achievable by each size fraction, to identify the reacting species and the influence of operating parameters, and to analyze the effects of the treatment on the mineralogy and leaching behaviour of the slag.
In the present work, accelerated carbonation tests were carried out separately on EAF and AOD slags in order to maximize the CO2 uptake achievable under mild operating conditions, which were selected on the basis of the main findings of the previous study. These new results are here presented and discussed in comparison to the main findings reported in the previous paper [40].
Materials and Methods
The three types of analysed residues were sampled from an Italian stainless steel manufacturing plant and specifically from: the slag disposal site (mixture), just downstream from the electric arc furnace after metals removal (EAF slag) and after steel refinement by argon oxygen decarburization at the outlet of the desulfurization unit (AOD slag).
The first type of material (mixture) was relatively heterogeneous in its grain size distribution (d10 = 0.082 mm, d50 = 0.425 mm and d90 = 1.68 mm) and was hence subdivided by sieving, discarding the 2 mm oversize fraction, into the following particle size fractions: class A 2–0.425 mm (45.9% wt), class B 0.425–0.177 mm (25.9% wt), class C 0.177–0.105 mm (12.8% wt), class D <0.105 mm (16.4% wt). Only with the aim of testing the effect of grain size on the results of the accelerated carbonation tests, a part of class A was milled in a ball mill equipped with corundum jars and milling spheres to a particle size below 0.425 mm. The EAF slag, which presented a grain size distribution similar to that of the mixture, was milled, using the equipment described above, to achieve a particle size below 0.150 mm. The AOD slag instead, was considerably more homogeneous and displayed a fine grain size (90% of the particles <0.150 mm); hence the only type of pre-treatment applied to this material consisted in the removal by sieving of the fraction above 0.150 mm. The pre-treatment strategy for the EAF slag was selected on the basis of the results obtained for the mixture [40], which indicated particle size as the crucial parameter influencing CO2 uptake, and also with the purpose of comparing the maximum sequestration capacity of this type of residue with that obtained for the AOD slag under the same operating conditions.
Characterization of each type of slag included the determination of its elemental composition, calcite content, mineralogy and leaching behaviour. The elemental composition was determined by alkaline digestion with Li2B4O7 in platinum melting pots at 1,050°C, followed by dissolution of the molten material in a 10% HNO3 solution and measurement of element concentrations using an atomic absorption spectrometer (AAS) equipped with an air-acetylene flame, a graphite furnace and a hydride generation system. The carbonate content was evaluated by calcimetry analysis of HCl-digested slag using a Dietrich-Frühling calcimeter. The mineralogical composition was evaluated by powder X-ray diffraction (XRD) analysis with Cu Kα radiation using a Philips Expert Pro diffractometer (equipped with a copper tube operated at 40 kV and 40 mA) with an angular step of 0.02° held for 2 s with 2ϑ spanning from 5 to 85°. The leaching behaviour of each type of slag was investigated applying the standard EN 12457-2 batch compliance test. Eluate concentrations of major and trace elements were determined by AAS analysis, whereas eluate anion concentrations were measured by ion chromatography testing. All chemical analyses were performed in triplicate except for the leaching experiments, which were run in duplicate.
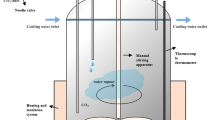
Batch accelerated carbonation tests were performed in a 150 ml pressurized stainless steel reactor placed in a thermostatic bath for temperature control up to 70°C. Temperature was monitored with a thermocouple, while gas humidity was maintained at 75% using a saturated NaCl solution in the reactor. In each run, three 1 g samples of dry slag were mixed with distilled water at specific L/S ratios, placed in tin foil containers and exposed to 100% CO2 for set times, ranging from 0.5 to 24 h. Initial wet carbonation tests were carried out on class D of the mixture to evaluate the influence of temperature (varied from 30 to 50°C), pressure (from 1 to 10 bar) and the L/S ratio (from 0 to 0.6 l/kg) on the CO2 uptake kinetics of the slag. Accelerated carbonation tests were then performed, under the optimal conditions determined for class D (50°C and L/S ratio of 0.4 l/kg), on the other fractions of the mixture, including milled (<0.425 mm) class A to evaluate the influence of composition and specific surface on the carbonation yield, as well as on the milled EAF and on the AOD slag. For the latter two types of residues, carbonation experiments at different CO2 pressures (1, 3 and 10 bar) were also carried out to assess and compare the effect of this parameter on carbonation kinetics, which had not been analyzed in detail for the slag mixture at 50°C. After treatment with CO2, the samples were oven-dried at 50°C and analyzed by calcimetry testing to measure the CO2 uptake obtained by the slag upon carbonation. The mineralogy and leaching behaviour of the treated samples were also investigated applying the same methods as those employed for the untreated slag.
Results and Discussion
Stainless Steel Slag Characterization
The elemental composition of each type of analyzed slag is reported in Table 1. Results proved to be in good agreement with data reported for these specific types of residues in prior studies [6, 9, 41, 42]. The elemental content of the EAF slag was overall similar to the average composition of the mixture for most elements (i.e.: Al, Cr, Cu, Fe, Mg, Mo and V), in accordance to the fact that EAF slag is the main type of slag generated during stainless steel manufacturing (~90 kg out of a total of ~150 kg residues per ton of produced steel). The AOD slag presented a considerably lower content of Al, Cr, Fe, Mo, Ni and V compared to the EAF slag, as indicated also by previous studies [41, 42]. The Ca content of all types of slag was very significant (>35% dry wt), particularly in the finest size fractions of the mixture and in the AOD slag. Also Mg concentrations resulted higher in the finest grain size classes of the mixture. All types of the tested slag however, presented a lower Mg content than values generally reported in the literature for stainless steel slag (50–100 g/kg wt) [6, 9, 41, 42]. Significant concentrations of Fe, Cr, Al, and V were measured in the coarser particle size fractions of the mixture and in the EAF slag. Ni concentrations were lower than those reported in other studies (2–4.5 g/kg) [6], probably due to differences in stainless steel manufacturing. The initial calcite contents of the different types of slag, as shown in Table 1, ranged from 2 to 3% wt for the coarser fractions of the mixture to 4–5% wt for the AOD slag and the finest particle size classes of the mixture.
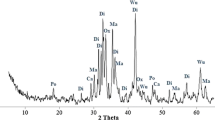
Several crystalline oxide and silicate phases were identified by XRD analysis in each type of slag, as shown in Fig. 1. In all the analyzed samples of untreated stainless steel slag a predominance of silicate phases was retrieved, in agreement with the results of previous studies on these types of residues [6, 9]. For the mixture and EAF slag these phases included dicalcium silicate, merwinite, akermanite, gehlenite, forsterite and also cuspidine, a typical species formed during slag hydration after fluorine addition, whereas the only silicate phase found in the AOD slag was dicalcium silicate. By comparison with XRD patterns reported in the literature for the different polymorphic phases of dicalcium silicate (e.g. [43]), the XRD spectra of Ca2SiO4 found in all of the analyzed samples (AOD slag in particular) was associated to the γ-phase. It is well known in fact, that during the cooling of steel manufacturing slag, and especially of the slag generated during steel refining, the polymorphic transformation of β-Ca2SiO4 to γ-Ca2SiO4 occurs and causes an increase in the phase volume, resulting in the disintegration of the slag into a fine powder (e.g. [44]). The type of Ca2SiO4 polymorph formed in the steel slag is an important issue to assess the reuse potential of the material. Slag with a high content of the γ-phase has in fact shown considerably poorer cementing properties than steel slag with a predominance of other polymorphs [45]. Different types of oxide phases were also detected in each type of slag, as shown in Fig. 1. Chromium oxide and magnetite showed similar peaks in all samples, while the mixture and the EAF slag showed varying contents of periclase, Cr–Mg oxide, Ca–Al–Fe oxide and crystalline silica phases (quartz and cristobalite). Calcite peaks were detected in untreated samples of the EAF slag and more appreciably in class D of the mixture, whereas calcium fluoride, one of the additives used in steel manufacturing and refining, was found in all samples and especially in the AOD slag.
The results of the EN 12457-2 compliance test on the different types of untreated slag, reported in Figs. 2 and 3, indicated that the pH of the eluates was rather similar in all samples, displaying highly alkaline values (>12; see Fig. 2a). The leaching of major and trace elements though, differed substantially depending on the type of examined slag and its particle size. The mixture exhibited a noticeable decrease in Ca, Cr and Mg leaching with particle size (see Fig. 2b–e), whereas Si leaching was negligible from all classes (see Fig. 3f). Ca and Mg leaching from milled EAF and AOD slag were comparable (see Fig. 2b, e), in accordance to the similar total contents of these elements and particle size distributions of the tested samples, while Cr and Fe leaching from milled EAF slag (see Fig. 2c, d) were noticeably higher. The only other species apart from Cr that showed relevant eluate concentrations as compared to compliance values, such as those set for landfill disposal, were chloride and fluoride (see Fig. 3). The leaching of both anions was considerably higher from the AOD slag compared to the milled EAF slag. Fluoride leaching, in particular, resulted above the acceptance criteria for non-hazardous waste landfills (15 mg/l).
CO2 Uptake Kinetics and Conversion Yield
The main results of the accelerated carbonation tests are summarized in Figs. 4 and 5. The CO2 uptake obtained by each tested sample upon carbonation was calculated applying Eq. 1, based on the results of calcimetry analysis on carbonated (CO2final) and untreated samples (CO2initial) expressed as CO2 weight percent contents.
In Fig. 4, the results of the carbonation experiments carried out on the stainless steel slag mixture are shown. In particular, in Fig. 4a, b, the effects of varying processing conditions, namely the operating temperature and the L/S ratio, on the CO2 uptake of the finest particle size class of the mixture are reported. Temperature, see Fig. 4a, was one of the parameters together with grain size, that seemed to mostly affect slag reactivity towards CO2, leading to CO2 uptakes just below 13% wt in 2 h. This effect was ascribed to the enhancement of silicate dissolution, indicated as an important factor in slurry-phase steel slag carbonation, for which an optimal temperature value of 150°C was reported [31]. In this study, the highest CO2 uptakes were achieved with a L/S ratio of 0.4 l/kg, as shown in Fig. 4b. This higher value compared to that indicated in prior studies (0.125 l/kg) [9], could be due to differences in slag composition, since in Johnson’s study portlandite was identified in the slag, whereas in this study no hydrated compounds were detected, indicating that part of the water added to the slag may have served for hydration of the oxide and silicate phases, other than for solvation of CO2 and Ca2+ ions. Variations in CO2 pressure at an operating temperature of 30°C did not appear to significantly affect the CO2 uptake or reaction kinetics of class D of the mixture [40], in agreement with the results of carbonation tests carried out under similar operating conditions on fly ash from waste incineration [27]. For a reaction time of 2 h, under the most favourable operating conditions tested (T = 50°C, P = 3 bar and L/S ratio = 0.4 l/kg), the CO2 uptake capacity of the slag mixture decreased considerably with increasing particle size, as shown in Fig. 4c. The reduction of the specific surface of the slag with increasing particle size was assumed as the main mechanism behind the decrease in slag reactivity towards CO2. This hypothesis was confirmed by the results of accelerated carbonation tests on milled (d < 0.425 mm) class A slag (see Fig. 5c), which presented CO2 uptakes similar to class C (0.105 < d < 0.177 mm), indicating that, also under the operating conditions investigated in this study, as reported by Huijgen et al. [31], intensive milling could contribute in enhancing the carbonation yield.
In Fig. 5a and b, the CO2 uptake kinetics at different operating pressure values for, respectively, milled EAF and AOD slag are shown. For the EAF slag (see Fig. 5a), milling (<0.150 mm) proved indeed very effective in increasing the CO2 uptake of the slag compared to the results obtained for the mixture, which, as previously discussed, presented a similar composition and mineralogy to the EAF slag. Reaction kinetics proved relatively fast, achieving similar CO2 uptake values for treatment times longer than 2 h, which proved in good agreement with values reported by previous wet carbonation studies on milled stainless steel slag [9, 17]. Similarly to what found for class D of the mixture at 30°C [40], pressure did not appear to appreciably affect reaction kinetics of the milled EAF slag, apart from at short processing times (<1 h) for which 1 bar CO2 pressure resulted in a ~30% reduction in CO2 uptake compared to higher pressures (see Fig. 5a). The AOD slag (see Fig. 5b) was the type of tested residue that exhibited by far the highest reactivity with CO2, with uptakes above 30% wt for an operating pressure of 10 bar and a processing time of 8 h. For this type of slag, reaction kinetics appeared to be influenced by CO2 pressure, in particular at intermediate operating times (4–24 h), with a 10 bar CO2 pressure resulting in the highest CO2 uptakes, probably due to the enhancement of dicalcium silicate dissolution. These values can be compared with the result (48% wt final CO2 uptake) of previous wet carbonation experiments applied in five cycles at ambient temperature and 2 bar CO2 pressure to β-Ca2SiO4, re-milled after each carbonation cycle to remove the product coating acting as a diffusion barrier against the advancement of the carbonation front [46]. The uptake achieved in the present study for the AOD slag, considering a Ca2SiO4 content of ~80% wt, appears hence relevant, taking into account that in this case no mechanical pre-treatment was applied.
The Ca conversion yields (η) resulting from the carbonation treatment, calculated as the ratio between the measured CO2 uptake and the amount of potentially reactive Ca phases (both expressed in terms of equivalent Ca content; see Eq. 2), varied greatly depending on the type of slag. Only Ca phases were considered in the calculation of the conversion yields, since the Mg content of the slag was an order of magnitude lower than the Ca content, and secondly because relevant CO2 uptakes by Mg oxide or silicate phases has not been reported in prior carbonation studies on steel slag, neither under mild nor under enhanced operating conditions [9, 10, 31].
The following Ca to carbonate maximum conversion yields were estimated for the different particle size fractions of the mixture: ~25% for class D, ~15% for class C, ~8% for class B, ~3 or 13% for un-milled or milled class A, respectively. For the milled EAF and the AOD slag, conversion yields are reported in Fig. 5a, b respectively, on the right hand side y-axis. Maximum conversion yields of 50% were achieved for the milled EAF slag, whereas for the AOD slag maximum values were similar (70% wt) to values reported by Huijgen et al. [31] for finely milled steel slag carbonated in slurry-phase at 100°C and 19 bar. The considerably higher conversion yields resulting for the milled EAF slag compared to the finest fraction of the mixture, could be correlated not only to the higher content of unreactive Ca-containing phases in class D of the mixture, but also to the mechanical activation effect exerted by the milling pre-treatment on the EAF slag.
Effects of Carbonation on Slag Properties
The mineralogy of the different types of slag showed to be noticeably affected by the carbonation treatment, as reported in Fig. 1. The diffraction patterns of carbonated samples of the mixture and EAF slag (see Fig. 1a–c) indicated the disappearance of phases such as Ca–Al–Fe oxide and periclase, a relevant reduction in peak intensities of dicalcium silicate, cuspidine, merwinite and chromium oxide, whereas no evident variations in the contents of akermanite, forsterite, Cr–Mg oxide, silica, magnetite and calcium fluoride. The only phase for which a noteworthy increase in peak intensity was retrieved after carbonation was calcite, indicating that, although MgO showed to decrease upon carbonation, only calcium carbonates could be identified. These results are in good agreement with findings by Johnson et al. [9], that evidenced the increase of calcite, the decrease of periclase, portlandite and partly also of dicalcium silicate, while an unmodified pattern for akermanite and silica. For the AOD slag (see Fig. 1d), carbonated samples exhibited a predominance of calcite peaks, a very relevant decrease in Ca2SiO4, the disappearance of CrO and MgO and a roughly unvaried content of magnetite and CaF2, in good accordance with the estimated Ca conversion yields achieved by the treatment.
The leaching behaviour of each type of tested slag was variously affected by the carbonation treatment, as shown in Figs. 2 and 3. The pH of the eluates of the EN 12457-2 tests carried out on all samples of carbonated slag (see Fig. 3a) were lower than those of the untreated samples, in particular for the residues that showed the highest reactivity with CO2 (class D of the mixture, EAF and AOD slag). Calcium concentrations (see Fig. 2b) in the eluates of the carbonated slag were also considerably reduced compared to the untreated materials, in accordance with the increase of calcite measured in the treated solid residues. Conversely, Si concentrations (see Fig. 2f) increased in the eluates of all carbonated samples, in particular for the EAF and AOD slags, indicating that indeed part of the silicate minerals in the original slag reacted during the carbonation treatment, forming a more readily soluble Si-containing phase. These results are in good agreement with findings by Chen et al. [10] on steel slag carbonated under similar operating conditions. Mg and Fe release (see Fig. 3d, e) appeared not to be noticeably affected by the carbonation process, since the observed differences fell within the range of variability of leaching test results for heterogeneous materials. Nevertheless, the results retrieved for the milled EAF slag, i.e. decrease in Fe leaching and slight increase in Mg release upon carbonation, were also reported in previous studies [39, 47]; further testing, employing pH-dependence leaching tests for example, is hence required to confirm these data. The effects of carbonation on Cr leaching (see Fig. 3c) differed depending on the type of slag: it decreased notably from all classes of the mixture apart from class A and D, whereas it increased from the EAF and AOD slag. While such differences may be correlated to the different oxidation states of Cr [8, 39, 47], in our case it was not possible to verify such a hypothesis due to the lack of direct measurements of Cr(III) and Cr(VI) contents. An indirect indication on which Cr form was prevalent for the tested material may be gained from the observation of the shape of the pH-dependent Cr leaching curve, which is currently being investigated. Regarding the effects of carbonation on soluble salts leaching, as shown in Fig. 4, contrasting results were found for the milled EAF and for the AOD slag, specifically on fluoride mobility, see Fig. 3b. Fluoride release appeared higher in the eluates of carbonated samples compared to those of untreated milled EAF slag, while it exhibited the opposite behaviour (decrease upon carbonation) in the eluates of the AOD slag. The higher concentrations of fluorides detected in the eluates of the carbonated EAF slag may be correlated to the decrease of cuspidine detected in the XRD patterns of the slag samples after carbonation (see Fig. 1c), indicating that as a consequence of the treatment, fluorides may have formed a more soluble phase. However, further investigations are needed to verify this theory and to clarify the effects of carbonation on fluoride mobility for the AOD slag, which did not exhibit any fluoride-containing crystalline phase except for CaF2 that did not show to be affected by the carbonation treatment (see Fig. 1d).
Conclusions
In this paper the main effects of accelerated carbonation on different types of humidified stainless steel slag are reported. The results of this study demonstrated that, even under mild operating conditions (50°C and 3 bar CO2), this process was able to achieve relevant CO2 uptakes, particularly for specific types of slag, and to affect several properties of the material.
Chemical and mineralogical characterization data indicated that all the types of slag exhibited a high potential reactivity with CO2, owing particularly to high Ca contents (35–50% wt), mainly in the form of silicates and specifically of Ca2SiO4.
The results of preliminary carbonation experiments carried out on different particle size fractions of a mixture of stainless steel slag showed that one of the parameters that most affected the CO2 uptake achieved by the slag upon the treatment was grain size. An increase in operating temperature from 30 to 50°C also exerted a positive effect on enhancing the CO2 uptake of the slag, while the L/S ratios that gave the highest CO2 uptakes were in the range of 0.2–0.4 l/kg. These operating conditions were applied to investigate the reactivity of milled (d < 0.150 mm) EAF slag and of AOD slag. Maximum CO2 uptakes of 130, 180 and 300 g CO2/kg slag were achieved for class D of the mixture, milled EAF and AOD slag, respectively. Assuming that roughly 90 kg of EAF slag, 50 kg of AOD slag and 300 kg of CO2 are generated per ton of produced steel, this process would have the potential of sequestering over 10% of CO2 emissions from a typical EAF steel manufacturing plant, amounting to a total of 0.4 Mt CO2/year in Italy, considering a 2 Mt/year slag production. This figure may be deemed interesting also in consideration of the mild operating conditions applied and the absence of processing water consumption and treatment, in comparison to slurry-phase treatment. Future experiments should be aimed at assessing the feasibility of further optimization of processing conditions, as for example increasing CO2 pressure to enhance the kinetics of AOD slag carbonation or applying a more intensive milling pre-treatment to increase the conversion yields achieved by EAF slag carbonation. It should be noted that all the reported results were obtained with 100% CO2, i.e. assuming capture of the CO2 from the flue gas before slag carbonation. Hence, an important aspect yet to analyze is the feasibility of applying this treatment to directly sequester the CO2 emitted from steel plants avoiding the capture step. To this aim, specific steel slag carbonation tests with CO2 concentrations typical of emissions from steel manufacturing plants (~10 to 20 vol%) should be performed.
Regarding the effects of the treatment in terms of slag valorization, carbonation appeared to exert positive effects on several material properties. Owing to a considerable content of γ-Ca2SiO4, as well as of free MgO, none of the slag samples investigated possessed suitable characteristics for reuse in civil engineering applications. Upon carbonation instead, XRD analysis of treated slag samples showed a relevant decrease in Ca and Mg oxide phases. In addition, an aspect that could be further investigated, is the ability of accelerated carbonation to enhance the mechanical and structural properties of the slag, which may be an interesting effect in view of the potential utilization of the treated material in engineering applications. Regarding the effects of accelerated carbonation on the environmental behaviour of the different types of steel slag, all types of treated slag exhibited a noteworthy pH reduction, a decrease of Ca and an increase of Si leaching. Depending on the type of slag, carbonation exerted a different effect on chromium and fluoride leaching, which were the only trace compounds in the eluates of these types of residues that showed relevant concentrations with respect to landfill acceptance criteria. Further testing will hence be necessary to elucidate the effects of this treatment on the leaching behaviour of the above mentioned contaminants.
References
OECD (Organization for Economic Cooperation and Development): Iron and steel industry report 2000. OECD, Paris (2002)
Rynikiewicz, C.: The climate change challenge and transitions for radical changes in the European steel industry. J. Clean. Prod. 16, 781–789 (2008)
Proctor, D.M., Fehling, K.A., Shay, E.C., et al.: Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 34, 1576–1582 (2000)
Federacciai (Federation of Italian Steel Companies).: The Italian steel industry key statistics 2009. http://www.federacciai.it/MenuSx/siderurgia2009.pdf. Accessed 6 Sep 2010
Motz, H., Geiseler, J.: Products of steel slags an opportunity to save natural resources. Waste Manage. 21, 285–293 (2001)
Shen, H., Forssberg, E.: An overview of recovery of metals from slags. Waste Manage. 23, 933–949 (2003)
Costa, G., Baciocchi, R., Polettini, A., Pomi, R., Hills, C.D., Carey, P.J.: Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues. Environ. Monit. Assess. 135, 55–75 (2007)
Van Gerven, T., Van Keer, E., Arickx, S., Jaspers, M., Wauters, G., Vandecasteele, C.: Carbonation of MSWI—bottom ash to decrease heavy metal leaching in view of recycling. Waste Manage. 25, 291–300 (2005)
Johnson, D.C., Macleod, C.L., Hills, C.D.: Solidification of stainless steel slag by accelerated carbonation. Environ. Technol. 24, 671–678 (2003)
Chen, Q., Johnson, D.C., Zhu, L., Yuan, M., Hills, C.D.: Accelerated carbonation and leaching behaviour of the slag from iron and steel making industry. J. Univ. Sci. Technol. Beijing 14, 297–301 (2007)
Lackner, K.S., Wendt, C.H., Butt, D.P., Joyce, E.L., Sharp, D.H.: Carbon dioxide disposal in carbonate minerals. Energy 20, 1153–1170 (1995)
Huijgen, W.J.J., Comans, R.N.J.: Carbon dioxide sequestration by mineral carbonation, literature review. ECN-C-03-016, Petten (2003)
Sipilä, J., Teir, S., Zevenhoven, R.: Carbon dioxide sequestration by mineral carbonation, literature review update 2005–2007. Åbo Akademi University, Faculty of Technology, Heat Engineering Laboratory report VT 2008-1 (2008)
Reddy, K.J., Gloss, S.P., Wang, L.: Reaction of CO2 with alkaline solid wastes to contaminant mobility. Water Res. 28, 1377–1382 (1994)
Tawfic, T.A., Reddy, K.J., Gloss, S.P.: Reaction of CO2 with clean coal technology ash to reduce trace element mobility. Water Air Soil Poll. 84, 385–398 (1995)
Jia, L., Anthony, E.J.: Pacification of FBC ash in a pressurized TGA. Fuel 79, 1109–1114 (2000). (21)
Johnson, D.C.: Accelerated carbonation of waste calcium silicate materials. SCI Lecture Papers Series (2000)
Montes-Hernandez, G., Pérez-López, R., Renard, F., Nieto, J.M., Charlet, L.: Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 161, 1346–1354 (2009)
Gunning, P.J., Hills, C.D., Carey, P.J.: Accelerated carbonation treatment of industrial wastes. Waste Manage. 30, 1081–1090 (2010)
Reddy, K.J., Drever, J.I., Hausfurther, V.R.: Effects of a CO2 pressure process on the solubilities of major and trace elements in oil shale solid wastes. Environ. Sci. Technol. 25, 1466–1469 (1991)
Uibu, M., Uus, M., Kuusik, R.: CO2 mineral sequestration in oil-shale wastes from Estonian power production. J. Environ. Manage. 90, 1253–1260 (2009)
Back, M., Kuehn, M., Stanjeck, H., Peiffer, S.: Reactivity of alkaline lignite fly ashes towards CO2 in water. Environ. Sci. Technol. 42, 4520–4526 (2008)
Pérez-López, R., Montes-Hernandez, G., Nieto, J.M., Renard, F., Charlet, L.: Carbonation of alkaline paper mill waste to reduce CO2 greenhouse gas emissions into the atmosphere. Appl. Geochem. 23, 2292–2300 (2008)
Huntzinger, D.N., Gierke, J.S., Kawatra, S.K., Eisele, T.C., Sutter, L.L.: Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ. Sci. Technol. 43, 1986–1992 (2009)
Fernández Bertos, M., Li, X., Simons, S.J.R., Hills, C.D., Carey, P.J.: Investigation of accelerated carbonation for the stabilization of MSW incinerator ashes and the sequestration of CO2. Green Chem. 6, 428–436 (2004)
Prigiobbe, V., Polettini, A., Baciocchi, R.: Gas–solid carbonation kinetics of air pollution control residues for CO2 storage. Chem. Eng. J. 148, 270–278 (2009)
Baciocchi, R., Costa, G., Di Bartolomeo, E., Polettini, A., Pomi, R.: The effects of accelerated carbonation on CO2 uptake and metal release from incineration APC residues. Waste Manage. 29, 2994–3003 (2009)
Baciocchi, R., Costa, G., Lategano, E., Marini, C., Polettini, A., Pomi, R., Postorino, P., Rocca, S.: Accelerated carbonation of different size fractions of bottom ash from RDF incineration. Waste Manage. 30, 1310–1317 (2010)
Iizuka, A., Fujii, M., Yamasaki, A., Yanagisawa, Y.: Development of a new CO2 sequestration process utilizing the carbonation of waste cement. Ind. Eng. Chem. Res. 43, 7880–7887 (2004)
Shtepenko, O.L., Hills, C.D., Coleman, N.J., Brough, A.: Characterization and preliminary assessment of a sorbent produced by accelerated mineral carbonation. Environ. Sci. Technol. 39, 345–354 (2005)
Huijgen, W.J.J., Witkamp, G.J., Comans, R.N.J.: Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 39, 9676–9682 (2005)
Teir, S., Eloneva, S., Fogelholm, C.J., Zevenhoven, R.: Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production. Energy 32, 528–539 (2007)
Eloneva, S., Teir, S., Salminen, J., Fogelholm, C.J., Zevenhoven, R.: Steel converter slag as raw material for precipitation of pure calcium carbonate. Ind. Eng. Chem. Res. 47, 7104–7111 (2008)
Kodama, S., Nishimoto, T., Yamamoto, N., Yogo, K., Yamada, K.: Development of a new pH—swing CO2 mineralization process with a recyclable reaction solution. Energy 33, 776–784 (2008)
Lekakh, S.N., Rawlins, C.H., Robertson, D.G.C., Richards, V.L., Peaslee, K.D.: Kinetics of aqueous leaching and carbonization of steelmaking slag. Metall. Mater. Trans. B 39B, 125–134 (2008)
Bonenfant, D., Kharoune, L., Sauvé, S., Hausler, R., Niquette, P., Mimeault, M., Kharoune, M.: CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind. Eng. Chem. Res. 47, 7610–7616 (2008)
Young, J.F., Berger, R.L., Breese, J.: Accelerated curing of compacted calcium silicate mortars on exposure to CO2. J. Am. Ceram. Soc. 57, 394–397 (1974)
Papadakis, V.G., Vayenas, C.G., Fardis, M.N.: Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 46, 1333–1338 (1991)
Huijgen, W.J.J., Comans, R.N.J.: Carbonation of steel slag for CO2 sequestration: leaching of products and reaction mechanisms. Environ. Sci. Technol. 40, 2790–2796 (2006)
Baciocchi, R., Costa, G., Polettini, A., Pomi, R.: Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Proc. 1, 4859–4866 (2009)
Teir, S.: Fixation of carbon dioxide by producing carbonates from minerals and steelmaking slags. Doctoral dissertation, Helsinki University of Technology (2008)
Shi, C.: Steel slag—its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 16, 230–236 (2004)
Gou, Z., Chang, J., Zhai, W.: Preparation and characterization of novel bioactive dicalcium silicate ceramics. J. Eur. Ceram. Soc 25(9), 1507–1514 (2005)
Lopatin, D.V., Chizhikova, V.M.: Crystal-chemical stabilization of dicalcium silicate. Steel Transl 37, 191–195 (2007)
Reddy, A.S., Pradhan, R.K., Chandra, S.: Utilization of basic oxygen furnace (BOF) slag in the production of a hydraulic cement binder. Int. J. Miner. Process. 79, 98–105 (2006)
Shtepenko, O.L., Hills, C.D., Brough, A., Thomas, M.: The effect of carbon dioxide on β-dicalcium silicate and Portland cement. Chem. Eng. J. 118, 107–118 (2006)
Suer, P., Lindqvist, J.E., Arm, M., Frogner-Kockum, P.: Reproducing ten years of road ageing—accelerated carbonation and leaching of EAF steel slag. Sci. Total Environ. 407, 5110–5118 (2009)
Acknowledgments
The authors would like to acknowledge the financial support received by the Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA) within the framework agreement on R&D activities with the Italian Ministry of Economic Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baciocchi, R., Costa, G., Di Bartolomeo, E. et al. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valor 1, 467–477 (2010). https://doi.org/10.1007/s12649-010-9047-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-010-9047-1