Abstract
The density functional theory computation has been carried out for the analysis of 5-methyluracil with help of a standard program of DFT/Gaussian-09 & GAR2PED. Recorded spectra (Raman & IR) of 5-methyluracil have been studied for their fundamental vibrational frequencies in the light of DFT calculations at the level B3LYP/6-311++ G**-09. In study of normal modes, the GAR2PED program has been applied to compute PEDs. The charge transfer of 5-methyluracil has been computed applying the HOMO and LUMO level energy analysis. HOMO–LUMO study for energy-gap helps in possibility of charge transfer within bio-molecule. The ESP plotting and electron density mappings for MEP have been analyzed for charge distribution concepts in molecule on the location of nucleophilic and electrophilic reactions. The density functional theory method has been applied to optimize for Mulliken/APT charges, molecular thermodynamics properties and structure of 5-methyluracil. This study has been done for the internal modes of methyl (–CH3) group substituent on C5 atom of ring, that this has arisen the splitting of frequencies for distribution of the fundamental mode between two species.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The 5-methyl-uracil is one of the aromatic organic molecules shaped as heterocyclic. Uracil normally occurs as a main component of ribonucleic acid (RNA). For the DNA, uracil is removed through thymine; namely as 5-methyl-uracil. The halo-uracils act as anti-tumor drugs against certain tumors. The uracils or their derivatives significantly exchange the chemical and spectroscopic behaviors in vivo/bio-activity. Due their significant role in pharmacology, the halo-pyrimidines are mostly used by extensively in medical community as; 5-fluorouracil as an anti–Cancer drug; 5-trifluoro-methyluracil & 5-iodouracil as an anti-viral activity. Thus, the studies of pyrimidine ring derivatives are most interesting for their spectroscopic properties and bio-activities [1,2,3], and such are usually utilized for anti–Carcinogenic and anti–HIV drugs [4,5,6,7,8]. As fluorinated pyrimidine antimetabolite, the 5-fluoro-uracil is well-known drug as for anti–Carcinogenic, which is usually treated in tumors or colorectal carcinoma [4, 5]. Here, hydrogen on site of C5 atom of pyrimidine is replaced by halogens is tested for anti–HIV/tumors drugs [6, 8]. This article is a void study of normal vibrations of the bio-molecule for substituted mass & electro-negativity effect of methyl (–CH3) group at uracil’s ring. The study of 5-substituteduracils [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] has been done through the several authors, but there are required addition study at C5 atom of uracil’s ring, that is perfectly undecided, and so far, it has been needed the further scientific study.
The main object of present study is to carry out the optimization of structural geometries, APT/Mulliken atomic charges and normal modes employing DFT method at basis set 6-311++G** of Gaussian-09 [24]. All of the normal modes have been computed with related parameters obtained by Gauss View-5.09 [25] of G-09 [24] and PEDs from the GAR2PED [26]. Now, it has also found out bio-molecular reactivity, electron density transfer and intra-molecular bonding employing HOMO–LUMO calculations as well as with other analysis.
2 Experimental details
2.1 Experiment
A small amount of 5-methyluracil crystalline spectral grade powder of Aldrich Chem. Co. was used to record Raman and IR spectra without any further purification at room temp (23 °C). FTIR spectrum as given Fig. 1a was recorded on normal temp (23 °C) within range of 400–4000 cm−1 on a FTIR (spectrophotometer model-5300). Raman spectra as given Fig. 2a was recorded on normal temperature with help of Raman Spex-1877 spectrophotometer within range of 200–4000 cm−1 through an excitation source with the 4880 A0 line of Ar+ laser. The resolution of spectrophotometer has a better than 2 cm.−1
2.2 Theoretical
Here, DFT calculation of 5-methyluracil was done at level of B3LYP/6-311+ +G** of G-09 program [24] with the optimized geometry at minimizing energies to respect of all geometrical parameters. The fundamental frequencies have been optimized with help of computed modes through visualization software of Gauss View-5.09 [25] as well as obtained related other parameters from G-09 program [24] & PEDs through GAR2PED [26]. This computation has been done for sake of bond lengths/angles and normal modes in wave numbers with their IR intensities (as shown Fig. 1b as well as Raman scattering activities and depolarization ratio (as shown Fig. 2b of 5-methyluracil. These computations have been used to produce thermodynamics data and related parameters of molecule as given Fig. 3a, b. Molecular Electrostatic Potentials (MEPs) have been calculated with visual representation for the reactive of electrophilic/ nucleophilic attack of biological activities. The analysis of HOMO–LUMO has been calculated with help of G-09 [24] for the study of charge transfer possibility in bio-molecule.
3 Results with discussion
3.1 Molecular charge
These have been discussed like Mulliken charges that can be found through an overlap contribution of computed APT charges. Cioslowski defined this charges depend upon the APT invariants [27]. The generalized atomic polar tensor (APT) charges can be computed with help of the DFT and those are directly related to observed intensities of the IR. The features helped to obtain the molecular charges through APT & GAPT charges. [28,29,30].
3.1.1 Atomic polar tensor charge
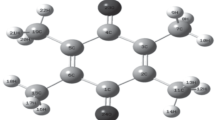
This is an addition of charge flux tensor & charge tensor that has a model of charge–Charge flux [31]. In molecule, these charges could be representative of all molecular chemical properties [32, 33]. Atomic labeling schemes of the 5-methyluracil are shown in Fig. 3b. APT charge at the atom to respective site of 5-methyluracil molecule has been optimized by DFT/B3LYP/6-311+ +G** [24] as summarized Table 1. In this table, electro-negativity of each atom is optimized to other −ve charge. APT charge of 5-methyluracil has been explained below to respected atom. Here, except C4 [as on C5 atom of ring of 5-methyl-uracil as shown in Fig. 3a] atom holds the negative ATP charge − 0.295694 a.u., but the rest 3 carbon atoms of ring C1, C3 and C5 have +ve charge as 1.334375, 1.133897 and 0.459049 a.u. to respected carbon atoms, but only one of the C4 atom having more negative charge (− 0.295694 a.u.) of ring atoms holds the reactant property than other the carbon atoms. Here the oxygen O10 and O11 hold the −ve APT charges as − 0.922440 & −.837151 a.u. of the bio-molecule to corresponding atoms. And the nitrogen N2 and N8 hold the −ve APT charges as − 0.726627 & − 0.737466 a.u. for the site of respective atoms of 5-methyluracil. For O & N atoms, they hold −ve APT charge for sake of the high electro-negativity. In molecule, 3 H atoms (H6, H7, H9) of 5-methyluracil on pyrimidine possess +ve APT charges; 0.053014, 0.219223 & 0.243731 a.u. and other three H atoms (H13, H14, H15) bear likely to be not similar -ve APT charges; − 0.001003, − 0.000858 & − 0.008466 a.u. to respective atoms. As well as C12 atom has positive APT charge 0.086416 a.u. on atom. Here, this could be found the C1 atom having highest charge than the other C12, C3, C4 and C5 carbon atoms. Here, it has been found that the hydrogen atoms (H6, H7, H9) on ring are attached to N atoms with +ve APT charges.
3.1.2 Mulliken charge
Here, the charges optimizations have a crucial role in DFT for optimization of molecular structural geometry; because of atomic charges affect the polarizability, electronic structure, dipole moment, and much more behaviors of molecule [31]. The Mulliken charges [31] have the main role in the DFT computation that it depends on the basis set. In the molecule, the natural charges come out on the surface of atoms that it has the indication of all quantum and molecular chemical behaviors [32, 33]. The Mulliken charges at the atoms are shown in Fig. 3b of 5-methyluracil have been optimized at the level 6-311++G**[24], which are tabulated in the Table 1. In which, it have been found that the 3 carbon atoms C1, C3 and C5 of pyrimidine ring have the +ve charges as 0.342391, 0.104118 & 0.190074 a.u. to respective carbons, except C4 atom in the ring [as on C5 atom ring of 5-methyl-uracil as shown in Fig. 3a] bears highest positive Mulliken charge 0.434665 a.u. for the reason of the methyl (–CH3) group. The O10 and O11 atoms have the negative Mulliken charges with their magnitudes of − 0.344641 and − 0.317117 a.u. on the respective atomic site. Similarly as, the N2 and N8 atoms of 5-methyluracil have the negative Mulliken charges with their magnitudes of − 0.397268 and − 0.360144 a.u. to corresponding atomic site. Thus, all oxygen & nitrogen atoms have very larger electro-negativity. There are three hydrogen (H6, H7, H9) of 5-methyluracil at pyrimidine ring have the positive charges with their magnitudes of 0.182282, 0.368440 and 0.342692 a.u. respect to the atoms and other three H atoms (H13, H14, H15) bear +ve Mulliken charges as 0.179201, 0.179207 & 0.132458 a.u. to respective hydrogen, in which H15 is not similar to other two hydrogen atomic charges. The out of ring atom C12 has −ve Mulliken charge −0.656211 a.u. at this atomic site. Hence, this is a notice that in all of the five carbon atoms (C1, C3, C4, C5, C12), the C12 atom has highest negative charge instead of C12 atom as discussed in the APT charges.
3.2 Molecular structural geometry
For the optimization of structural geometry, atomic scheme of 5-methyl-uracil bio-molecule is given in the Fig. 3b. The optimized geometrical structural values of 5-methyl-uracil have been summarized in the Table 2. The results advise that if methyl (CH3) group is replaced the H atom on C4 of the ring [as on C5 atom of ring of 5-methyluracil as shown in Fig. 3a] on uracil pyrimidine ring. The evaluated results of ring of 5-methyluracil, the structural geometry shows in pyrimidine ring that all of bonds are having partial double bond nature. As optimized Table 2, bond lengths of pyrimidine of 5-methyluracil, all of C–N bonds are having a descending order as (N2–C3, N8–C1, N2–C1, C5–N8), are mostly similar in their magnitudes to each molecules lie between bond length ~ 1.41 Å to ~ 1.38 Å, are lying in a plane of pyriminide ring. As well as, the two ring, C3–C4 and C4=C5 bonds are in the same plane of ring as the order of (C3–C4) > (C4=C5) in the magnitude ~ 1.47 Å and ~ 1.35 Å respectively. The outer most C4–C12 bond length is the largest C–C bond, 1.4999 Å than other. Thus, it has been found that the C–N–C, C–C–C, C–C–N as internal angles of pyrimidine ring have not same to each other like as smallest \(\alpha\)(N2–C1–N8), angle value 112.570 and largest \(\alpha\)(C1–N2– C3), angle value 128.100 of the ring, as well as in the twenty six dihedral angles are ~ 00 ± 0.1 or ~ 1800 ± 1.0, and it is show that these 12 atoms of 5-methyluracil, C1, N2, C3, C4, C5, N8, O10, O11, H6, H7, H9 and C12 are in a same plane. Rest three hydrogen atoms, H13, H14, H15, are not lying in the same plane. Here, it is shown that these geometrical parameters remain same, and all of the atoms in pyriminide ring are having in the plane.
3.3 Vibrational assignments
Recently, the biological significance of the bio-molecules and its derivatives are found to be highly interesting for sake of its bio-activities [1,2,3]. Here in their spectral investigation, the vibrational assignment of the substituent as methyl (CH3)-group has been made for the fifth place of uracil’s ring. This study is a wide spectral investigation for 5-methyluracil on fifth position at uracil’s ring with due respect to mass and electro-negativity of hydrogen < methyl (CH3) and this explains the theoretical and experimental study to the 5-methyl-uracil molecule under these properties. Vibrational study of the pyrimidine or its derivatives is most similar with benzene ring. This assignment has been made especially for planarity/non-planarity of group of atoms substitution on C5 position of ring that might arise the vibrational frequencies with effect of their mass & electro-negativity of substituted groups at C4 atom [as on C5 atom ring of 5-methyluracil as shown in Fig. 3 a] on the ring of uracil. All of the normal frequencies have been optimized in the light of calculated frequencies through visualization program of Gauss View-5.09 [25] of G-09 [24] and PEDs are calculated by the software GAR2PED [26]. 5-Methyluracil molecule having the Cs symmetry bears 15 atoms and 39 fundamental frequencies which are expected for appearing in the spectra of IR & Raman. The optimization of 5-methyluracil molecule has been made for vibrational frequencies in wave numbers with the intensities of IR (in shown Fig. 1b), the scattering activities of Raman and Raman bands depolarization ratio (as shown in Fig. 2b) are summarized in the Table 3. In the systematic study, the calculated and recorded normal frequencies of 5-methyluracil has been discussed together with PEDs as given Table 3 & Fig. 3b in these sections:
3.3.1 CH 3 group (9 modes)
CH3 group splits nine internal of vibrations, as in-planar five modes are νs(CH3)-sym. stretch, νas(CH3)-anti-sym. stretch, δs(CH3)-sym. deformation/scissor, δas(CH3)-anti-sym. deformations, ρ|| (CH3)-rocking and non-planar/out-of-plane four modes are δas(CH3)-anti-sym. deformation, νas(CH3)-anti-sym. stretch, ρ⊥(CH3)-rocking/wagging, τ(CH3)-torsion/twist respectively. These group frequencies are usually localized characteristics modes and mixed with other modes i.e. intermolecular hydrogen bonding and ring modes. These ν(C–H) vibrations are found to be lying between region 2800–3200 cm−1 [18]. The νs(CH3) and νas(CH3) modes in-plane have been calculated the unscaled frequencies 3033 and 3108 cm−1 which have been recorded for IR peaks at 2900 & 2980 cm−1, similarly the Raman bands at 2900 & 2990 cm−1 having the strong intensities in both IR and the Raman. In-plane, the δs(CH3) and δas(CH3) modes are computed at 1067 & 1491 cm−1 and that these are recorded the band peaks for IR at 1067 & 1480 cm−1 respectively but Raman band at 1475 cm−1 with the strong peak for δas(CH3) mode, in which the δs(CH3) is treated as umbrella modes to have strongly mixed up with the rings modes. The ρ|| (CH3) mode in-plane is calculated at 1022 cm−1 and that this has been recorded at 1024 cm−1 for the IR followed by Raman band at 1010 cm−1with strong intensity for rocking mode. Here, it could be seen that all these modes are supported to observed results and mixed up with rings modes, C=O modes and hydrogen bonding modes as shown in PEDs (Table 3).
The non-planar modes, νas(CH3) and δas(CH3) are computed at 3086 and 1469 cm−1and that these normal modes are recorded at 2925 & 1470 cm−1 for IR with Raman bands at 2925 & 1445 cm−1, in which one of δas(CH3) mode is mixed up in other modes i.e. ring vibrations, C=O mode, hydrogen bonding. Similarly, the ρ⊥(CH3) and τ(CH3) modes are optimized at 1423 & 142 cm−1 and which are recorded for Raman band at 1424 & 205 cm−1 respectively and IR peak at 1426 cm−1 for the ρ⊥(CH3), which are heavily mixed up with the other vibrations.
3.3.2 C–CH 3 (3 modes)
For stretching mode, the ν (C–CH3) has been found to be at the higher frequencies region compare to aromatic amines. In comparative research of 5-methyluracil (thymine), the ν (C–CH3) mode was reported at ~ 1250 cm−1 in the refs. [3, 18]. But here, the ν (C4–CH3) mode has been calculated at 1223 cm−1 and this has been observed for IR at 1220 cm−1 with Raman band at 1222 cm−1 that this is heavily mixed up together the other ring vibrational modes. In-plane, the β (C4–CH3) bending mode has been reported between the 300–250 cm−1 as reported refs [3, 18]. At present, this mode has been found mostly near & below to ~ 300 cm−1. Calculated mode for β(C4–CH3) has been optimized at 277 cm−1and observed Raman peak at 280 cm−1 that is quietly below for the reason of mass & electro-negativity of CH3 group. And out of plane, the γ(C4 –CH3) mode is optimized at 290 cm−1, but observed Raman peak at 320 cm−1 is mixing together with the hydrogen bonding.
3.3.3 C–H (3 modes)
These stretching vibrations of C–H bond are really the highly characteristic peaks in bio-molecules analysis [3] and the mode appears between the ranges of 3000–3300 cm−1[19]. The modes in-plane vibrations ν(C5–H6) and β (C5–H6) have been calculated at 3193 & 1370 cm−1 and which are recorded at 3070 & 1370 cm−1 in the IR and the Raman peaks at 3075 & 1370 cm−1 to respective vibrations, and in which of ν(C5–H6) frequency has the strong existence in the PEDs showing with the characteristic appearance of band. The γ (C5–H6) vibration in non-planar has been calculated at 906 cm−1 and this is observed for IR at 910 cm−1. At present, it is found that the mode of C–H stretching is highly sensitive for the reason of molecule isolation in the Ar matrix than the solid crystal phase [19]. This could be noticed that these assigned results have a similar agreement to reported refs [9,10,11,12,13,14,15,16,17,18,19,20,21].
3.3.4 N–H (6 modes)
As the ν(C–H) vibrations, the N–H bond stretching vibrations are found to be also appearing with strong characteristic peaks in analysis of bio-molecules [3] and have found between the region of 3200–3600 cm−1, and where the discrepancies have been arisen between theoretical & experimental values in vibrational frequencies of the N–H due to involvement of some other vibrations [19]. Stretching modes in-plane ν (N8–H9) and ν (N2–H7) have been optimized at 3640 & 3596 cm−1, but here the vibrational frequencies have been observed for the IR at 3250 & 3140 cm−1 respectively having the strong characteristic bands appearance in the PEDs. In-plane vibrational modes β (N8–H9) and β (N2–H7) are optimized at 1499 & 1409 cm−1 and that these have been recorded in IR 1498 & 1494 cm−1 with Raman peaks at 1500 & –- cm−1 for respective modes. The γ (N2–H7) and γ (N8–H9) modes in non-planar have been computed at 668 & 550 cm−1 and these are recorded in the IR at 664 & 550 cm−1 and in the Raman bands at 645 & 560 cm−1 respectively. So far, this has been noticed that ν (N–H) vibration holds the highly sensible as ν(C–H) mode for the reason of molecule isolation in Ar matrix compare to solid crystal phase [19]. Here, it is found that the above result have a similar agreement with the refs [9,10,11,12,13,14,15,16,17,18,19,20,21].
3.3.5 C=O (6 modes)
Normal frequencies of 5-methyluracil hold the six C=O vibrations as; ν(C1=O10), ν(C3=O11), β(C1=O10), β(C3=O11), γ(C1=O10) & γ(C3=O11). Between the spectral 1600–1800 cm−1 range of uracil or their derivatives have an involvement and congestion of the stretch (C=O) and stretch of (C=C) bond in this region [12,13,14,15,16,17,18]. The (C=O) stretching vibrations have been affected with the interaction of hydrogen bonding and shifted annoying of Fermi resonance [15,16,17,18]. Here, ν (C1=O10) and ν(C3=O11) modes are computed at 1799 & 1753 cm−1 respectively, but have mixing together with other ring vibrations as given in PEDs, as well as the IR frequency is observed at 1800 cm−1 corresponding to ν (C1=O10) Raman band as refs [15,16,17,18]. In-plane for β(C1=O10) & β(C3=O11) bending vibrations have been optimized at 389 & 606 cm−1 and that these have been recorded for IR at 400 & –- cm−1 with Raman bands at –- & 610 cm−1 to corresponding bands as in support of the results of refs [12,13,14,15,16,17,18]. In non-planar vibrations, γ(C1=O10) and γ(C3=O11) modes are calculated at 753 & 765 cm−1 and these corresponding vibrations have been recorded for IR at 750 & 752 cm−1 with Raman bands at 752 & 765 cm−1 respectively, whereas these have a mixing together with the rings vibrations and H–bonding modes.
3.3.6 Ring vibrations (12 modes)
Like the ring of phenyl vibrations, pyrimidine ring vibrations of 5-methyluracil has the 12 normal vibrations as; the 6 normal vibrations for the ring stretching, 3 normal vibrations in-plane ring deformation and 3 normal vibrations in out-of-plane ring deformation. The ring stretching vibrations are very complicated having the combination of stretching bonds (C=C, C–C, C–N) [15,16,17,18]. Here, For the reason of spectral congestions of carbonyl (C=O) stretch vibration, H- bonding and ring stretch vibrations have been found between the range 1800–700 cm−1 as well as the addition to stretch (C=C) vibration has been found between the range 1800–1600 cm−1 of the reported refs [15,16,17,18,19,20,21,22,23]. For the present work, all of the six ring stretch vibrations have been optimized at 1695 for ν(C4=C5), 1417, 1196, 1149 (for Kekule mode), 964 and 733 cm−1(for the most popular ring breathing vibration), and consequently the 1670, 1418, 1196, 1150, 962 and 730 cm−1 are recorded with the strong intensities of IR corresponding for Raman bands at 1690, 1190, 1148, & 734 cm−1 respectively having the medium strong intensities. Kekule vibration (ν14 as benzene ring Kekule mode) of 5-methyluracil [18] was reported at ~ 1150 cm−1; here in this study, it has been assigned with the same vibration. Now here a most popular ring breathing vibration of 5-methyluracil has been found at ~ 733 cm−1 which has been shifted above up to ~ 3 cm−1 compare to previous assignment of 5-methyluracil at ~ 730 cm−1 of the ref. [18]. In-plane, the 3 normal vibration of ring deformation in-plane have been optimized at 803 ( trigonal bending vibration), 546 & 461 cm−1 and subsequently these have been recorded at 810, 540 & 460 cm−1 in the IR and Raman bands at 806, –- & 460 cm−1 to respective modes. Trigonal bending vibration of 5-methyluracil [18] had been reported at ~ 800 cm−1, that is found to be same with our result. Now the last 3 normal vibrations in out-of-plane ring deformation have been calculated at 391, 146 & 108 cm−1 and such optimized vibrations of ref. [18] had been reported at 390, 161 & 142 cm−1 to the respected modes. Here, this has been noticed that the ring deformation vibrations for out-of-plane have been corrected possibly in lower region due to the PEDs.
3.4 Analysis of HOMO & LUMO
Molecular orbital’s (MOs) theory has an importance in molecular quantum chemical calculation to investigate the electronic and optical behaviors [34]. The 5-methyluracil bears 15 atoms with 66 electrons which occupy 33 MOs and parent molecule uracil holds 12 atoms with 58 electrons occupying 29 MOs, where each MO has 2 electrons holding the opposite spins are known as α (↑ up spin) or β (↓ down spin). Energy gap with analysis of HOMO–LUMO computation of 5-methyluracil molecule has been worked out the stability & reactivity. HOMO & LUMO analysis suggest the transfer of charge distribution probability in bio-molecule, consequently energy-gap levels supports to study of activity in pharmacological properties. In ring, HOMO plays as the electron donor, that is associated with C–C, C–N or C–H bonds, still LUMO plays like the electron acceptor, it is associated with C=C bond of pyrimidine. Thus, electronic transition of HOMO toward LUMO motivates pyrimidine ring bond (C=C) to ring bond (C–C & C–N) of bio-molecule. Energy-gap is energy level difference of HOMO & LUMO that measures the stability and reactivity which is generally lowest excitation energy in a bio-molecule. So far, this motivates the transition from ground level (as HOMO) to first excited level (as LUMO) & vice-versa. A smaller energy gap excites easily and has responsible to internal charge transfer takes place in bio-activity of bio-molecule. A higher energy band gap has higher chemical kinetic stability and lower reactivity. So that, the any activated feasible reaction should have small energy-gap [35, 36]. Here transition energy between HOMO & LUMO has been computed as − 5.4192 eV for 5-methyluracil which corresponds a transition of ground level to lowest excited level. The energy gap shows the kinetic stability and chemical reactivity for the molecule. The visualized energy levels with frontier view of the HOMO–LUMO for 5-methyluracil is given as Fig. 4. HOMO/LUMO energy levels (as Table 4) have been computed as − 6.9934 & − 1.5742 eV for lowest energy of 5-methyluracil. Here, an optimized energy gap is − 5.4192 eV for 5-methyl-uracil. Hence, this has been clearly shown an occurrence of charge transfer (CT) within molecule. Energy-gap b/w MOs of ground level EHOMO to first excited level ELUMO had shown the bio-activity for intra charge transfer (ICT) [37, 38].
3.5 Analysis of molecular electrostatic potential(MEP)
The surface of MEP plots of 5-methyluracil have been visualized through the Fig. 5a, that is a most important computation normally applied to investigate the intermolecular characterization with action modes of drug of the tiny molecule. In fact, the spatial distributions of MEPs have the chemical activities of an agent on active sites. The MEPs are associated with molecular chemical reactivity, electro-negativity charges and dipole moment. These are a crucial visualization method to represent the relative polarity through mapping the molecular electron densities (ED) of a molecule. The MEPs surface plots are shown by various colors combination as for molecular electrostatic potential; the red area for the most −ve, the blue area for the most +ve and the green area for zero potential. Hence, a negative MEP shows for the attraction of proton/cation with red as the concentrated ED, but the positive MEP represents for repulsion of the proton or cations by the blue colored as atomic nuclei regions for low concentrated ED. In Fig. 5a, the −ve region (red) show nucleophilic reactivity, and electrophilic reactivity has shown with blue color for +ve region of the MEPs. This could be observed by the Fig. 5a that the O11 atom has been found in orange region, but other O10 atom has been found in partially red color region that shows the electronegative area and this is an active place of nucleophilic attack. And now, the position of 3 hydrogen atoms at the pyrimidine is most +ve area and it is the active place of electrophilic attack. Here, green color shows zero MEP. The MEP surface in Fig. 5a, this has clearly visualized that a place near the corners of methyl (CH3) group represent the area of partially negative MEP and rest of corners of the group are most +ve MEP (Fig. 5a).
(a) Molecular Electrostatic Potentials plot of 5-methyluracil (b) ESP iso-surface contour map of 5-methyluracil (c) Visualization of the total density mapping for 5-methyluracil (d) Visualization of the ESP array-plot for 5-methyluracil (e) Visualization of the total density array-plot for 5-methyluracil
For iso-surface plots of electrostatic potentials (ESPs) for 5-methyluracil have been shown in Fig. 5b, the ESP is the useful and an important explanation of the charge distribution in molecule like the visual variably charged area. These charge distributions show the all information about that how these molecules act and react with another [39, 40]. ESPs are associated with electron density(ED) that this explains electrophilic effect or nucleophilic reactions and with H-bonding interaction [41, 42].
As Fig. 5b, the −ve electrostatic potential shows with the proton attraction through high ED (as red colored), but +ve ESP represents the proton repulsion where low ED exists (as blue colored). ESP iso-surface holds the specified fractional value of electron density probability in bio-molecule. In Fig. 5b, the ED iso-surface has been given with different colors upon respective contour iso-surfaces. In the present study, the ESPs are in increasing order with these colors; red, orange, yellow, green & blue. Such surface explains a charge density and chemical reactivity of molecule. For different surfaces, the different electrostatic potential values have shown with these coloring area; red as most -ve ESP area, blue as most +ve ESP area and green for the area of zero ESP. Graphically electrostatic potential has been described through Connolly [43,44,45,46] for a series of values. The mapped electron density iso-surface are shown in the Fig. 5b of 5-methyluracil.
Here, the Fig. 5c represents the visualized total density mapping. Similarly, the ESP as well as total density array-plot for 5-methyluracil have been represented through the visualization in Fig. 5d and e. The visual demonstration of chemical activity and comparative atomic reactivity has been shown in bio-molecule. The shown in Fig. 5d for the locality of atoms of pyrimidine ring in 5-methyluracil, here the yellow colored lines stage with electronegative that these are found to be anywhere more closer as nucleophilic properties and anywhere small closer behave as the electrophilic properties.
3.6 Thermodynamic functions
The thermodynamic functions are associated with the translational, rotational and vibrational entropies of molecule. In DFT computation, the optimized frequency has been applied to produce thermodynamic properties for 5-methyluracil, that have been summarized in the Table 5. The optimized thermodynamic data have been utilized to modify the exp. thermodynamic data at zero K temperature for an effect of the zero-point vibrational energy (ZPE). Here, a scaling factor could apply to correct the overestimation. In this study, ZPE and Gibb free energy have been optimized at − 454.161531 & − 454.194099 a.u. for 5-methyluracil. And computed entropy of bio-molecule can be helpful to modify the data of experimental thermodynamic information for neglect of residual entropy present at zero K temperature for the crystal phase. And now, respected translational, rotational & vibrational entropies have been optimized as 40.408, 28.687 & 18.111 calmol−1 K−1 for 5-methyluracil; and also total entropy of bio-molecule holds the value 87.207 cal-mol−1 K−1. Here the molecular dipole moment bears the value 4.5322 Debyes.
So far, these above thermodynamic values might be useful for the forthcoming related study of bio-molecules. The optimized data in Table 5 may be needful in an estimation molecular chemical reactions and thermodynamic values to related bio-molecule. This study has a fruitful for all of thermodynamic computations have been optimized only in the gas phase, but they could not be applied for the solution.
4 Conclusions
The three hydrogen atoms at pyrimidine ring of 5-methyluracil have found to be directly attached to the N atoms as well as bear the +ve APT & Mulliken charges. To all of five C atoms (C1, C3, C4, C5, C12), the C1 atom has highest +ve APT charge, but C12 atom holds a highest -ve Mulliken charge. Here, the out of three hydrogen atoms (H13, H14, H15), H15 has not had similar Mulliken atomic charge as two other atoms, but these hydrogen atoms have not same APT charges. The optimized geometrical structures represent that all of the atoms in the ring as well as on pyrimidine ring of molecule are lying in the same plane except three hydrogen atoms.
All the 39 modes have been assigned as 30 modes for pyriminide ring and 9 modes for methyl (CH3) group using observed Raman and IR spectra as well as computed vibrations by Gaussian-09 and GAR2PED. Here, Kekule vibration (ν14) of 5-methyluracil has been optimized at ~ 1150 cm−1 as well as a most popular ring breathing vibration at ~ 733 cm−1. So far, some of normal modes have been corrected by this study of PEDs for the reported study [18]. As well as here the mixing of the above corresponding modes with other modes has been shown in PEDs. The complexity of 9 modes for CH3 group has been clearly assigned to have the mixing with the other modes.
The computations of HOMO–LUMO & energy-gap have been made a suggested study for the charge distribution probability, charge transfer and pharmacological activeties in bio-molecule. The MEPs and ESPs plots represent that the locality of 2 oxygen atoms are −ve potential regions where these are active place for nucleophilic attacks, as well as the one corner of CH3 group has partially −ve potential. And such way, the surrounding of 3 hydrogen atoms at pyrimidine are most +ve area and these are the active location of electrophilic attack.
References
M D Kirnos, I Y Khudykov, N I Alexandrashikina and B F Vanyushin Nature 270 369 (1977)
F Michel, M Hanna, R Green, D P Bartel and J W Szostak Nature 342 391 (1989)
W. B. Person, Krystyna Szczpaniak, Vibrational Spectra and Structure, 20, Edited by J.R. Durig, Chap. 5 (1993)
S. Farquharson, C. Shendre, F. E. Inscore, Paul Maksymiuk, A.Gift, J. Raman Spectrosco. 36, 208 (2005)
M A Graham, G W Lockwood, D Greenslade, S Brienza, M Bayssas and E Gamelin Clin. Cancer Res. 6 1205 (2000)
T Nakajjma Word J. Surgery 19 570 (1995)
BE Billinghurst, R Yeung and GR Loppnow J. Phys. Chem. A 110 6185 (2006)
H O Kim, S K Ahn, A I Alves and I W Beach J. Med. Chem. 35 1987 (1992)
V K Rastogi, Chattar Singh, M Vaibhav Jain and Alcolea Palafox J. Raman Spectrosco. 31 1005 (2000)
V Krishnakumar and R Ramasamy Spectrochim. Acta 66A 503 (2007)
Soujanya Yarasi, Brant E Billinghurst and Glen R Loppnow J. Raman Spectrosco. 38 117 (2007)
M Alcolea Palafox, G Tardajos, A Guerrero-Martínez, V K Rastogi, D Mishra, S P Ojha and W Kiefer Chem Phys. 340 17 (2007)
V K Rastogi, M A Palafox, L Mittal, N Peica, W Kiefer, K Lang and S P Ojha J. Raman Spectrosco. 38 1227 (2007)
V K Rastogi, M Alcolea Palafox, A Guerrero-Martínez, G Tardajos, J K Vats, I Kostova, S Schlucker and W Kiefer J. Mol. Struct: THEOCHEM 940 29 (2010)
J S Singh J. Mol. Struct. 876 127 (2008)
J S Singh Spectrochim Acta A (G.B.) 117 502 (2013)
J S Singh Spectrochim. Acta A (G.B.) 130 313 (2014)
J S Singh Spectrochim Acta A (G.B.) 137 625 (2015)
L Lapinski, M J Nowak, D C Bienkob and D Michalska Phys. Chem. Chem. Phys. 4 1123 (2002)
Barbara Morzyk-Ociepa and Danuta Michalska Spectrochim Acta, A 59 1247 (2003)
W B Person, K Szczepaniak and Jozef S Kwiatkowski Int J. Quantum Chem. 90 995 (2002)
P M El’kin, M A Erman and O V Pulin J. Appl. Spectrosc. 73 4 485 (2006)
Myong Yong Choi and Roger E Miller J. Phys. Chem. A 111 2475 (2007)
M. J. Frisch et al., Gaussian 09, Revision C.01, G. Inc., Wallingford, CT, (2010)
R. Dennington II, T. Keith, J.Millam, GaussView, Version 4.1.2, Semichem Inc., Shawnee Mission, KS, (2007)
J M I Martin and C Van Alsenoy GAR2PED (Belgium: University of Antwerp) (1995)
J Cioslowski JACS 111 8033 (1989)
M Gussoni J. Mol. Struct. 141C 63 (1986)
W B Person and J H Newton J. Chem. Phys. 61 1040 (1974)
A Milano and C Castiglioni J. Mol. Struct. Theochem. 955 158 (2010)
M M C Ferreira and E Suto J. Phys. Chem. 96 8844 (1992)
M J S Dewar The Molecular Orbital Theory of Organic Chem (New York, NY (USA): Mc. Graw–Hill and Inc.) (1969)
Charles Alfred Coulson, R. McWeeny, Coulson's Valence, Oxford University Press, (1979)
H Pir, N Günay, D Avcı and Y Atalay Spectrochim. Acta A 96 916 (2012)
D E Manolopoulos, J C May and S E Down Chem. Phys. Lett. 181 105 (1991)
V. Dixit and R.A. Yadav Biochem Pharmacol (Los Angel), 4, 183 (2015)
L Padmaja, C Ravi Kumar, D Sajan, I H Joe, V S Jayakumar and G R Pettit J. Raman Spectrosc. 40 419 (2009)
S Sagdinc and H Pir Spectrochim Acta, A 73 181 (2009)
N Ozdemir, B Eren, M Dincer and Y Bekdemir Mol. Phys. 108 13 (2010)
P Politzer and J S Murray Theor. Chem. Acc. 108 13 (2002)
F J Luque, J M Lopez and M Orozco Theor. Chem. Acc. 103 343 (2000)
N Okulik and A H Jubert Internet electron J. Mol. Des. 4 17 (2005)
B Chattopadhyay, S Basu, P Chakraborty, S K Choudhuri and A K Mukherjee J. Mol. Struct. 932 90 (2009)
U C Singh and P A Kollman J. Comput. Chem 5 129 (1984)
M L Connolly Science 221 709 (1983)
S Muthu and U Maheswari Spectrochim. Acta 92 154 (2012)
Acknowledgements
Corresponding author is highly grateful to Prof. A. Pradhan, dept. of phys, IIT, Kanpur for the needful help.
Funding
No any funding agency has supported for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, J.S., Khan, M.S. & Uddin, S. Raman and IR spectra and DFT/G-09 molecular analysis with vibrational study and related other parameters of 5-methyluracil. Indian J Phys 97, 1037–1053 (2023). https://doi.org/10.1007/s12648-022-02480-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-022-02480-3