Abstract
In this study, rare earth element terbium doped Ni0.4Cu0.2Zn0.4TbxFe2−xO4 (where X = 0.0 to 0.1 in steps of 0.025) were synthesiesed by citric acid as catalyst via auto-combustion route. The structural, morphological, spectral and magnetic properties are explored and studied in detail. The ferrite samples were characterized by energy dispersive X-ray analysis (EDAX), X-ray diffraction of XRD, field emission scanning electron microscopy, transmission electron microscopy, Fourier transformed Infrared spectroscopy, UV–Vis spectroscopy (UV), Raman spectroscopy and vibrating sample magnetometer techniques. The EDAX analysis authenticates the formation of required stoichiometric ferrite samples. The formation of spinel cubic structure was validated by XRD technique. The lattice constant of all the ferrite was found to be declined with upsurge in Tb3+ content. The morphological investigations show that grain size of the ferrites remains in the extent 11–24 nm. The existence of two intrinsic IR absorption band of spinel lattice at υ1 (561–567) cm−1 and υ2 (534–542) cm−1 for NiCuZn ferrite system approves the presence of tetrahedral-A and octahedral-B sites over which the cations are distributed in spinel lattice. The UV–Vis measurements suggest that the as prepared and tempered samples at 900 °C of Ni0.4Cu0.2Zn0.4TbxFe2−xO4 (where X = 0.0 to 0.1 in steps of 0.025) ferrite possess diverse optical properties depending upon the composition of the samples. The Raman fingerprint affirms the spinel structure and variation in the cation distribution in synthesized nanomaterials. The frequency dependent dielectric constant of NiCuZn ferrites revealed the dielectric dispersion behavior in agreement with Maxwell–Wagner model. It is seen that saturation magnetization and magnetic moment of NiCuZn ferrites alters with the Tb3+ content and sintering temperature. The doping of rare earth element Tb3+ in NiCuZn ferrites significantly amends the structural and electromagnetic properties of the host material. Thus, Tb doped NiCuZn ferrites have major impact on structural, optical and magnetic properties, and it is good for multilayer chip inductor component application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites are pronounced materials for various applications such as information storage systems, magnetic core, electrochemical and microwave appliances in power generation and medical diagnostics, etc., due to their excellent electrical and magnetic properties [1,2,3]. These ferrites possesses elevated electrical resistivity saturation magnetization, high curie temperature and low eddy current losses make them convenient for multilayer chip inductor[4], recording heads, inductors, transformer cores, capacitor layers [5, 6]. Enrichment in structural and magnetic properties of Ni-spinel nano ferrites achieved, due to doping of magnetic, non-magnetic and rare earths ions [7,8,9]. Depending upon the element doped, cationic distribution altered which simultaneously influence on magnetic moments of synthesiesed nanomaterials [9]. Doping of rare earths metals in spinel ferrite leads to structural modifications in symmetry along with preserving the lattice strain. These reformations consequence into, amendment in properties of synthesiesed spinel ferrites [9]. Several investigators have made attempts to modify the structural, optical and magnetic properties of rare earth doped Ni-spinel ferrites [10, 11].
The NiCuZn ferrites doped with trivalent rare earth (RE) elements display good electromagnetic properties and adapted morphology. Slimani et al. [12] have given a report of considerable changes in magnetization data with Tm3+ substitution in NiCuZn ferrites. Patil et al. [13] have reported substantial decline in saturation magnetization and magnetic moment of NiCuZn ferrites with La3+ content. Roy et al. [14] observed enhanced initial permeability in (Ni0.25Cu0.20Zn0.55) La0.025Fe1.175O4 in ferrite powder along with controlled solvability in the framework of ~ 0.1 atom/unit. Kabbur et al. [15] have manufactured Ni0.25Cu0.30Zn0.45TbxFe2−xO4 (where x = 0.00 and 0.125 mol.) with glycerin as catalyst using sol–gel aided auto-combustion technique and noticed deviation in magnetic properties of synthesized ferrites due to super-exchange interactions. Due to difference in ionic radii Fe3+ and rare earth ion RE3+, deformation in spinel structure of ferrite occurs which advances properties of nanomaterials [16]. The 4f electrons in rare earth element are screened by outer 5s25p6 sub shells, which shows powerful spin orbit angular momentum coupling. To our knowledge, rarefied literatures was found on manufacture of Tb3+ doped NiCuZn ferrites and their influence on electromagnetic properties [17, 18].
As a host NiCuZn, we have chosen our prior reported stoichiometry Ni0.4Cu0.2Zn0.4Fe2O4 for Tb doping [19]. In the present work, a methodical study of Ni0.4Cu0.2Zn0.4TbxFe2−xO4 (where X = 0.0 to 0.1 in steps of 0.025) ferrites, synthesized by sol–gel auto-combustion is carried out. We report here, the morphological and electromagnetic possessions of synthesized ferrites.
1.1 Experimental details
1.1.1 Ferrite synthesis
The analytical grade chemicals nickel nitrate (Ni (NO3)3·6H2O), copper nitrate (Cu (NO3)3·3H2O), zinc nitrate (Zn (NO3)3·6H2O), ferric nitrate (Fe (NO3)3·9H2O) and rare earth element terbium (Tb (NO3)3·5H2O) were used. Citric acid (C6H8O7·H2O) was used as a fuel agent. Stoichiometric amounts of each chemicals necessary for the preparation of Ni0.4Cu0.2Zn0.4TbxFe2−xO4 (where X = 0.0 to 0.1 in steps of 0.025), were solubilized in 50 ml of Milli-Q water. A reaction mixture was prepared and obtained a homogeneous mixture upon stirring to ample time. The pH of the final solution was fine-tuned to 7 using liquid ammonia. The solution was warmed to get the viscous gel. The auto-combustion reaction, in conclusion converts the viscous gel into powder. The powder was then grounded and pre-sintered at 200 °C for 2 h. The pellets were fabricated using this powder by hydraulic press. Finally, the pellets were sintered at 200 °C for 2 h and used for characterization.
1.1.2 Characterization techniques
-
X-ray powder diffraction patterns are recorded employing CuKα (λ = 1.5406 Ǻ) line.
-
Fourier transform infrared spectra (FTIR) for the powders are recorded on i-Spirit, Schimadzu in the interval of 400–4000 cm−1 by KBr pellet means.
-
UV–Vis spectra were recorded using JASCO SpectrophotometerV-670.
-
The Raman spectra were recorded by means of in Via Raman from Renishaw with spatial resolution of ~ 1 µm.
-
Morphological observations were analyzed using by scanning electron microscopy (SEM Model: JEOL-JSM 6360) and transmission electron microscopy (TEM Model: PHILIPS CM 200) with operating voltages 20–200 kv with resolution 2.4 Ǻ.
-
Vibrating sample magnetometer (VSM Model: EG&G PAR 4500) was used to record hysteresis behavior of ferrites.
-
LCR-Q meter (Model: HIOKI IM3536) was used to measure dielectric parameters with frequency in the range 20 Hz–8 MHz at room temperature.
2 Results and discussions
2.1 X-ray diffraction
The X-ray diffraction patterns recorded for the synthesized ferrite nanocrystals are shown in Fig. 1(a) and (b). The existence of the most intense peaks for all the samples indexed as (220), (311), (222), (400), (422), (511) and (440). These peaks are found to associate with single-phase cubic spinel structure (JCPDS card no. 39-1277).
The lattice constant was observed to vary with upsurge in Tb3+ content due to its larger ionic radius (0.98 Ǻ) as compared to Fe3+ (0.67 Ǻ). There are other factors like electronic configuration, an ionic radius which affects rare earth ions in spinel ferrites and causes variation in lattice constant [6]. The minor phases of TbFeO3 at 360 are discovered in accordance with JCPDS card No: 33-0661 [20, 21].
Figure 2 shows the crystallite size was observed to enhance with the sintering temperature and increase of terbium concentration. Usually rare earth ions are present at the grain boundaries which causes barrier in the grain growth and that results into increase in crystal size and unit cell parameters. The computed values of lattice parameter are in close agreement in standard data.
2.2 Surface morphology: (EDX, SEM and TEM)
Figure 3(a) and (b) shows the energy dispersive x-ray spectra (EDX) of Tb3+ substituted NiCuZn ferrites. The elemental analysis by x-ray peaks approves the elements that have been utilized in the fabrication and as per their stoichiometry. Compositional atomic percentage of all the prepared ferrite samples is given in Tables 1 and 2. The entire EDX peak corresponds to Ni, Cu, Zn, Fe, Tb and O elements.
The purity was confirmed by the EDX profile and quantitative results. The EDX results show the presence of Ni, Zn, Cu, Fe, Tb, O elements in the sample with expected ratio, and no other extra elements were observed; this validate the formation of pure NiCuZnTbFe2O4.
The scanning electron microscope images of the samples achieved after heat treatment at 500 °C and 900 °C presented in Fig. 4(a) and 4(b) shows the nanocrystalline character, and crystallinity increases as annealing temperature increases. The SEM images show appearance of spherical structure with a large number of pores, and this might be due to the fact that the copper, nickel and zinc are non-miscible with each other and terbium. This may be ascribed to the fact that the growth of the crystal takes place in distinct orientations. The crystallographic images clearly signify that the particles are assembled on top of each other because of their conjoint magnetic interactions. The ferrite composition comprises small cubic grains. As Tb content increases the grain shape gradually converts into homogeneous cubic shape.
The calculation of average grain size (d) is made from method of linear intercepts [22]. Average grain size (d) was observed in the scale of 10–25 nm.
Figure 5(a) and 5(b) shows micrographs of TEM of nanocrystalline ferrite for as prepared and annealed samples at 500 °C and 900 °C. The crystalline size upsurges with increase in temperature which is in agreement with TEM data.
According to Fig. 5(a) and 5(b), the particle is seen to be in spherical appearance and unevenly distributed with crystallite size of 10 to 20 nm, and electron diffraction pattern confirms the spinel structure of ferrite with numerous lines (220), (311), (222), (400), (422), (511) and (440) depicted from TEM data. Agglomeration of nanocrystals may be due to the tendency of nanoparticles to aggregate, to achieve a low free energy state by bring down the specific superficial area by minimizing the interfaces with other particles. Average particle size calculated from TEM images is similar with the crystallite size obtained from XRD.
2.3 Spectroscopic studies
2.3.1 Fourier transforms Infrared spectral analysis
The obtained powders of ferrite material were researched by Fourier transform Infrared spectroscopy. The FTIR spectra in the frequency range 400–4000 cm−1 are shown in Fig. 6(a) and 6(b). The difference in positions of the bands results due to differences in the distances for the octahedral and tetrahedral ions. The high frequency band υ1 lies in the range 561.23–567.07 cm−1 while the low frequency band υ2 lies in the range 534.28–542.00 cm−1. According to the study of vibration spectra by Waldron, the υ1 band arises due to intrinsic vibrations of the tetrahedral groups and υ2 band due to the intrinsic vibrations of octahedral groups. The change in the bond length of Fe3+–O2− changes band positions of υ1 and υ2 [23]. These two bands are influenced by stoichiometry, synthesis technique and sintering temperature. Their positions can be altered due to change in bond length of Fe3+–O2− complexes at tetrahedral-A site (1.89 Ǻ) and octahedral-B site (1.99 Ǻ). The expansion in the unit cell pushes Fe3+ ions away from O2− ions with increase in Tb3+ ions concentration, and it results in the increase in bond length. Beyond X = 0.050, the bond length decreases due to decrease in lattice constant and results into secondary phase formation of TbFeO4 and governs the grain growth at boundaries.
2.3.2 UV–Vis analysis
Figure 7(a) and (b) illustrates the optical absorption spectra for Ni0.4Cu0.2Zn0.4Tb0.1 Fe1.9O4 nanocrystals in the UV interval for the formulated samples at room temperature and heated at 500 °C and 900 °C. There are three bands of absorptions found at 481 nm, 490 nm and 573 nm in correlative with the XRD conclusions as depicted in Table 3. This absorption build upon the constitution and measure of heat content of the test sample as represented in Fig. 7(a) and (b). Three kinds of electronic shift take place in the spectra of optical absorption of Fe3+ substances [24]. As it is disclosed from Fig. 7(a) and (b) that for x = 0.1 composition, the electronic transition for the charge carry-over in the wavelength lies in the range of 480–580 nm prevails for the optical absorption characteristics of the test sample while the ligand field changeover in the interval of 755–770 nm prevails for the optical absorption features. The band gap energy is calculated by using formula
where h represents Planck’s constant, c is the velocity of light in vacuum, and \(\lambda\) is wavelength. The band gap decreases from 2.579 to 2.164 eV as sintering temperature increases for wavelength max1, and it decreases from 1.633 to 1.610 eV for wavelength max2. The outcomes show composition and annealing measure of heat content of the test sampling put major impact on its optical characteristics [25].
2.3.3 Raman study
Raman spectroscopy is non-devastating material characterization technique, and it is very responsive to structural disorder. It provides an important tool to investigate the surface and structural properties of the material. Figure 8(a) and (b) shows the Raman spectra of all samples annealed at the temperature 500 °C and 900 °C in the frequency range 200–1200 cm−1 at room temperature. Three first order Raman active modes (A1g + Eg + T2g) are observed at ambient conditions. Tables 4 and 5 show all the observed vibration frequencies for different compositions. The A1g mode is caused by symmetric stretching of oxygen atoms along Fe–O bonds. Eg mode is due to symmetric bending of oxygen with respect to Fe, and T2g mode is because of symmetric stretching of Fe [26]. In cubic spinel ferrites, active modes at above 600 cm−1 usually relate to the motion of oxygen in tetrahedral group (A-site) [27], and the other low frequency modes signify the characteristics of the octahedral group (B-site) of ferrites [28,29,30]. Enlargement of modes in Raman spectra is caused by the strain advancement and shrinking in grain size [30, 31]. The enlargement and shift of Raman spectra are also caused by the scattering of nanocrystalline ferrite phonons with q > 0. The mass deviation between the four ions (Ni2+, Zn2+, Cu2+, Fe3+) separates the A1g mode in different energy measures. Out of all the metal ions, the most lighter ion Ni2+ responds around 684.07 cm−1 for X = 0.025, and the most heavier ion Fe3+ responds nearly at 638 cm−1 and intermediate mode of Cu2+ ion around 547 cm−1. The doping of rare earth element terbium ion and its concentration, alter the relative intensity and peak position of the modes. The Raman frequency dependent on Fe (NiCuZn)-O and bond length. The intensity of the highest wavelength Raman mode around 697.25 cm−1 reduces with terbium content up to 671.87 cm−1. Phonons with q > 0 take part in Raman scattering process and results into peak position and enlargement of the peak width. The Raman signal intensity step up as sintering temperature rises as shown in Fig. 8(a) and (b). The shift toward lower wave number is the result of the crystalline disorder, and sign of presence of grain boundaries which are large as compared to synthesized nanomaterials. Also, the enlargement of the Raman signal affirms the crystalline disorder. These two effects are generally found in polycrystalline materials and are assigned to the confinement of optical phonons in small crystalline particles [32].
2.4 Magnetic characterization
2.4.1 Vibrating sample magnetometer (VSM)
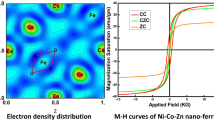
VSM analysis of Tb3+ substituted NiCuZn ferrites is shown in Fig. 9. The typical soft magnetic behavior of the synthesized ferrite material is observed from hysteresis loop. The hysteresis loops of the investigated samples are used to determine magnetic parameters such as the saturation magnetization (MS), coercivity (Hc), remnant magnetization (Mr) and magneton number (nB). Tb3+ incorporation has strongly affected the magnetic parameters. The coercivity is also influenced by crystallite and particle size. When volume of particle reduces in such a way that it reduces in a single domain then its change in magnetization takes place by rotation, and this varies coercivity. The coercivity is a measure of magnetization reversal process under the action of applied field and depends strongly on particle size and crystalline anisotropy constant [33, 34]. The experimental magnetic moment nB (obs) was calculated using the relation
The saturation magnetization (Ms) for pure sample (x = 0.00) at room temperature was 34.76 emu/gm. The saturation magnetization for Tb3+ substituted ferrite samples was observed to fluctuate from 15 to 89 emu/gm with increase in Tb3+ concentration and sintering temperature. The decrease in the magnetization was also observed with increase in Tb3+ concentration. Though the magnetic moment for Tb3+ (9.5 μB) is higher than the replaced Fe3+ (5.0 μB) ions. This happens due to effects of ionic radii, and several results were reported by researchers [35,36,37]. Ionic radius of rare earth ion Tb3+ (0.93 Ǻ) is larger which results into structural alteration and magnetization in spinel ferrites.
Remnant magnetization (Mr) values vary between 5.92 to 26.56 emu/gm and point out the soft hysteresis nature of the synthesized ferrite samples. The calculated nB values were listed in Table 6. The nB values for pure NiCuZn ferrites at 500 °C are 3.76 and at 900 °C is 1.22. The nB values show inhomogeneous trend with increase in Tb3+ substituting level at 500 °C and 900 °C. The variation between nB and Ms was observed which leads to the magnetization of the B-site strengthening and then weakening for corresponding composition. Hence the A–B exchange interaction is strengthening and weakening with Tb3+ substitution and dislocate the structure of collinear spins in the NiCuZn nano synthesized ferrite systems at the B-site [38]. The coercivity shows variation tendency of crystallite site. The values of squareness ratio ‘R’ are shown in Table 6. Usually the nanoparticles are considered to be in multimagnetic domain for R ≥ 0.5 and in single magnetic domain when R < 0.5 [39, 40].
2.4.2 Dielectric parameters (ε′, ε′′, tanδ)
The dielectric constant of the sample can be calculated using the equation,
where A = area of pellet, c = Capacitance measured by LCR, d = Thickness, ε0 = Absolute permittivity.
Koop’s and Maxwell Wagner theory well elucidate the dielectric behavior of ferrites [41], the variation of Tb concentration on dielectric properties of mixed ferrites shown in Fig. 10. It shows the dielectric constant decreases with the increase in frequency the reason behind of it is with the increase in temperature results less number of pairs for hole and electron hopping as a result charges getting build up across the grain boundaries and resistance increases; this may be the reason behind low dielectric constant value in Tb rich concentration [42].
The variation of tanδ with frequency can be seen from Fig. 11, and that tanδ decreases with employed frequency. This may be due to decrease in magnetization which decreases domain wall motion as magnetization(emu/g) increases with rare earth doped Terbium concentration. Thus, losses are found to decrease [30].
It was reported in [35] that the loss factor was considered to be attributed to domain wall resonance. At higher frequencies, losses are found to be low since domain wall motion is suppressed, and magnetization is pushed to change by rotation for Terbium substituted NiCuZn ferrite.
Figure 12(a) and (b) shows the dielectric permittivity decreases with increase in frequency, the reason behind is, with the increase of Tb concentration the Fe2+ ions forms a minor number of pairs for hole and electron hoping consequently charges getting buildup across the grain boundaries and resistance intensifies and hence permittivity declines.
Figure 12(b) shows the variation of permittivity with frequency at room temperature. It shows that permittivity for all the samples declines with increase in frequency and ultimately becomes constant at elevated frequencies. This decrease in permittivity is more rapid in the depleted frequency region and becomes sluggish as the applied frequency increases. This behavior is subjected to dielectric polarization under the application of AC field.
3 Conclusion
Ni0.4Cu0.2Zn0.4Tb0.1 Fe1.9O4 (where X = 0.0 to 0.1 in steps of 0.025) was developed by sol–gel auto-combustion technique. The developed material demonstrates distinct crystal structure that corresponds with the standard reference spectra. It shows the partial immersion of Tb3+ ions in the spinel lattice, and other ions were reversed into secondary phases on grain boundaries. The lattice constant ‘a’ decreased with increasing Tb3+ ion concentration. The crystalline size is in the range of 11–24 nm. The presence of Ni, Zn, Cu, Fe, Tb and O elements is noticed with predictable ratio without any extra elements. Morphological studies indicated an agglomeration of nano materials in fabricated ferrites. Structural distortion is seen in lattice, due to larger Tb3+ ions substituted smaller Fe3+ ions resulting in magnetization due to cation distribution at sub-lattice. This is correlated with observations from magnetic hysteresis loops. The SAED pattern depicts that after annealing and rare earth Tb3+ ion substitution, the nano particles are united.
The observed vibrational modes correspond to metal–oxygen bonding. The samples annealed at 500 °C and 900 °C possess dissimilar optical properties depending upon the composition of the samples. The presence of symmetric and asymmetric stretching as well as symmetric bending in the materials is observed.
The decrease in the initial permeability was found with increase in concentration of Tb3+ ions. Magnetic analysis designates a typical change in magnetization with Tb doping concentration with maximum saturation magnetization (Ms) at X = 0.1 annealed at 900 °C. Dielectric dispersion and permittivity studies were explicated on the base on Koop’s phenomenological theory. The results obtained of Tb doped NiCuZn ferrites are highly convincing for MLCI applications due to low sinter ability (< 900 °C), soft magnetic nature, high permeability and low dielectric loss at high frequency. Moreover, fabricated fine-grained microstructure of ferrite is capable for MLCI’s to use it as a thinner, internal ferrite layers.
References
H Harzali et al J. Magn. Magn. Mater. 419 50 (2016)
Y Slimani, E Hannachi, F Ben Azzouz and M Ben Salem J. Superconduct. Novel Magn. 32 511 (2019)
A. Baykal, N Kasapoğlu, Y Köseoğlu, M S Toprak and H Bayrakdar J. Alloys Compd. 464 514 (2008)
Z Yue, L Li, J Zhou, H Zhang, Z Ma and Z Gui J. Mater. Lett. 44 279 (2000)
S Ramesh B Chandra Sekhar, P S V Subba Rao and B Parvatheeswara Rao Ceram Int. 40 8729 (2014)
N Varalaxmi and K V Sivakumar Mater. Sci. Eng. 184 88 (2014)
M Mozaffari, J Amighian and E Darsheshdar J. Magn. Magn. Mater. 350 19 (2014)
P Yaseneva, M Bowker and G Hutchings Phys. Chem. Chem. Phys. 13 18609 (2011)
K Srinivasamurthy, V Angadi, S Kubrin, S Matteppanavar, P M Kumar and B Rudraswamy Ceram. Int. 44 18878 (2018)
A. A. Ati, Z. Othaman and A. Samavati J. Mol. Struct. 1052 177 (2013).
T Vigneswari and P Raji J. Mol. Struct. 1127 515 (2017)
Y Slimani, M A Almessiere, M Sertkol, S E Shirsath, A Baykal, M Nawaz, S Akhtar, B Ozcelik and I Ercan Ultrasonics - Sonochemistry 57 203 (2019)
B B Patil, A D Pawar, D B Bhosale, J S Ghodake, J B Thorat and T J Shinde J. Nanostruct. Chem. 9 119 (2019)
P K Roy and J Bera Mater. Res. Bull. 42 77 (2007)
S M Kabbur et al J. Magn. Magn. Mater. 473 99 (2019)
S E Shirsath, R H Kadam, S M Patange, M L Mane, A Ghasemi and A Morisako Appl. Sci. Lett. 100 042407 (2012)
S E Shirsath, S M Patange, Y Yasukawa, X Liu and A Morisako IEEE Trans. Magn. 50 1 (2014)
Y-J Siao and X Qi J. Alloys Compoud. 691 672 (2017)
V V Awati, S M Rathod, S E Shirsath and M L Mane J. Alloys Compoud. 553 157 (2013)
M Juniad et al J. Magn. Magn. Mater. 419 338 (2016)
N Rezlescu and E Rezlescu J. Phys. IV FRANCE 7 C1-553 (1997)
K Chandrababu and W Madhuri J. Magn. Magn. Mater. 420 109 (2016)
R D Waldron Phys. Rev. 99 1727 (1955)
Skoog et al Principles of Instrumental Analysis, vol. 6 (Thomson Books Co. Ltd.) p 169 (2007)
G K Pradhan and K M Parida ACS Appl. Mater. Interfaces 3 317 (2011)
O N Shebanova and P Lazor J. Solid State Chem. 174 424 (2003)
Z Wang, D Schiferl, Y Zhao H St and C O Neill J. Phys. Chem. Solids 64 2517 (2003)
K Mohit et al 407 935 (2012)
Y Qu, H Yang, N Yang, Y Fan, H Zhu and G Zou Mater. Lett. 60 3548 (2006)
P Chandra Mohan, M P Srinivasan, S Velmurugan and S V Narasimhan J. Solid State Chem. 184 89 (2011)
T Yu, S CTan, Z X Shen, L W Chen, J Y Lin and A K See Appl. Phys. Lett. 80 2266 (2002)
J Pal Singh, R C Srivastava, H Agarwal and R Kumar J. Raman Spectrosc. 42 1510 (2011)
A B Nawale, N S Kanhe, K R Patil, S V Bhoraskar, V L Mathe and A K Das J. Alloys Compds. AIP Conf. Proc. 1591 603 (2014)
M M Rashad, E M El-Sayed, M M Moharam, R M Abou-Shahba and A E Saba J. Alloys Compd. 486 759 (2009)
T Sodaee, A Ghasemi, E Paimozd, A Paesano Jr. and A Morisako J. Elect. Mater. 42 2771 (2013)
F Cheng et al J. Appl. Phys. 85 2782 (1999)
C Murugesan and G Chandrasekaran RSC Adv. 5 73714 (2015)
J P Singh, H Kumar, A Singhal, N Sarin, R C Srivastava and K H Chae Appl. Sci. Lett. 2 3 (2016)
M A Almessiere et al Ultrason. Sonochem. 58 104654 (2019)
Y Slimani et al J. Mater. Sci. Mater. Electron. 30 9143 (2019)
R S Devan, Y D Kolekar and B K Chougule J. Phys. Condens. Matter. 18 9809 (2006)
E Veena Gopalan Ph.D. thesis (Department of Cochin University of Science and Technology) (2015)
Acknowledgement
This work was supported by BOD, Savitribai Phule Pune University, Pune for financial assistance under the research project (Proposal No: 15SCI000326). Authors would like to acknowledge DST for the accessibility of instruments procured under DST-FIST program at Chandmal Tarachand Bora College, Shirur File No: SR/FST/COLLEGE/068/2017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Awati, V., Badave, K. & Bobade, D. Effect of Tb3+ substitution on structural, optical and magnetic properties of NiCuZnFe2O4 prepared by sol–gel route. Indian J Phys 96, 89–101 (2022). https://doi.org/10.1007/s12648-020-01955-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-020-01955-5