Abstract
The neurotrophin growth/differentiation factor 5 (GDF5) is studied as a potential therapeutic agent for Parkinson’s disease as it is believed to play a role in the development and maintenance of the nigrostriatal system. Progress in understanding the effects of GDF5 on dopaminergic neurones has been hindered by the use of mixed cell populations derived from primary cultures or in vivo experiments, making it difficult to differentiate between direct and indirect effects of GDF5 treatment on neurones. In an attempt to establish an useful model to study the direct neuronal influence of GDF5, we have characterised the effects of GDF5 on a human neuronal cell line, SH-SY5Y. Our results show that GDF5 has the capability to promote neuronal but not dopaminergic differentiation. We also show that it promotes neuronal survival in vitro following a 6-hydroxydopamine insult. Our results show that application of GDF5 to SH-SY5Y cultures induces the SMAD pathway which could potentially be implicated in the intracellular transmission of GDF5’s neurotrophic effects. Overall, our study shows that the SH-SY5Y neuroblastoma cell line provides an excellent neuronal model to study the neurotrophic effects of GDF5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterised by the selective and progressive loss of the dopaminergic neurones of the substantia nigra pars compacta (SNpc) and the presence of intraneuronal proteinaceous inclusions known as Lewy bodies within the surviving neurones (Braak et al. 2003; Cookson 2005). Clinical symptoms usually appear when ~50% of dopaminergic neurones in the SNpc are lost, leading to a depletion of dopamine in the corpus striatum. Most of the available therapies aim to reduce the symptoms of the disease but cannot stop the progressive neurodegeneration or promote survival of the remaining neurones. Neuroprotective therapy could offer ways of preserving these neurones and, when administered with symptomatic treatments, could improve the long-term outcome for patients. Several compounds are being investigated as potential neuroprotectants (Toulouse and Sullivan 2008).
A group of dimeric proteins known for their neurotrophic properties has recently attracted much attention. Glial cell line-derived neurotrophic factor (GDNF) and neurturin (NRTN) have produced potent neurotrophic effects on dopaminergic neurones in vivo and in vitro. They have been shown to protect cultured dopaminergic neurones from a variety of insults (Akerud et al. 1999; Horger et al. 1998; Lin et al. 1993) and prevent 6-hydroxydopamine-(6-OHDA) and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-(MPTP) induced nigrostriatal damage in animal models (Gasmi et al. 2007a, b; Herzog et al. 2007; Kordower et al. 2006). Intraputamenal injections of GDNF were initially successful in open-label trials but this was not replicated in a randomized double-blind trial (Gill et al. 2003; Patel et al. 2005; Slevin et al. 2005). Differences in the selection of patients, catheter design and drug dosage may explain the discrepancy. Intraputamenal injection of an adeno-associated virus type 2 (AAV2)-based NRTN expression vector initially proved to be very efficient. Results from an open-label trial showed that 6 months after receiving the injection, patients showed a 25% reduction in their “off” medication UPDRS score, a 50% reduction in their “off” time and an increase in periods without dyskinesia (Marks et al. 2006). However, an 18 month assessment of a double-blind trial of intraputamenal AAV2-NTN showed only minor clinical improvements (Bartus 2009).
Growth/differentiation factor 5 (GDF5) is a member of the TGFB superfamily, that is related to GDNF and NRTN. In its active state, GDF5 forms a dimer that has binding affinity for various cell surface receptors; the bone morphogenetic proteins (BMP) receptors and the orphan receptor ROR2. Binding of GDF5 to BMPR1a or BMPR1b recruits BMPR2 to form a serine/threonine kinase receptor dimer that activates the SMAD family of nuclear transcription factors, SMAD 1/5/8 and the co-factor SMAD4 (ten Dijke et al. 2000). GDF5 has higher affinity for BMPR1b than BMPR1a (Nishitoh et al. 1996). Alternatively, the BMPR1b receptor can form a heterodimer with the ROR2 tyrosine kinase receptor in the presence or absence of GDF5 (Sammar et al. 2004). It has been shown that formation of this receptor complex inhibits SMAD signalling, most likely by sequestering BMPR1b and therefore providing a negative modulation loop (Sammar et al. 2004, 2009).
GDF5 is expressed in many regions of the brain, including the midbrain. It is expressed in the ventral mesencephalon (VM) from embryonic day (E) 12, peaking at E14, the time of peak dopaminergic neurogenesis (Clayton and Sullivan 2007; O’Keeffe et al. 2004b). In vitro studies have shown that GDF5 treatment of VM cultures promotes the survival and the morphological differentiation of dopaminergic neurones and protects cultured dopaminergic neurones from MPP+-induced cell death, suggesting that it may play a role in the development and maintenance of the nigrostriatal system (Krieglstein et al. 1995; O’Keeffe et al. 2004a). In vivo studies using the 6-OHDA-lesioned rat model of PD have shown that intracerebral injection of GDF5 protects the nigrostriatal pathway (Hurley et al. 2004; Sullivan et al. 1997, 1999). Furthermore, GDF5 was found to be as effective as GDNF in promoting the survival and functional integration of embryonic VM grafts in 6-OHDA-lesioned rats (Sullivan et al. 1998).
It remains unclear whether GDF5’s neurotrophic effects are mediated by direct actions on the dopaminergic neurones or are the results of secondary signalling from the surrounding glia. Most experiments conducted so far involved primary cultures or the use of animal models which do not allow separation of these effects. An article by Wood et al. (2005) where primary cultures were treated with 5-FdU to prevent the growth of glial cell suggested that at least part of the dopaminergic neurotrophic effects of GDF5 maybe through direct action on neurones; but otherwise, very little information is available. The experiments presented here aimed to assess the direct effects of GDF5 in a neuronal model, the SH-SY5Y cell line. Our results show that some of the neurotrophic effects of GDF5 can be reproduced in this model, mainly its capacity to promote neuronal survival and differentiation and that the SH-SY5Y is an ideal system for studying the effects of GDF5 on these parameters.
Materials and Methods
Cell Culture
SH-SY5Y cells were maintained in Dulbecco’s Modified Eagle’s Medium:Ham’s F12 mixture (1:1, DMEM:F12, Sigma-Aldrich) supplemented with 10% foetal calf serum (Sigma-Aldrich), 100 mM l-glutamine (Sigma-Aldrich), 100 U/ml penicillin and 10 μg/ml streptomycin (Sigma-Aldrich) in a 37°C humidified atmosphere supplemented with 5% CO2. Where indicated, the cells were treated with 100 ng/ml recombinant human GDF5 (rhGDF5, Biopharm GmbH) or 10 μM retinoic acid (RA, Sigma-Aldrich).
Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
For RT-PCR, total RNA was extracted from SH-SY5Y. RNA was extracted using the method described by Berk and Sharp (Berk and Sharp 1977). RNA samples were treated with RQ1 DNAse (Promega) for 20 min at room temperature before being neutralised. Complementary DNA (cDNA) synthesis was performed using 1 μg RNA following the ImProm-II™ kit protocol (Promega Inc.). Negative controls where the reverse-transcriptase was left out of the reaction were also prepared (RT−). PCR was performed using the primers and conditions indicated in Table 1. Aliquots of the reactions were electrophoresed on 1.5% agarose gels and photographed.
Immunocytochemistry
10,000 SH-SY5Y cells were seeded in 24-well plates and grown for 7 days in the presence of GDF5 (100 ng/ml). Control cultures were left untreated. Cells were fixed in 4% paraformaldehyde or ice-cold methanol for 15 min followed by permeabilization in 0.2% Triton-X. Immunodetection was performed using the following antibodies: mouse monoclonal antibodies to BMPR1A (1:500, R&D Systems), BMPR1B (1:000, R&D Systems), BMPR2 (1:1000, R&D Systems), ROR2 (1:500, R&D Systems), TH (1:500, NovoCastra Laboratories), and rabbit polyclonal antibodies to DAT1 (1:500, Santa Cruz biotechnology), SMAD 1/5/8 (1:1000, Santa Cruz biotechnology) and phospho-SMAD 1/5 (1:1000, Cell Signalling Technology). Alexa 488-conjugated donkey anti-rabbit and Alexa 594-conjugated donkey anti-mouse secondary antibodies (1:1500, Molecular Probes) were used. Cells were counterstained with DAPI. The cells were imaged on an Olympus IX70 inverted microscope. The fluorescence intensity of individual cells stained for phospho-SMAD 1/5/8 was measured using the Image J analysis software (Rasband, WJ, http://rsb.info.nih.gov/ij/). The relative fluorescence intensity was calculated as the intensity of individual cells after substraction of the background noise. Results were compared using a Student’s t-test.
Immunoblotting
Total protein extracts were prepared by homogenizing cells in protein extraction buffer (70 mM TRIS–HCl pH 6.8, 10% glycerol, 3% SDS and 700 mM 2-mercaptoethanol) followed by centrifugation at 14000 g. 20 μg were electrophoresed on 10% SDS–polyacrylamide gels and were transblotted to nitrocellulose membranes. Immunodetection was performed using rabbit polyclonal antibodies to SMAD 1/5/8 (1:2000, Santa Cruz biotechnology) and phospho-SMAD 1/5/8 (1:2000, Cell Signalling Technology) or rabbit polyclonal anti-actin (1:10000, Santa Cruz Biotechnology) antibodies. Results were visualized by chemiluminescence.
Cell Growth Assay
50,000 SH-SY5Y cells were seeded in 6-well plates. Cells were collected 2, 4 and 7 days after seeding, stained with trypan blue and counted using a haemacytometer. Growth curves were plotted and differences were assessed by performing a one-way ANOVA followed by a Dunnett’s post-hoc test.
Measurement of Cellular Morphology
10 to 15 microscopic fields were randomly selected from three independent experiments, photographed using an Olympus IX70 inverted microscope and all cells in each photograph were measured. Neurite branching was assessed by counting the numbers of “nodes” per cell. Primary nodes were considered branches from the cell body, and secondary nodes were considered branches of primary neurites. The length of the neuritic arborisation was estimated using standard stereological procedures (Mayhew 1992). A line grid was superimposed on the microscopic images and the number of times a neurite intersects the grid was recorded. The neurite length was calculated using the following formula;
where a is the number of times the neurite intersect the grid lines, T is the distance between the gridlines on the magnified image (taking into account the magnification factor). Results were compared using a Student’s t-test.
Neuroprotection Assays
100,000 cells per well were seeded in 24-well plates and grown in the presence or absence of rhGDF5 (100 ng/ml). After 24 h, half of the wells were treated for 1 h with 50 μM 6-OHDA. The cells were then rinsed three times in saline solution and fed either with culture medium or culture medium supplemented with 100 ng/ml rhGDF5 for an additional 24 h. This treatment modality produced six experimental groups (Fig. 4a). Following the second incubation, an MTT assay was performed to assess cell viability. Thiazolyl Blue Tetrazolium Bromide (MTT) was added to cells at a concentration of 0.5 mg/ml in the culture medium and incubated for 3 h. Cell culture medium was removed and the cells were lysed using a mixture of Isopropanol:HCl (24:1). Absorbance was measured at 540 nm with a reference wavelength of 690 nm. The mean of 9 independent samples were calculated for each group and results were compared using an ANOVA followed by Tukey’s post-hoc analysis.
Results
GDF5 Signalling Machinery is Expressed in SH-SY5Y Cells
To assess their suitability as a neuronal model to further study the neurotrophic properties and downstream signalling pathways of GDF5, human neuroblastoma SH-SY5Y cells were tested for the expression of the various GDF5 receptors, BMPR1a, BMPR1b, BMPR2 and ROR2. RT-PCR experiments revealed that the mRNA for each of the four receptors is expressed in SH-SY5Y cells (Fig. 1a). Immunocytochemistry confirmed that all four receptors are expressed on the surface of SH-SY5Y cells; although cell surface expression of BMPR1a was low compared with the other receptors (Fig. 1b). Results also showed that the intracellular machinery for signal processing, SMAD proteins 1, 5 and 8, are present in both unphosphorylated (inactive) and phosphorylated (active) forms (Fig. 2a). Controls performed for each secondary antibody confirmed the specificity of the staining (data not shown).
Expression of GDF5 receptors in SH-SY5Y cells. a Representative gel electrophoresis of PCR products for BMPR1a, BMPR1b, BMPR2 and ROR2. MW Molecular weight marker, + RT-positive reaction, − RT-negative control. b Representative images showing immunocytochemical staining for the cell surface receptors for GDF5 (BMPR1a, BMPR1b, BMPR2, ROR2) as well as a negative control in which the primary antibody was omitted. The cells were counterstained with DAPI. Phase contrast images were also taken at 60× magnification (scale bar 10 μm)
Expression of SMAD proteins in SH-SY5Y cells. a Representative images showing immunocytochemical staining for SMAD 1/5/8 and phosphorylated SMAD 1/5/8 proteins in untreated SH-SY5Y and GDF5-treated (100 ng/ml for 7 days) cells. Images were taken at 20× magnification (scale bar 50 μm). b The relative immunofluorescence intensity of untreated and GDF5-treated cells expressing phospho-SMAD 1/5/8. Data are presented as the mean relative fluorescence intensity ± SEM. (***P < 0.001 vs. untreated cells, Student’s t-test, n = 120 cells for each group). c Western blot showing the effects of GDF5 treatment on SMAD proteins phosphorylation
Given that the cells express the receptors and downstream transcription factors necessary for GDF-5 signalling, we next assessed whether GDF5 induced a physiological effect in SH-SY5Y cells, by quantifying the relative intensity of phosphorylated SMAD 1, 5, 8 present in the nucleus. Results showed that treatment with 100 ng/ml GDF5 for 7 days resulted in a significant (35%) increase in phospho-SMAD 1/5 signal intensity compared with untreated cells (P < 0.001, Fig. 2a and b). An immunoblot confirmed these findings. Protein extracts from GDF5-treated and untreated SH-SY5Y cells were probed with antibodies against SMAD 1/5/8 or their phosphorylated form. Comparison to an actin control revealed an increase in phosphorylated SMAD proteins in the GDF5-treated sample (Fig. 2c). These data suggest that GDF5 should be able to actively induce changes in gene expression as a result of nuclear accumulation of phospho-SMAD proteins, and this may induce phenotypic changes in the cells.
GDF5 Inhibits the Growth of SH-SY5Y Cells
To assess any potential phenotypic changes, we examined the growth and differentiation of the SH-SY5Y cells. Firstly we examined the growth rates of these cells in response to retinoic acid and GDF5. To assess the capacity of GDF5 to induce post-mitotic growth arrest of SH-SY5Y cells, growth rates were measured over a period of 7 days in the presence of 100 ng/ml GDF5. We used cells grown in 10 μM RA as a positive control, as RA has been shown to induce the post-mitotic neuronal differentiation of SH-SY5Y cells and inhibit their growth rates (Pahlman et al. 1995; Pahlman et al. 1984). As expected, RA strongly inhibited the growth of SH-SY5Y cells after 4 and 7 days of treatment (P < 0.001, Fig. 3). Interestingly, our results show that treatment with GDF5 also resulted in growth inhibition (Fig. 3). While the difference is not as strong as the one elicited by RA, it is nonetheless significant compared with the control after 4 days and remains significant at 7 days (P < 0.05, Fig. 3).
GDF5 Stimulates Neuronal Differentiation of SH-SY5Y
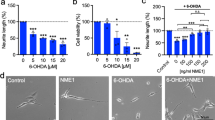
While the results above maybe an effect of GDF5 on cellular differentiation, they could also be a consequence of mitotic inhibition without differentiation. One of the morphological features of maturing neurones is the development of a neuritic arborisation and GDF5 has been shown to promote neurite outgrowth in primary VM cultures (O’Keeffe et al. 2004a). To assess the effect of GDF5 on neuronal differentiation, we analysed the number of primary and secondary neurites on GDF5-treated, RA-treated and untreated SH-SY5Y cells (Fig. 4a). Our results show that after 7 days of treatment, 100 ng/ml GDF5 induced an increase in the number of neurites compared with untreated controls (Fig. 4b). While the difference remained close to significance levels for the number of primary neurites (P = 0.067), it reached statistical significance for second order neurites (P < 0.05, Fig. 4b). The control retinoic acid-treated cells showed a significant increase in the number of primary neurites (P < 0.05) while there was a non-significant increase in the number of secondary neurites (Fig. 4b). Furthermore, GDF5-treated cells showed a 27% increase in total neurite length compared with control cells (P < 0.05, Fig. 4c) while the RA-treated cells showed a 42% increase in total neurite length (P < 0.001). Altogether, these results suggest that GDF5 induces differentiation rather than simply inducing growth arrest.
GDF5 induces morphological changes in SH-SY5Y cells. a Phase contrast microphotographs of untreated, GDF5-treated (100 ng/ml), and RA-treated (10 μM) SH-SY5Y cells. b The numbers of primary and secondary neurites in untreated, GDF5-treated (100 ng/ml), and RA-treated (10 μM) cells after 7 days. Data are presented as the mean ± SEM of 60 cells from 3 experiments (*P < 0.05 compared with untreated cells, One-way ANOVA with Dunnett’s post-hoc analysis). c The length of the neuritic arborisations in untreated, GDF5-treated (100 ng/ml), and RA-treated (10 μM) cells after 7 days. Data are presented as the mean ± SEM length per cell of 60 cells from 3 experiments (*P < 0.05 and ***P < 0.001 compared with untreated cells, one-way ANOVA with Dunnett’s post-hoc analysis)
Treatment with GDF5 Does Not Affect Tyrosine Hydroxylase Expression
Previous results from our lab showed that treatment of primary VM cultures with GDF5 leads to an increased survival/number of DA neurones (O’Keeffe et al. 2004a). SH-SY5Y cells have been reported by some groups to readily express tyrosine hydroxylase (TH, the rate-limiting enzyme in the synthesis of DA) (Gomez-Santos et al. 2002; McMillan et al. 2007), while others failed to demonstrate expression (Mastroeni et al. 2009). To assess whether the neuroblastoma cell line model used here could recapitulate the results obtained using primary cultures, we analysed TH expression by RT-PCR, immuncytochemistry and immunoblotting in cultures maintained in the presence of 100 ng/ml GDF5 for 4–7 days. Using adult rat midbrain extracts or cryosections as positive controls, we were unable to demonstrate the presence of TH expression in SH-SY5Y cells in the presence or absence of GDF5 (data not shown).
Treatment with GDF5 Protects SH-SY5Y Cells from 6-OHDA-Induced Toxicity
Having shown that GDF5 stimulates neuronal maturation we next investigated its neuroprotective properties. GDF5 has been previously shown to protect DA neurones from 6-OHDA-induced neurotoxicity both in vitro and in vivo (O’Keeffe et al. 2004a; Sullivan et al. 1997, 1998, 1999). To assess whether SH-SY5Y cells could represent a good cellular model to study the neuroprotective properties of GDF5, we demonstrated that SH-SY5Y cells express the dopamine transporter (DAT) involved in the uptake of 6-OHDA, on their surface (data not shown). Various treatment modalities with GDF5 and 6-OHDA were devised (Fig. 5a) and neuronal survival was assessed using MTT assays.
Neuroprotective effects of GDF5 on SH-SY5Y cells. a Experimental groups assessed. b Cell viability in each treatment group after 48 h, as measured by MTT assays. Mean absorbance values ± SEM are presented, with untreated SH-SY5Y cells (group 1) considered as 100% viability (*P < 0.05 compared to group 1, $ P < 0.05 compared to group 2, ANOVA with post-hoc Tukey’s test)
Cell viability was decreased by 34% following a 6-OHDA treatment (50 μM, 1 h) compared with untreated controls (group 2 vs. group 1, P < 0.01, Fig. 5b). Continuous GDF5 treatment significantly protected cells from 6-OHDA-induced toxicity (group 3 vs. group 2, P < 0.05, Fig. 5b). There was no observable difference between groups 1 and 3 (100% vs. 98% viability). GDF5 treatment applied only before or only after the 6-OHDA insult (groups 4 and 5, respectively) conferred significant neuroprotection (group 4 vs. group 2 P < 0.05 and group 5 vs. group 2, P < 0.05, Fig. 4b). With the exception of group 2, there was no difference in viability between group 1 and any of the other groups (Fig. 5).
Discussion
The neuroprotective properties of GDF5 have been well documented in primary neuronal cultures and in in vivo models of Parkinson’s disease. However, due to the mixed cell populations of these models, it is not possible to determine whether the effects are direct or if they are mediated through other cell types, such as glial cells. In an attempt to establish a cell line model to further study the neuroprotective effects of GDF5, we have characterised the neuroblastoma cell line SH-SY5Y with regards to the expression of GDF5 surface receptors and its responsiveness to this neurotrophin.
This study confirmed that all four types of GDF5 receptor are expressed on SH-SY5Y cells. In addition, we showed that the principal signal transduction machinery for GDF5, SMAD proteins 1, 5 and 8, are present in SH-SY5Y cells and is activated in response to GDF5 treatment. This neuroblastoma cell line has previously been shown to respond to neuronal differentiating agents by exiting the mitotic cycle and acquiring a more complex dendritic arborisation (Pahlman et al. 1984, 1995). Our results showed that, while the effect of GDF5 is not as strong as that of RA, cell growth was nonetheless significantly inhibited when the cells were grown in the presence of GDF5. Analysis of the dendritic arborisation revealed that treatment with GDF5 for 7 days resulted in the development of a more extensive neurite network, particularly at secondary branching points.
Some authors have previously reported that TH is readily expressed by SH-SY5Y cells, while others reported that TH is not expressed in undifferentiated SH-SY5Y cells (Gomez-Santos et al. 2002; Mastroeni et al. 2009; McMillan et al. 2007). In addition, GDF5 has previously been reported to induce DA differentiation of rat primary VM cultures (O’Keeffe et al. 2004a). The absence of TH induction following GDF5 application found in the present article could be due to a variety of factors, including clonal variations, defects in signalling pathways or in the TH gene promoter or to the dosage of GDF5 used. Gomez-Santos et al. (2002) previously showed that in SH-SY5Y cells, TH expression is induced through the activation of SMAD2/3 and not SMAD1/5/8. The latter group of SMADs are the targets of the BMPR pathway (ten Dijke et al. 2000) suggesting that the induction of the dopaminergic phenotype in primary VM cultures may have been an indirect effect of GDF5 treatment. This is further supported by results from Castelo-Branco et al. (2006) showing that secretion of Wnt5a by VM glia is an important event in the differentiation of VM dopaminergic neurones and that blockade of this signal results in reduced DA differentiation. Interestingly, Wnt5a is a ligand for ROR2 and induces homodimerization of the receptor on the cell surface (Liu et al. 2008). While it is obvious that the SH-SY5Y cells do not recapitulate the events observed in GDF5-induced primary VM dopaminergic differentiation, we propose that the stimulation of TH expression observed in the O’Keeffe study was ultimately achieved via signals secreted from surrounding cells in primary cultures. For example, Wnt5a secreted by glial cells could have acted on the neurones but ultimately, GDF5 stimulation did not directly influence neuronal dopaminergic differentiation.
In our final series of experiments, we demonstrated that, while SH-SY5Y cells do not reproduce all the dopaminergic features of primary VM cultures, they nonetheless represent an excellent model to study the neuroprotective effects of GDF5. We confirmed that the SH-SY5Y cells express the dopamine transporter protein DAT. Although some authors have shown that 6-OHDA neurotoxicity requires neuronal uptake by DAT in vivo (Glinka et al. 1997; Storch et al. 2004), results obtained from primary cell cultures and cell lines, including SH-SY5Y, suggest that 6-OHDA neurotoxicity in vitro maybe mediated independently of DAT (Abad et al. 1995; Michel and Hefti 1990; Rosenberg 1988; Storch et al. 2000). Notwithstanding the mechanism, SH-SY5Y cells remain susceptible to the neurotoxic effects of 6-OHDA (Lopes et al. 2010; Storch et al. 2000). Our results showed that continuous treatment with GDF5 (group 3) could prevent neurotoxicity induced by 6-OHDA. Cells treated with GDF5 before a 6-OHDA pulse resisted its toxic effects (group 4, neuroprotection) but most importantly, application of GDF5 after the 6-OHDA pulse rescued most of the cells from the neurotoxic insult (group 5, neurorescue). Considering that neuroprotection following the onset of the disease is one of the major therapeutic avenues for the treatment of PD, our results suggest that GDF5 has enormous potential and the establishment of the model described here will greatly facilitate the elucidation of the pathways and intracellular machinery by which it confers neuroprotection.
GDF5 is one of the most potent neurotrophins characterised to date in animal models of PD but its molecular characterisation has been hindered by the lack of a good cellular model. While it remains an imperfect model for the characterisation of dopaminergic effects, our results show that SH-SY5Y cells are well suited to study some of the molecular events associated with GDF5 signalling and its role in neuronal differentiation and neuroprotection.
References
Abad F, Maroto R, Lopez MG et al (1995) Pharmacological protection against the cytotoxicity induced by 6-hydroxydopamine and H2O2 in chromaffin cells. Eur J Pharmacol 293:55–64
Akerud P, Alberch J, Eketjall S et al (1999) Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem 73:70–78
Bartus RT (2009) CERE-120 (AAV2-NTN) for Parkinson’s Disease: Review of Progress and Future Plans. American Society of Cell and Gene Therapy Meeting, San Diego
Berk AJ, Sharp PA (1977) Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell 12:721–732
Braak H, Del Tredici K, Rub U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Castelo-Branco G, Sousa KM, Bryja V et al (2006) Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol Cell Neurosci 31:251–262
Clayton KB, Sullivan AM (2007) Differential effects of GDF5 on the medial and lateral rat ventral mesencephalon. Neurosci Lett 427:132–137
Cookson MR (2005) The biochemistry of Parkinson’s disease. Annu Rev Biochem 74:29–52
Gasmi M, Brandon EP, Herzog CD et al (2007a) AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis 27:67–76
Gasmi M, Herzog CD, Brandon EP et al (2007b) Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol Ther 15:62–68
Gill SS, Patel NK, Hotton GR et al (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595
Glinka Y, Gassen M, Youdim MB (1997) Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl 50:55–66
Gomez-Santos C, Ambrosio S, Ventura F et al (2002) TGF-beta1 increases tyrosine hydroxylase expression by a mechanism blocked by BMP-2 in human neuroblastoma SH-SY5Y cells. Brain Res 958:152–160
Herzog CD, Dass B, Holden JE et al (2007) Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord 22:1124–1132
Horger BA, Nishimura MC, Armanini MP et al (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18:4929–4937
Hurley FM, Costello DJ, Sullivan AM (2004) Neuroprotective effects of delayed administration of growth/differentiation factor-5 in the partial lesion model of Parkinson’s disease. Exp Neurol 185:281–289
Kordower JH, Herzog CD, Dass B et al (2006) Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60:706–715
Krieglstein K, Suter-Crazzolara C, Hotten G et al (1995) Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. J Neurosci Res 42:724–732
Lin LF, Doherty DH, Lile JD et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Liu Y, Rubin B, Bodine PV et al (2008) Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem 105:497–502
Lopes FM, Schroder R, Junior ML et al (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94
Marks WJ, Verhagen Metman L, Starr PA, et al (2006) Trophic factor gene transfer in Parkinson’s disease: preliminary outcomes from the phase I CERE-120 study. American Neurological Association, 131st Annual Meeting, Chicago, IL
Mastroeni D, Grover A, Leonard B et al (2009) Microglial responses to dopamine in a cell culture model of Parkinson’s disease. Neurobiol Aging 30:1805–1817
Mayhew TM (1992) A review of recent advances in stereology for quantifying neural structure. J Neurocytol 21:313–328
McMillan CR, Sharma R, Ottenhof T et al (2007) Modulation of tyrosine hydroxylase expression by melatonin in human SH-SY5Y neuroblastoma cells. Neurosci Lett 419:202–206
Michel PP, Hefti F (1990) Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res 26:428–435
Nishitoh H, Ichijo H, Kimura M et al (1996) Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem 271:21345–21352
O’Keeffe GW, Dockery P, Sullivan AM (2004a) Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol 33:479–488
O’Keeffe GW, Hanke M, Pohl J et al (2004b) Expression of growth differentiation factor-5 in the developing and adult rat brain. Brain Res Dev Brain Res 151:199–202
Pahlman S, Ruusala AI, Abrahamsson L et al (1984) Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ 14:135–144
Pahlman S, Hoehner JC, Nanberg E et al (1995) Differentiation and survival influences of growth factors in human neuroblastoma. Eur J Cancer 31A:453–458
Patel NK, Bunnage M, Plaha P et al (2005) Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 57:298–302
Rosenberg PA (1988) Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci 8:2887–2894
Sammar M, Stricker S, Schwabe GC et al (2004) Modulation of GDF5/BRI-b signalling through interaction with the tyrosine kinase receptor Ror2. Genes Cells 9:1227–1238
Sammar M, Sieber C, Knaus P (2009) Biochemical and functional characterization of the Ror2/BRIb receptor complex. Biochem Biophys Res Commun 381:1–6
Slevin JT, Gerhardt GA, Smith CD et al (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102:216–222
Storch A, Kaftan A, Burkhardt K et al (2000) 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: independent of mitochondrial energy metabolism. J Neural Transm 107:281–293
Storch A, Ludolph AC, Schwarz J (2004) Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J Neural Transm 111:1267–1286
Sullivan AM, Opacka-Juffry J, Hotten G et al (1997) Growth/differentiation factor 5 protects nigrostriatal dopaminergic neurones in a rat model of Parkinson’s disease. Neurosci Lett 233:73–76
Sullivan AM, Pohl J, Blunt SB (1998) Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson’s disease. Eur J Neurosci 10:3681–3688
Sullivan AM, Opacka-Juffry J, Pohl J et al (1999) Neuroprotective effects of growth/differentiation factor 5 depend on the site of administration. Brain Res 818:176–179
ten Dijke P, Miyazono K, Heldin CH (2000) Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci 25:64–70
Toulouse A, Sullivan AM (2008) Progress in Parkinson’s disease-where do we stand? Prog Neurobiol 85:376–392
Wood TK, McDermott KW, Sullivan AM (2005) Differential effects of growth/differentiation factor 5 and glial cell line-derived neurotrophic factor on dopaminergic neurons and astroglia in cultures of embryonic rat midbrain. J Neurosci Res 80:759–766
Acknowledgments
The authors would like to thank Dr Jens Pohl from Biopharm GmbH for the generous gift of GDF5, Dr Gerard O’Keeffe from University College Cork for his comments, and the Higher Education Authority (Ireland) for funding through the PRTLI3 programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toulouse, A., Collins, G.C. & Sullivan, A.M. Neurotrophic Effects of Growth/Differentiation Factor 5 in a Neuronal Cell Line. Neurotox Res 21, 256–265 (2012). https://doi.org/10.1007/s12640-011-9266-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-011-9266-7