Abstract
Cryptosporidium parvum is an important coccidian parasite that could infect the intestine, respiratory and biliary tracts of man and animals. This study aims to test the potential therapeutic and prophylactic effects of a natural herbal agent (Asafoetida) versus the nowadays drug of choice (Nitazoxanide). Fifty bred female, white Albino mice of CDI strain were divided into 5 groups; group I (GI): immunosuppressed, infected with C. parvum and treated with Asafoetida, group II (GII): immunosuppressed, prophylactically treated with Asafoetida for 7 days prior to infection, group III (GIII): immunosuppressed, infected and treated with Nitazoxanide, group IV (GIV): immunosuppressed and infected (Positive control), group V (GV): immunosuppressed and non infected (Negative control). Parasitological and histopatholgical examinations of the stool, ileocaecal and liver specimens were performed for the study groups. GI showed reduction of the mean oocyst count in stool with improvement of the pathological changes at the ileocaecal region with preservation of hepatic architecture. Results of GI were better than GII and GIV but not as good as GIII. GII showed the least improvement among the test groups. GIII showed the best response between the test groups. GIV show no statistical significant difference between the mean oocyst count in the mice stool at the time of infection and 7 days after infection. It was therefore concluded that Asafoetida is a promising natural therapeutic and prophylactic agent against cryptosporidiosis while, Nitazoxanide is the best chemotherapeutic agent against cryptosporidiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryptosporidium parvum (C. parvum) is an obligate intracellular coccidian protozoon parasite causing a disease called cryptosporidiosis. In developing countries, it is considered to be the most significant waterborne organism (Haque et al. 2003). It accounts for the majority of parasitic intestinal infections worldwide (Doganci et al. 2002). Nevertheless, many infection outbreaks occurred in the developed countries, treated water in swimming and wading pools had strong associations with such outbreaks (Craun et al. 2005). Cryptosporidium can maintain viability and infectivity in moist and cool situations for several months (Dawson et al. 2004). Many strains of Cryptosporidium were reported in human patients, such as C. parvum, C. hominis and C. bovis (Helmy et al. 2013). Cryptosporidium spp. infection in animals leads to an economic loss and becomes an important source for zoonotic infection. Clinically, C. parvum may precipitate severe life-threatening diarrhea in immunocompromised hosts (Guk et al. 2005). Moreover, a case control study was performed in United Kingdom and Sweden, revealed that Cryptosporidium infection may lead to persistent symptoms in immunocompetent patients (Hunter et al. 2004 and Insulander et al. 2013). Post infectious irritable bowel syndrome was strongly suggested to be the cause of the persistent symptoms (Marion et al. 2006 and Khaldi et al. 2009). Another study reported reactive arthropathy following cryptosporidiosis (Hay et al. 1987). Nitazoxanide (Nanazoxid) is the drug of choice for treatment of cryptosporidiosis as it helps to alleviate diarrhea by attacking the parasite metabolic processes. In immunocomromised patients, a combination of Azithromycin (Zithromax) along with one of the antiprotozoal drugs may be used (Rosenblatt 1992). Although, Nitazoxanide was an important innovative drug treatment for cryptosporidiosis in children, it has a limited efficacy in compromised or malnourished patients. This raises an important question about the proper management for these patients (Hayley et al. 2015). There are few available drugs against cryptosporidiosis like Azithromycin, Paromomycin, Roxithromycin and antiretroviral drugs (Gargala 2008). Unfortunately, these drugs are not effective to kill the infectious oocyst with probable side effects in addition to restricted availability in developing countries. Furthermore, many reports about drug toxicity, resistance and failure made the prescription of such synthetic antimicrobials non preferable for doctors. So, it is a great challenge for the health professionals to find a novel, effective, safe and inexpensive drug alternatives (Rosenblatt 1992).
It noteworthy, the medicinal herbs were used for treatment of different parasitic infections all over the world so many years ago (Behnke et al. 2008). In the herbal medicine, Asafoetida was used as a mucokinetic, diuretic, sedative and antiparasitic agent. In traditional therapies, Asafoetida was used in a dose of 0.2–0.5 g daily (Mohammadi et al. 2009 and Sadraei et al. 2003). Different studies proved that the aqueous extract, resin extract from the stems and roots and essential oil of Asafoetida was effective treatment for intestinal worms, lead to inhibition of Trichomonas vaginalis growth in vitro, and decrease the Schistosoma mansoni worm load in the experimental animals respectively (Bhattarai 1992; Ramadan and Al Khadrawy 2003; Ramadan et al. 2004).
With this introduction in mind, this study aimed to evaluate the potential therapeutic and prophylactic effects of the methanol extract of Asafoetida on white albino mice that were experimentally immunosuppressed and infected with C. parvum and compare the therapeutic effect with Nitazoxanide.
Aim of the work
Evaluating the potential therapeutic and prophylactic effects of Asafoetida in murine cryptosporidiosis.
Materials and methods
Animals
Animal source and handling
Laboratory bred female, white Albino mice of CDI strain, about 4–6 weeks old, weighing 20–25 g, were obtained from Theodor Bilharz Animal house. Animal experiments were performed in the biological unit of Theodor Bilharz Research Institute (TBRI), in a well-ventilated plastic cage with clean wood-chip bedding in conditioned rooms (27 ± 2 °C) and away from direct sunlight, ensuring good sanitary condition. All applied experiments on animals were carried out according to the internationally valid guidelines after the approval of the institutional ethical committee.
Animal groups
Fifty laboratory bred white albino female mice were used in this study.
Mice were divided into 5 groups; 10 mice each:
- Group I:
-
immunosuppressed, infected and treated with Asafoetida
- Group II:
-
immunosuppressed and received Asafoetida for 7 days prior to infection (as prophylaxis)
- Group III:
-
immunosuppressed, infected and treated with Nitazoxanide
- Group IV:
-
immunosuppressed and infected (Positive control)
- Group V:
-
immunosuppressed and non infected (Negative control)
Immunosuppression
Mice were administered with 0.25 ug/g/day of dexamethasone sodium phosphate (Dexazone) orally by gavages using esophageal tube. Dexazone was given daily for 2 weeks prior to oral inoculation with Cryptosporidium oocysts and was maintained weekly during the whole experiment for all groups (Rehquel et al. 1998).
Infection
Cryptosporidium parvum oocysts were obtained from the Animal Research Institute in Giza governorate, Egypt. Mice were orally infected with C. parvum oocysts via gavage using esophageal tube. A pilot study was performed to calculate the proper infecting dose by oral infection of mice with different doses of oocysts followed by stool examination 1 and 2 weeks after the infection; infecting each mouse by 500 oocysts didn’t establish infection, increasing the dose to 1000 oocysts succeeded to establish infection but further increasing the dose to 5000 oocysts caused the death of the mice.
Accordingly, the infection dose for (GI–GIV) was 1000 C. parvum oocysts that were dissolved in 200μL of PBS.
Drugs administration
-
Plant material
Asafoetida powder was purchased from a local market in Giza governorate, Egypt. The identification of the plant was authenticated at the Department of Botany, Faculty of Science, Cairo University, Egypt.
-
Preparation of methanol extract of Asafoetida
The dried powder of Asafoetida was soaked overnight in absolute methanol at room temperature. The pooled extracts were filtered, evaporated under reduced pressure using rotatory evaporator (Ghareeb et al. 2018).
GI received 5% concentrations of the methanol extract of Asafoetida. The extract of the natural herb was given for 7 successive days post infection. GII got the infection after a 7 days course of the methanol extract of Asafoetida prophylactic administration (Farhadi et al. 2016).
This dose was selected according to pilot study that revealed very weak effect using a concentration of 2.5% and the same responses on using a concentration of 5% and 10%, so we preferred to use the minimal effective dose.
GIII received oral suspension of Nitazoxanide in a dose of 65 mg. The drug was administered for 7 successive days post infection. The doses were calculated by extrapolation of human therapeutic doses to animal doses according to the table of (Barnes and Paget 1965).
Parasitological examination
Stool samples were collected and subjected to parasitological examination after staining by modified Zheil Nelsen (MZN) stain according to (Henricksen and Pohlenz 1981) using the oil immersion lens (× 100) to ensure that mice have been infected and to count C. parvum oocysts.
Oocyst count in stool was done in GI, GII, GIII and GIV 7 days post infection followed by sacrifice of the tested mice and for GV it was done at the same time as previous groups.
Histopathological examination
Sacrifice of all mice was done 7 days post infection and it was done at the same time for the mice of GV. The ileocecal region and liver were obtained and fixed in 10% buffered formalin solution, embedded in paraffin wax blocks that were sectioned then stained in the pathology lab of TBRI, staining was done using Hematoxylin and Eosin (H&E) to assess the pathological changes and explore any abnormal proliferation pattern.
Statistical analysis
Data were analyzed using paired sample t test, P value is considered significant if < 0.0001.
Ethical consideration
This study was approved by Scientific Research Ethics Committee of TBRI.
Results
Parasitological examination for the stool of different study groups
GI, GII and GIII showed highly significant statistical difference between the mean oocyst count in the stool of mice before and after treatment (GI: 91.4 and 25.10 respectively) (GII: 96.56 and 48.11 respectively) and (GIII: 92.2 and 3.0 respectively). The study revealed that GI showed improvement more than GII and GIV but less than GIII. GII showed the least improvement among the test groups. GIII showed the best response between the test groups with marked reduction of the mean oocyst count in the stool. GIV show no statistical significant difference between the mean oocyst count in the mice stool at the time of infection and 7 days after infection (Table 1).
Histopathological examination of sections from ileocaecal junction and livers of the study groups
-
GI
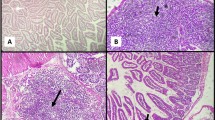
Examination of small intestinal sections at ileocaecal junction revealed partial improvement of the histopathological changes which follow cryptosporidiosis infection in the form of mild villous blunting with mild decrease villous height to crypt length ratio. There was partial healing of intestinal mucosa with focally ulcerated mucosa, mild goblet cell depletion and mild inflammatory infiltration in the lamina propria. Sections examined from liver revealed preserved architecture with slightly expanded portal tracts showing mild lymphocytic infiltrate as shown in Fig. 1.
a Section of small intestine in GI showing partial villous blunting with partial healing and focal ulceration of intestinal mucosa “red arrows” and mild decrease in the ratio between villous height to crypt length “black arrow” (H&E stain ×200). b Section of liver in this group showing preserved architecture and slightly expanded portal tracts showing mild lymphocytic infiltrate “black arrow heads” (H&E stain ×200) (colour figure online)
-
GII
Examination of small intestinal sections at ilocecal junction revealed mild improvement of the histopathological changes that follow cryptosporidiosis infection including partial villous blunting and broadening with moderate decrease in villous height to crypt length ratio. There were ulcerations in the intestinal mucosa with partial healing, moderate goblet cell depletion and moderate inflammatory infiltration in the lamina propria. Sections examined from liver revealed preserved architecture with expanded portal tracts showing moderate lymphocytic infiltrate as shown in Fig. 2.
a Section of small intestine in GII showing partial healing of the intestinal mucosa with areas of ulceration “black arrows” moderate goblet cell depletion and moderate inflammatory infiltration in the lamina propria “red arrows” (H&E stain ×200). b Liver section of this group with “black arrow heads” showing moderate lymphocytic infiltrate of portal tracts (H&E stain ×200) (colour figure online)
-
GIII
Examination of sections from ileocaecal junction revealed significant improvement of the histopathological changes following cryptosporidiosis infection including restoration of the normal villous architecture with preserved villous height to crypt length ratio. The mucosal lining becomes intact with healing of the surface epithelium, preserved brush border of the villi and minimal surface erosions. Goblet cells were preserved with no depletion and minimal inflammatory infiltration in the lamina propria was detected. No cryptosporidium oocysts could be detected. Examination of liver sections showed preserved architecture and slightly congested sinusoids as shown in Fig. 3.
a Section of small intestine in GIII revealed healing of intestinal surface epithelium with minute surface erosions “red arrows” (H&E stain ×200). b Sections examined from liver in this group showed preserved hepatic architecture and slightly congested sinusoids and central veins “black arrow heads” (H&E stain ×200) (colour figure online)
-
GIV
Examination of sections from ileocaecal junction revealed significant histopathological changes in small intestinal villi architecture as a result of cryptospodiosis infection. These changes were in the form of variable grades of villous atrophy with shortened broad villi and decreased ratio between villous height to crypt length. Mucosal ulceration and goblet cell depletion were detected together with inflammatory infiltrate of lamina propria formed mainly of lymphocytes, plasma cells and macrophages with lymphoid follicles hyperplasia. Cryptosporidum oocysts were detected along the brush border of the villi and in the intestinal lumen. Examination of liver sections revealed slightly congested sinusoids, scattered foci of spotty necrosis and interface activity with expanded portal tracts showing moderate lymphocytic infiltration forming porto-central bridges with interface activity and moderate fibrosis as shown in Fig. 4.
a Section of small intestine from GIV showing significant villous blunting and ulceration “red arrows” (H&E stain ×200). b Marked inflammatory infiltrate in the lamina proria with lymphoid follicle hyperplasia “black arrows” (H& E stain ×100). cCryptosporidium oocysts (tiny purple stained structures) detected in the intestinal lumen and along brush border of the villi “blue arrows” (H&E stain ×1000 “oil immersion”). d Section of liver in GIV showing expanded portal tracts with marked inflammation and moderate fibrosis “black arrow heads” (H&E stain ×200) (colour figure online)
-
GV
Examination of sections from ileocaecal junction revealed normal architecture of small intestinal villi with average length and width. The brush border was intact, well formed and goblet cells are of average number. Examination of liver sections revealed preserved hepatic architecture with hepatocytes arranged in regular plates around central vein with portal tracts showing normal structures Fig. 5.
Discussion
Cryptosporidium parvum although causes self-limiting diarrhea in immunocompetent patients, it leads to severe and life-threatening diarrhea in immunocompromised hosts (Guk et al. 2005).
The side effects and the increasing resistance to the available antiparasitic drugs used in the treatment of C. parvum make the researchers face a great challenge to find a natural herbal alternative that is not toxic and easily obtained in adequate quantities to replace the standard drugs in the market (Rayan et al. 2005).
According to the results of the present study, treatment with the methanol extract of the natural herb Asafoetida could effectively reduce Cryptosporidium parasites in experimentally infected mice and improve the histopathological changes of small intestinal villi and preservation of liver architecture with slightly expanded portal tracts showing mild lymphocytic infiltrate following cryptosporidiosis. The antiprotozoal properties of Asafoetida were previously assessed against other protozoal parasites. The effects of Asafoetida herbal extract against Leishmania major promastigotes was tested, and revealed an important anti-leishmanial effect of this herb (Barati et al. 2010). This is in congruence with authors who proved reduction of the Trichomonas vaginalis parasite load by 90% within 1 h of exposure by giving the herbal extract of Asafoetida at the concentration of 2 mg/ml (Sarkari et al. 2009). Lethal effects against Giardia cysts were also reported by the aqueous and alcoholic extracts of Asafoetida (Rezaiemanesh and Shirbazou 2012). The antihelminthic effects of Asafoetida were tested by many authors who proved the anti-trematode effects of Asafoetida through the oral administration of Asafoetida essential oil. It notably reduced the number of eggs and worms in infected rats with Schistosoma mansoni (Ramadan et al. 2004). In another study, the anti-cestode effects of Asafoetida against Hymenolepis nana were proved through administration of 17.7 mg/ml of Asafoetida extract for 2 weeks with health improvement in around 90% of the infected rats (Maraghi and Soghra 1991). This is similar to a study that proved a significant decrease in the number of Hymenolepis nana in the experimental animals receiving the methanol extract of Asafoetida compared to control subjects (Youssefi et al. 2012). Nevertheless, the herbal extract fails to show a significant effect against nematode Syphacia obvelata, which exclude the antinematode effect of this medicinal herb (Farhadi et al. 2016).
The prophylactic doses of methanol extract of Asafoetida showed significant reduction of the mean oocyst count in the stool of infected mice 7 days after the infection, mild improvement of the histopathological changes at the ileocaecal region, preserved liver architecture with expanded portal tracts showing moderate lymphocytic infiltrates. A review and meta-analysis declared that Nitazoxanide led to important evidence of oocyst clearance compared with placebo. The effect was not significant for HIV-seropositive participants. The absence of effective treatment for immunocompromized patients made an alarm for the importance of preventive interventions (Abubakar et al. 2007).
Nitazoxanide showed the best parasitological and histopathological responses in this study by significantly reducing the mean oocyst count in the stool of infected mice 7 days after treatment and also restore the normal villous and liver architectures following cryptosporidiosis. Nitazoxanide (NTZ) was described as a drug with broad activity against protozoa and helminthes. NTZ and its two metabolites, tizoxanide (TZ) and tizoxanide-glucuronide (TZglu) were proved to suppress the growth of C. parvum in a concentrations < 10 mg/L (Theodos et al. 1998; Gargala et al. 2000 and Cai et al. 2005). Activity in the intestine is the role of NTZ and TZ. TZglu is excreted in high concentrations in bile and it is believed to be responsible for the activity of the drug against cholangitis due to disseminated cryptosporidiosis in immunocompromised patients. Clinical and parasitological cures from cryptosporidiosis were reported in immunocompetent adults, adolescents and children that were treated by a 3 days course of NTZ (Rossignol 2001; Rossignol 2006 and Amadi et al. 2002). Fifty-nine percent of adult AIDS patients with cryptosporidial diarrhea showed a persistent clinical response while on treatment with NTZ. Reports stated that doses up to 3000 mg/day or for long duration of treatment were non safe (Rossignol 2006).
Conclusion
Results of the present research indicated that the methanol extract of Asafoetida has promising therapeutic and prophylactic effects against C. parvum. Menthol extract of Asafoetida is cheap, easily obtained in suitable quantities and help to avoid the adverse reactions caused by chemotherapeutic drugs. Furthermore, complementary research regarding the side effects of this medicinal herb or its compounds will present it as useful therapeutic and prophylactic agent against C. parvum for the immunocompromized patients.
References
Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR (2007) Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol 63(4):387–393
Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P (2002) Effect of NTZ on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360(9343):1375–1380
Barati M, Sharifi A, Sharifi Far S (2010) Antileishmanial activity of Artemisia aucheri, Ferula asafoetid and Gossypium hirsutum extracts on Leishmania major promastigotes in vitro. J Artesh Med Univ 8(3):166–172
Barnes JM, Paget GE (1965) Mechanisms of toxic action. Prog Med Chem 4:18–38
Behnke JM, Buttle DJ, Stepek G, Lowe A, Duce IR (2008) Developing novel anthelmintics from plant cysteine proteinases. Paras Vec 1(1):1–18
Bhattarai N (1992) Folk anthelmintic drugs of central Nepal. Int J Pharmacol 30(2):145–150
Cai X, Woods KM, Upton SJ, Zhu G (2005) Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob Agents Chemother 49(11):4437–4442
Craun GF, Calderon RL, Craun MF (2005) Outbreaks associated with recreational water in the United States. Int J Environ Health Res 15:243–262
Dawson DJ, Samuel CM, Scrannage V, Atherton CJ (2004) Survival of Cryptosporidium species in environments relevant to foods and beverages. J Appl Microbiol 96:1222–1229
Doganci T, Araz E, Ensari A, Tanyuksel M, Doganci L (2002) Detection of Cryptosporidium parvum infection in childhood using various techniques. Med Sci Monit 8:223–226
Farhadi A, Youssefi MR, Abouhosseini TM (2016) Evaluation of the anticestode and antinematode Effects of the methanol extract of ferula asafoetida on experimentally infected rats. J Babol Univ Med Sci 18(6):47–51
Gargala G (2008) Drug treatment and novel drug target against cryptosporidium. Parasite 15:275–281
Gargala G, Delaunay A, Li X, Brasseur PH, Favennec L, Ballet JJ (2000) Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J Antimicrob Chemother 46(1):57–60
Ghareeb MA, Mohamed T, Saad AM, Refahy LA, Sobeh M, Wink M (2018) HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J Pharm Pharmacol 70:133–142
Guk SM, Seo M, Park YK, Oh MD, Choe KW, Kim JL et al (2005) Parasitic infections in HIV-infected patients who visited Seoul National University Hospital during the period 1995–2003. Korean J Parasitol 43:1–5
Haque R, Huston CD, Hughes M, Houpt E, Petri WA (2003) Cryptosporidiosis. N Engl J Med 348:1565–1573
Hay EM, Winfield J, McKendrick MW (1987) Reactive arthritis associated with Cryptosporidium enteritis. Br Med J (Clin Res Ed) 295:248
Hayley BS, Gayatri MD, Alejandro PhD, Clinton A (2015) Treatment of Cryptosporidium: what we know, gaps, and the way forward. Curr Trop Med Rep 2(3):181–187
Helmy YA, Krücken J, von Samson-Himmelstjerna G, Zessin KH (2013) Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet Parasitol 193(1–3):15–24
Henricksen S, Pohlenz J (1981) Staining of Cryptosporidium by a modified Ziehl-Neelsen technique. Acta Vet Scand 22:594–596
Hunter PR, Hughes S, Woodhouse S, Raj N, Syed Q, Chalmers RM et al (2004) Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis 39:504–510
Insulander M, Silverlas C, Lebbad M, Karlsson L, Mattsson JG, Svenungsson B (2013) Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol Infect 14:1009–1020
Khaldi S, Gargala G, Le Goff L, Parey S, Francois A, Fioramonti J (2009) Cryptosporidium parvum isolate-dependent post-infectious jejunal hypersensitivity and mast cell accumulation in an immunocompetent rat model. Infect Immune 77:5163–5169
Maraghi S, Soghra T (1991) In vitro and in vivo assay of Ferulla assa extract effects on Hymenolepis nana and comparison of it with Niclosamide. Jundisahpour J Med Sci 23:48–56
Marion R, Baishanbo A, Gargala G, Francois A, Ducrotte P, Duclos C et al (2006) Transient neonatal Cryptosporidium parvum infection triggers long- term jejunal hypersensitivity to distension in immunocompetent rats. Infect Immun 74:4387–4389
Mohammadi R, Sepahvand A, Mohammadi SR, Mirsafaei H, Shargh RN (2009) Antifungal activity of Ferula assafoetida against clinical agents of Mucormycosis. J Isfahan Med Sch 27(100):582–588
Ramadan NI, Al Khadrawy F (2003) The in vitro effect of assafoetida on Trichomonas vaginalis. J Egypt Soc Parasitol 33(2):615–630
Ramadan N, Abdel-Aaty H, Abdel-Hameed D, El Deeb H, Samir N, Mansy S et al (2004) Effect of ferula asafoetida on experimental murine Schistosoma mansoni infection. J Egypt Soc Parasitol 34(3):1077–1094
Rayan P, Stenzel D, McDonnell PA (2005) The effect of saturated fatty acids on Giardia duodenalis trophozoites in vitro. Parasitol Res 10:1432–1435
Rehquel T, David A, Belwett N, Manuel S, Carmona P (1998) C. parvum infection in experimentally infected mice: infection dynamics and effect of immunosuppression. Folkia Parasitol 45:101–107
Rezaiemanesh M, Shirbazou S (2012) In-vitro giardicidal effect of aqueous and alcoholic extracts of Asafoetida on Giardia lamblia cyst. J Birjand Univ Med Sci 19(1):22–23
Rosenblatt JE (1992) Antiparasitic agents. Mayo Clin Proced 67(3):276–287
Rossignol JF (2006) Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment Pharmacol Ther 24(5):887–894
Rossignol JF, Ayoub A, Ayers MS (2001) Treatment of diarrhea caused by C. parvum: a prospective randomized, doubleblind, placebo-controlled study of nitazoxanide. J Infect Dis 184(1):103–106
Sadraei H, Ghannadi A, Malekshahi K (2003) Composition of the essential oil of asafoetida and its spasmolytic action. Saudi Pharma J 11(3):136–140
Sarkari B, Hadisa T, Shahrbanoo A, Elahm F, Mehrangiz A (2009) In Vitro anti-Trichomonas activity of Freula assafoetida and garlic extracts. J Gorgan Uni Med Sci 11(3):13–17
Theodos CM, Griffiths JK, D’onfro J, Fairfield A, Tzipori S (1998) The efficacy of nitazoxanide against C. parvum in cell culture and in animal models. Antimicrob Agents Chemother 42(8):1959–1965
Youssefi MR, Abuhosseini Tabari M, Sadeghi Hashjin G, Kouhi MK (2012) Antiparasitic efficacy of worm wood (Artemisia absinthium) alcoholic extract on syphacia obvolata. Iran J Vet Med 6(1):47–50
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical consideration
The study was approved by the Research Ethics Committee, Theodor Bilharz Research Institute (TBRI). All the animal experiments were performed according to the national regulations for the Animal Ethics rules.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelmaksoud, H.F., El-Ashkar, A.M., Elgohary, S.A. et al. Potential therapeutic and prophylactic effects of Asafoetida in murine cryptosporidiosis. J Parasit Dis 44, 646–653 (2020). https://doi.org/10.1007/s12639-020-01241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01241-5